Co-Inoculation of Endophytes Bacillus siamensis TUR07-02b and Priestia megaterium SMBH14-02 Promotes Growth in Rice with Low Doses of Nitrogen Fertilizer
Abstract
:1. Introduction
2. Results
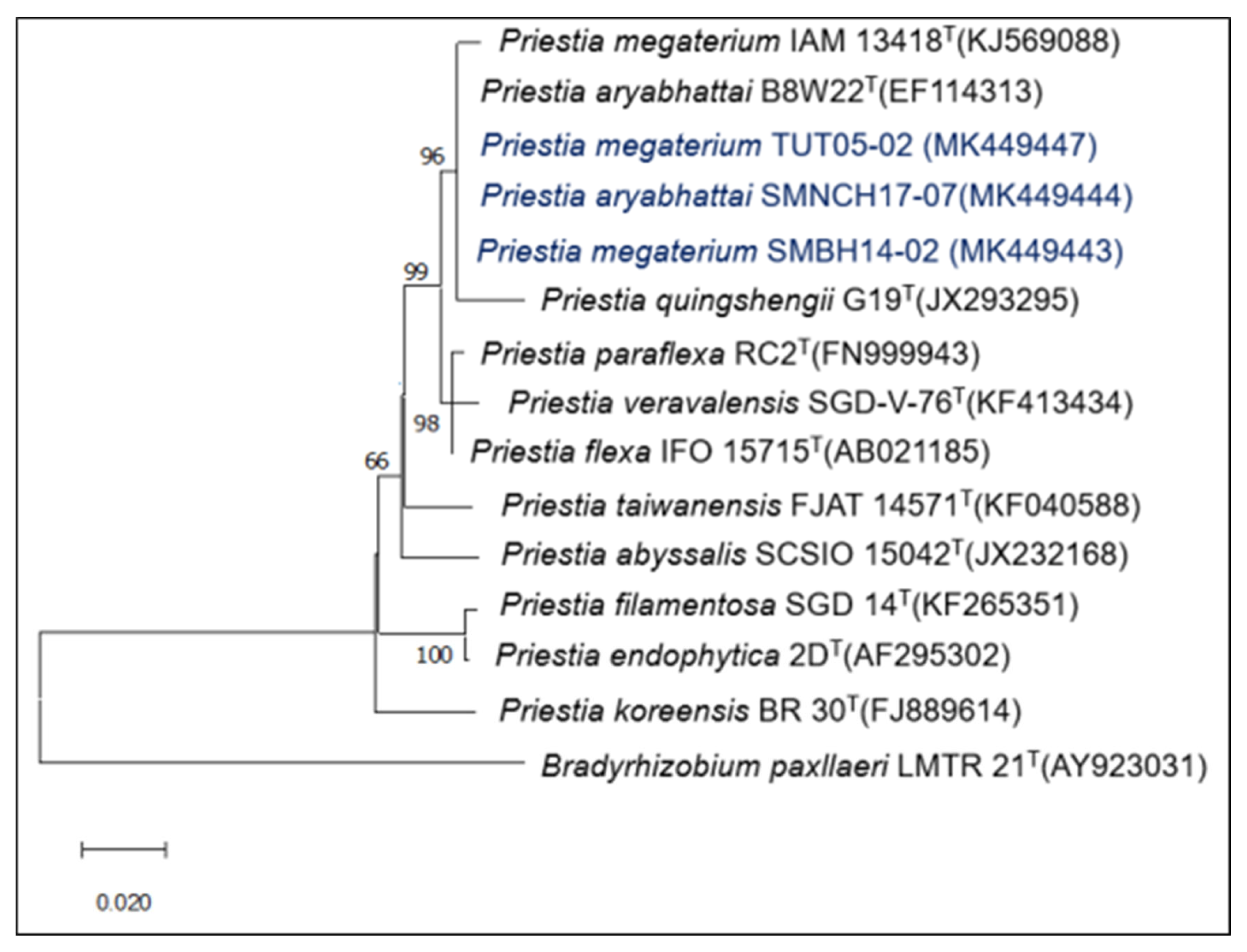
2.1. Strains under Study and Verification of Endophytic Colonization
2.2. Evaluation of Plant-Growth-Promoting (PGP) Bacterial Characteristics
2.3. Growth-Promotion Experiments
3. Discussion
3.1. Strains under Study and Verification of Endophytic Colonization
3.2. Evaluation of PGP Bacterial Characteristics
3.3. Growth-Promotion Experiments
4. Materials and Methods
4.1. Strains under Study
4.2. Verification of Endophyte Colonization
4.3. Evaluation of Growth-Promoting Characteristics
4.3.1. Determination of Indolic Compounds
4.3.2. Nitrogen Fixation
4.3.3. Solubilization of Ca, Fe, and Al Phosphates
4.3.4. Cellulase, Pectinase, and Chitinase Enzymes
4.4. Growth-Promotion Experiments
4.4.1. Germination
4.4.2. Effects of Inoculation on Agronomic Parameters
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Statistical Yearbook—World Food and Agriculture; FAO: Rome, Italy, 2021; 368 p., ISBN 978-92-5-134332-6. [Google Scholar]
- Mishra, A.K.; Mottaleb, K.A.; Khanal, A.R.; Mohanty, S. Abiotic stress and its impact on production efficiency: The case of rice farming in Bangladesh. Agric. Ecosyst. Environ. 2015, 199, 146–153. [Google Scholar] [CrossRef]
- Kumar, A.; Patel, J.S.; Meena, V.S.; Srivastava, R. Recent advances of PGPR based approaches for stress tolerance in plants for sustainable agriculture. Biocatal. Agric. Biotechnol. 2019, 20, 101271. [Google Scholar] [CrossRef]
- Rahman, C.R.; Arko, P.S.; Ali, M.E.; Khan, M.A.I.; Apon, S.H.; Nowrin, F.; Wasif, A. Identification and recognition of rice diseases and pests using convolutional neural networks. Biosyst. Eng. 2020, 194, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S. Sensing and uptake of nitrogen in rice plant: A molecular view. Rice Sci. 2019, 26, 343–355. [Google Scholar] [CrossRef]
- Han, M.L.; Lv, Q.Y.; Zhang, J.; Wang, T.; Zhang, C.X.; Tan, R.J.; Wang, Y.L.; Zhong, L.Y.; Gao, Y.Q.; Chao, Z.F.; et al. Decreasing nitrogen assimilation under drought stress by suppressing DST-mediated activation of Nitrate Reductase 1. 2 in rice. Mol. Plant 2022, 15, 167–178. [Google Scholar] [CrossRef]
- Lyu, Y.; Yang, X.; Pan, H.; Zhang, X.; Cao, H.; Ulgiati, S.; Wu, J.; Zhang, Y.; Wang, G.; Xiao, Y. Impact of fertilization schemes with different ratios of urea to controlled release nitrogen fertilizer on environmental sustainability, nitrogen use efficiency and economic benefit of rice production: A study case from Southwest China. J. Clean. Prod. 2021, 293, 126198. [Google Scholar] [CrossRef]
- Bora, K. Spatial patterns of fertilizer use and imbalances: Evidence from rice cultivation in India. Environ. Chall. 2022, 7, 100452. [Google Scholar] [CrossRef]
- Doni, F.; Suhaimi, N.S.M.; Mispan, M.S.; Fathurrahman, F.; Marzuki, B.M.; Kusmoro, J.; Uphoff, N. Microbial Contributions for Rice Production: From Conventional Crop Management to the Use of ‘Omics’ Technologies. Int. J. Mol. Sci. Int. J. Mol. Sci. 2022, 23, 737. [Google Scholar] [CrossRef]
- Ríos-Ruiz, W.F.; Torres-Chávez, E.E.; Torres-Delgado, J.; Rojas-García, J.C.; Bedmar, E.J.; Valdez-Nuñez, R.A. Inoculation of bacterial consortium increases rice yield (Oryza sativa L.) reducing applications of nitrogen fertilizer in San Martin region, Peru. Rhizosphere 2020, 14, 100200. [Google Scholar] [CrossRef]
- Zaghum, M.J.; Ali, K.; Teng, S. Integrated Genetic and Omics Approaches for the Regulation of Nutritional Activities in Rice (Oryza sativa L.). Agriculture 2022, 12, 1757. [Google Scholar] [CrossRef]
- Win, K.T.; Oo, A.Z.; Ohkama-Ohtsu, N.; Yokoyama, T. Bacillus Pumilus Strain TUAT-1 and Nitrogen Application in Nursery Phase Promote Growth of Rice Plants under Field Conditions. Agronomy 2018, 8, 216. [Google Scholar] [CrossRef] [Green Version]
- Shinjo, R.; Tanaka, A.; Sugiura, D.; Suzuki, T.; Uesaka, K.; Takebayashi, Y.; Kojima, M.; Sakakibara, H.; Takemoto, D.; Kondo, M. Comprehensive analysis of the mechanisms underlying enhanced growth and root N acquisition in rice by the endophytic diazotroph, Burkholderia vietnamiensis RS1. Plant Soil 2020, 450, 537–555. [Google Scholar] [CrossRef]
- Kumar, V.; Jain, L.; Jain, S.K.; Chaturvedi, S.; Kaushal, P. Bacterial endophytes of rice (Oryza sativa L.) and their potential for plant growth promotion and antagonistic activities. S. Afr. J. Bot. 2020, 134, 50–63. [Google Scholar] [CrossRef]
- Cheng, T.; Yao, X.Z.; Wu, C.Y.; Zhang, W.; He, W.; Dai, C.C. Endophytic Bacillus megaterium triggers salicylic acid-dependent resistance and improves the rhizosphere bacterial community to mitigate rice spikelet rot disease. Appl. Soil Ecol. 2020, 156, 103710. [Google Scholar] [CrossRef]
- Awan, S.A.; Ilyas, N.; Khan, I.; Raza, M.A.; Rehman, A.U.; Rizwan, M.; Brestic, M. Bacillus siamensis reduces cadmium accumulation and improves growth and antioxidant defense system in two wheat (Triticum aestivum L.) varieties. Plants 2020, 9, 878. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Bueis, R.; Mulas, R.; Gómez, X.; González-Andrés, F. Innovative liquid formulation of digestates for producing a biofertilizer based on Bacillus siamensis: Field testing on sweet pepper. J. Plant Nutr. Soil Sci. 2017, 180, 748–758. [Google Scholar] [CrossRef]
- Widawati, S. Isolation of Indole Acetic Acid (IAA) producing Bacillus siamensis from peat and optimization of the culture conditions for maximum IAA production. IOP Conf. Ser. Earth Environ. Sci. 2020, 572, 012025. [Google Scholar]
- Shen, N.; Li, S.; Li, S.; Zhang, H.; Jiang, M. The siderophore-producing bacterium, Bacillus siamensis Gxun-6, has an antifungal activity against Fusarium oxysporum and promotes the growth of banana. Egypt. J. Biol. Pest Control. 2022, 32, 34. [Google Scholar] [CrossRef]
- Hossain, M.T.; Khan, A.; Harun-Or-Rashid, M.; Chung, Y.R. A volatile producing endophytic Bacillus siamensis YC7012 promotes root development independent of auxin or ethylene/jasmonic acid pathway. Plant Soil 2019, 439, 309–324. [Google Scholar] [CrossRef]
- Feng, F.; Ge, J.; Li, Y.; He, S.; Zhong, J.; Liu, X.; Yu, X. Enhanced degradation of chlorpyrifos in rice (Oryza sativa L.) by five strains of endophytic bacteria and their plant growth promotional ability. Chemosphere 2017, 184, 505–513. [Google Scholar] [CrossRef]
- Durairaj, J.; Thankappan, S.; Prabina, B.J.; Binodh, A.K.; Jenita Rajammal, T.S. Rhizosphere engineering of rice with plant growth promoting rhizobacteria (PGPR) elicits crop growth and soil. In Communications in Soil Science and Plant Analysis; Taylor & Francis: Abingdon, UK, 2022; pp. 1–13. [Google Scholar]
- Liu, Z.; Zhang, X.; Li, L.; Xu, N.; Hu, Y.; Wang, C.; Shi, Y.; Li, D. Isolation and characterization of three plant growth-promoting rhizobacteria for growth enhancement of rice seedling. J. Plant Growth Regul. 2022, 41, 1382–1393. [Google Scholar] [CrossRef]
- Biedendieck, R.; Knuuti, T.; Moore, S.J.; Jahn, D. The “beauty in the beast”-the multiple uses of Priestia megaterium in biotechnology. Appl. Microbiol. Biotechnol. 2021, 105, 5719–5737. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Nuñez, R.A.; Ormeño-Orrillo, E.; Torres-Chávez, E.E.; Torres-Delgado, J.; Ríos-Ruiz, W.F. Caracterización genética de bacterias endofíticas de arroz (Oryza sativa L.) con actividad antimicrobiana contra Burkholderia glumae. Rev. Argent. De Microbiol. 2020, 52, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahi, M.; Pourbabaei, A.A.; Etesami, H.; Talebi, K. Diazinon degradation by bacterial endophytes in rice plant (Oryza sativa L.): A possible reason for reducing the efficiency of diazinon in the control of the rice stem–borer. Chemosphere 2020, 246, 125759. [Google Scholar] [CrossRef] [PubMed]
- Jing, R.; Li, N.; Wang, W.; Liu, Y. An endophytic strain JK of genus Bacillus isolated from the seeds of super hybrid rice (Oryza sativa L., Shenliangyou 5814) has antagonistic activity against rice blast pathogen. Microb. Pathog. 2020, 147, 104422. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Singh, S.; Gupta, A.R.; Gupta, A.; Singh, U.B.; Manzar, N.; Bhowmik, A.; Singh, H.B.; Saxena, A.K. Endophytic bacilli from medicinal-aromatic perennial Holy basil (Ocimum tenuiflorum L.) modulate plant growth promotion and induced systemic resistance against Rhizoctonia solani in rice (Oryza sativa L.). Biol. Control 2020, 150, 104353. [Google Scholar] [CrossRef]
- Hameed, A.; Shahina, M.; Lai, W.A.; Stothard, P.; Young, L.S.; Lin, S.Y.; Young, C.C. Draft genome sequence reveals co-occurrence of multiple antimicrobial resistance and plant probiotic traits in rice root endophytic strain Burkholderia sp. LS-044 affiliated to Burkholderia cepacia complex. J. Glob. Antimicrob. Resist. 2020, 20, 28–30. [Google Scholar] [CrossRef]
- Maqsood, A.; Shahid, M.; Hussain, S.; Mahmood, F.; Azeem, F.; Tahir, M.; Ahmed, T.; Noman, M.; Manzoor, I.; Basit, F. Root colonizing Burkholderia sp. AQ12 enhanced rice growth and upregulated tillering-responsive genes in rice. Appl. Soil Ecol. 2021, 157, 103769. [Google Scholar] [CrossRef]
- Wallner, A.; Busset, N.; Lachat, J.; Guigard, L.; King, E.; Rimbault, I.; Mergaert, P.; Béna, G.; Moulin, L. Differential genetic strategies of Burkholderia vietnamiensis and Paraburkholderia kururiensis for root colonization of Oryza sativa ssp. japonica and ssp. indica, as revealed by Tn-seq. bioRxiv 2022, arXiv:bioRxiv:04.14.488431. [Google Scholar] [CrossRef]
- Liu, M.; Philp, J.; Wang, Y.; Hu, J.; Wei, Y.; Li, J.; Ryder, M.; Toh, R.; Zhou, Y.; Denton, M.D.; et al. Plant growth-promoting rhizobacteria Burkholderia vietnamiensis B418 inhibits root-knot nematode on watermelon by modifying the rhizosphere microbial community. Sci. Rep. 2022, 12, 8381. [Google Scholar] [CrossRef]
- Mano, H.; Morisaki, H. Endophytic bacteria in the rice plant. Microbes Environ. 2008, 23, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Okunishi, S.; Sako, K.; Mano, H.; Imamura, A.; Morisaki, H. Bacterial flora of endophytes in the maturing seed of cultivated rice (Oryza sativa). Microbes Environ. 2005, 20, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Mano, H.; Tanaka, F.; Nakamura, C.; Kaga, H.; Morisaki, H. Culturable endophytic bacterial flora of the maturing leaves and roots of rice plants (Oryza sativa) cultivated in a paddy field. Microbes Environ. 2007, 22, 175–185. [Google Scholar] [CrossRef]
- Hossain, M.T.; Khan, A.; Chung, E.J.; Rashid, M.H.O.; Chung, Y.R. Biological control of rice bakanae by an endophytic Bacillus oryzicola YC7007. Plant Pathol. J. 2016, 32, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertani, I.; Abbruscato, P.; Piffanelli, P.; Subramoni, S.; Venturi, V. Rice bacterial endophytes: Isolation of a collection, identification of beneficial strains and microbiome analysis. Environ. Microbiol. Rep. 2016, 8, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Vílchez, J.I.; Yang, Y.; Yi, D.; Zhang, H. Measurements of root colonized bacteria species. Bio-protocol 2021, 11, e3976. [Google Scholar] [CrossRef]
- Ambreetha, S.; Chinnadurai, C.; Marimuthu, P.; Balachandar, D. Plant-associated Bacillus modulates the expression of auxin-responsive genes of rice and modifies the root architecture. Rhizosphere 2018, 5, 57–66. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A.; Hosseini, H.M. Indole-3-acetic acid (IAA) production trait, a useful screening to select endophytic and rhizosphere competent bacteria for rice growth promoting agents. MethodsX 2015, 2, 72–78. [Google Scholar] [CrossRef]
- Shen, F.T.; Yen, J.H.; Liao, C.S.; Chen, W.C.; Chao, Y.T. Screening of rice endophytic biofertilizers with fungicide tolerance and plant growth-promoting characteristics. Sustainability 2019, 11, 1133. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.L.; Yang, W.L.; Fang, W.W.; Zhao, Y.X.; Guo, L.; Dai, Y.J. The plant growth-promoting rhizobacterium Variovorax boronicumulans CGMCC 4969 regulates the level of indole-3-acetic acid synthesized from indole-3-acetonitrile. Appl. Environ. Microbiol. 2018, 84, e00298-18. [Google Scholar] [CrossRef] [Green Version]
- Dhungana, S.A.; Itoh, K. Effects of co-inoculation of Indole-3-acetic acid-producing and-degrading bacterial endophytes on plant growth. Horticulturae 2019, 5, 17. [Google Scholar] [CrossRef]
- Gan, D.; Feng, J.; Han, M.; Zeng, H.; Zhu, B. Rhizosphere effects of woody plants on soil biogeochemical processes: A meta-analysis. Soil Biol. Biochem. 2021, 160, 108310. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef] [Green Version]
- Walia, A.; Guleria, S.; Chauhan, A.; Mehta, P. Endophytic bacteria: Role in phosphate solubilization. In Endophytes: Crop Productivity and Protection. Sustainable Development and Biodiversity; Maheshwari, D., Annapurna, K., Eds.; Springer: Cham, Switzerland, 2017; pp. 61–93. [Google Scholar]
- Varga, T.; Hixson, K.K.; Ahkami, A.H.; Sher, A.W.; Barnes, M.E.; Chu, R.K.; Battu, A.K.; Nicora, C.D.; Winkler, T.E.; Reno, L.R.; et al. Endophyte-promoted phosphorus solubilization in Populus. Front. Plant Sci. 2020, 11, 567918. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.H.; Gururani, M.A.; Chun, S.C. Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol. Res. 2014, 169, 83–98. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Maiti, T.K.; Pramanik, K.; Ghosh, S.K.; Mitra, S.; De, T.K. The role of arsenic resistant Bacillus aryabhattai MCC3374 in promotion of rice seedlings growth and alleviation of arsenic phytotoxicity. Chemosphere 2018, 211, 407–419. [Google Scholar] [CrossRef]
- Hii, Y.S.; San, C.Y.; Lau, S.W.; Danquah, M.K. Isolation and characterisation of phosphate solubilizing microorganisms from peat. Biocatal. Agric. Biotechnol. 2020, 26, 101643. [Google Scholar] [CrossRef]
- Panda, B.; Rahman, H.; Panda, J. Phosphate solubilizing bacteria from the acidic soils of Eastern Himalayan region and their antagonistic effect on fungal pathogens. Rhizosphere 2016, 2, 62–71. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, J.; Goswami, S.; Pramanik, K.; Maiti, T.K.; Kar, R.K.; Dey, N. Growth promoting properties of Mycobacterium and Bacillus on rice plants under induced drought. Plant Sci. Today 2021, 8, 49–57. [Google Scholar] [CrossRef]
- Kong, P.; Hong, C. A potent Burkholderia endophyte against boxwood blight caused by Calonectria pseudonaviculata. Microorganisms 2020, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Malviya, M.K.; Li, C.N.; Solanki, M.K.; Singh, R.K.; Htun, R.; Singh, P.; Verma, K.K.; Yang, L.T.; Li, Y.R. Comparative analysis of sugarcane root transcriptome in response to the plant growth-promoting Burkholderia anthina MYSP113. PloS ONE 2020, 15, e0231206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic bacteria: A new source of bioactive compounds. 3 Biotech 2017, 7, 315. [Google Scholar] [CrossRef] [PubMed]
- Adeniji, A.A.; Babalola, O.O. Bacillus velezensis: Phylogeny, useful applications, and avenues for exploitation. Appl. Microbiol. Biotechnol. 2019, 103, 3669–3682. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guan, Y.; Dong, Y.; Zhao, L.; Rong, S.; Chen, W.; Lv, M.; Xu, H.; Gao, X.; Chen, R.; et al. Isolation and evaluation of endophytic Bacillus tequilensis GYLH001 with potential application for biological control of Magnaporthe oryzae. PLoS ONE 2018, 13, e0203505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberl, L.; Vandamme, P. Members of the genus Burkholderia: Good and bad guys. F1000Research 2016, 5, PMC4882756. [Google Scholar] [CrossRef] [PubMed]
- Shabanamol, S.; Divya, K.; George, T.K.; Rishad, K.S.; Sreekumar, T.S.; Jisha, M.S. Characterization and in planta nitrogen fixation of plant growth promoting endophytic diazotrophic Lysinibacillus sphaericus isolated from rice (Oryza sativa). Physiol. Mol. Plant Pathol. 2018, 102, 46–54. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, H.; Ren, Z.; Li, X.; Zhong, J.; Liu, E. Efficacy of Bacillus tequilensis strain JN-369 to biocontrol of rice blast and enhance rice growth. Biol. Control 2021, 160, 104652. [Google Scholar] [CrossRef]
- Kumar, K.V.K.; Yellareddygari, S.K.; Reddy, M.S.; Kloepper, J.W.; Lawrence, K.S.; Zhou, X.G.; Sudini, H.; Groth, D.E.; Raju, S.K.; Miller, M.E. Efficacy of Bacillus subtilis MBI 600 against sheath blight caused by Rhizoctonia solani and on growth and yield of rice. Rice Sci. 2012, 19, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Hua, K.; Xu, R.; Zeng, D.; Wang, R.; Dong, G.; Zhang, G.; Lu, X.; Fang, N.; Wang, D.; et al. The LARGE2-APO1/APO2 regulatory module controls panicle size and grain number in rice. Plant Cell 2021, 33, 1212–1228. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, S.; Bai, L.; Jiang, S.; Ding, G.; Wang, T.; Zhao, H.; Wang, J.; Liu, H.; Yang, L.; et al. Identification of candidate genes for panicle length in Oryza sativa L. ssp. japonica via genome-wide association study and linkage mapping. Euphytica 2022, 218, 16. [Google Scholar] [CrossRef]
- Gupta, R.; Noureldeen, A.; Darwish, H. Rhizosphere mediated growth enhancement using phosphate solubilizing rhizobacteria and their tri-calcium phosphate solubilization activity under pot culture assays in Rice (Oryza sativa). Saudi J. Biol. Sci. 2021, 28, 3692–3700. [Google Scholar] [CrossRef] [PubMed]
- Dhondge, H.V.; Pable, A.A.; Barvkar, V.T.; Dastager, S.G.; Nadaf, A.B. Rhizobacterial consortium mediated aroma and yield enhancement in basmati and non-basmati rice (Oryza sativa L.). J. Biotechnol. 2021, 328, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Liu, Y.; Wu, G.; Zeng, S.; Tran Thi, T.G.; Liang, L.; Liang, Y.; Dong, Z.; She, D.; Wang, H.; et al. Identification1 of a candidate gene for panicle length in rice (Oryza sativa L.) via association and linkage analysis. Front. Plant Sci. 2016, 7, 596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamad, H.R.; Zulkarami, B.; Halimi, M.S. Effects of inoculation of plant growth promoting rhizobacteria to minimize panicle grain shattering habit for increased yield of rice (Oryza sativa L.). Afr. J. Microbiol. Res. 2019, 13, 256–263. [Google Scholar] [CrossRef]
- Pedraza-Herrera, L.A.; Bautista, J.P.; Cruz-Ramírez, C.A.; Uribe-Vélez, D. IBUN2755 Bacillus strain controls seedling root and bacterial panicle blight caused by Burkholderia glumae. Biol. Control 2021, 153, 104494. [Google Scholar] [CrossRef]
- Jamily, A.S.; Koyama, Y.; Win, T.A.; Toyota, K.; Chikamatsu, S.; Shirai, T.; Uesugi, T.; Murakami, H.; Ishida, T.; Yasuhara, T. Effects of inoculation with a commercial microbial inoculant Bacillus subtilis C-3102 mixture on rice and barley growth and its possible mechanism in the plant growth stimulatory effect. J. Plant Prot. Res. 2019, 59, 193–205. [Google Scholar] [CrossRef]
- Yanni, Y.G.; Dazzo, F.B. Enhancement of rice production using endophytic strains of Rhizobium leguminosarum bv. trifolii in extensive field inoculation trials within the Egypt Nile delta. Plant Soil 2010, 336, 129–142. [Google Scholar]
- Padgham, J.L.; Sikora, R.A. Biological control potential and modes of action of Bacillus megaterium against Meloidogyne graminicola on rice. Crop Prot. 2007, 26, 971–977. [Google Scholar] [CrossRef]
- Mattos, K.A.; Pádua, V.L.; Romeiro, A.; Hallack, L.F.; Neves, B.C.; Ulisses, T.M.; Mendonça-Previato, L. Endophytic colonization of rice (Oryza sativa L.) by the diazotrophic bacterium Burkholderia kururiensis and its ability to enhance plant growth. An. Da Acad. Bras. De Cienc. 2008, 80, 477–493. [Google Scholar] [CrossRef] [Green Version]
- Hoagland, D.R. Mineral nutrition. In Laboratory Experiments in Plant Physiology; Labovitch, A., Anderson-Prouty, N., Ghosheh, S., Eds.; Macmillan Publishing Co. Inc.: New York, NY, USA, 1975; pp. 129–134. [Google Scholar]
- Barac, T.; Taghavi, S.; Borremans, B.; Provoost, A.; Oeyen, L.; Colpaert, J.V.; Vangronsveld, J.; Van der Lelie, D. Engineered endophytic bacteria improve phytoremediation of water-soluble, volatile, organic pollutants. Nat. Biotechnol. 2004, 22, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Gravel, V.; Antoun, H.; Tweddell, R.J. Effect of indole-acetic acid (IAA) on the development of symptoms caused by Pythium ultimum on tomato plants. Eur. J. Plant Pathol. 2007, 119, 457–462. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951, 26, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.S.; Xie, J.Y.; Wang, L.Y.; Chen, S.F. Paenibacillus maysiensis sp. nov., a nitrogen-fixing species isolated from the rhizosphere soil of maize. Curr. Microbiol. 2018, 75, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Baldani, J.I.; Reis, V.M.; Videira, S.S.; Boddey, L.H.; Baldani, V.L.D. The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: A practical guide for microbiologists. Plant Soil 2014, 384, 413–431. [Google Scholar] [CrossRef]
- Marra, L.M.; Oliveira, S.M.D.; Soares, C.R.F.S.; Moreira, F.M.D.S. Solubilization of inorganic phosphates by inoculant strains from tropical legumes. Sci. Agric. 2011, 68, 603–609. [Google Scholar] [CrossRef] [Green Version]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Mateos, P.F.; Jimenez-Zurdo, J.I.; Chen, J.; Squartini, A.S.; Haack, S.K.; Martinez-Molina, E.; Dazzo, F.B. Cell-associated pectinolytic and cellulolytic enzymes in Rhizobium leguminosarum biovar trifolii. Appl. Environ. Microbiol. 1992, 58, 1816–1822. [Google Scholar] [CrossRef] [Green Version]
- Degrassi, G.; Devescovi, G.; Kim, J.; Hwang, I.; Venturi, V. Identification, characterization and regulation of two secreted polygalacturonases of the emerging rice pathogen Burkholderia glumae. FEMS Microbiol. Ecol. 2008, 65, 251–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swift, R. Plant Growth-Promoting Bacteria from Western Australian Soils. Ph.D. Thesis, Murdoch University, Perth, Australia, 2016. [Google Scholar]
- Mia, M.B.; Shamsuddin, Z.H.; Mahmood, M. Effects of rhizobia and plant growth promoting bacteria inoculation on germination and seedling vigor of lowland rice. Afr. J. Biotechnol. 2012, 11, 3758–3765. [Google Scholar] [CrossRef]





| Strains | Root | Stem |
|---|---|---|
| Log 10 CFU g−1 | Log 10 CFU g−1 | |
| Priestia megaterium SMBH14-02 | 3.52 (± 0.04) B | 3.42 (± 0.01) C |
| Priestia aryabhattai SMNCH17-07 | 2.60 (± 0.02) C | 3.04 (± 0.04) E |
| Bacillus tequilensis SMNCT17-02 | 5.71 (± 0.15) A | 6.11 (± 0.03) A |
| Priestia megaterium TUT05-02 | 3.56 (± 0.13) B | 3.25 (± 0.02) D |
| Bulkholderia vietnamiensis TUR04-03 | 3.86 (± 0.02) B | 3.93 (± 0.01) B |
| Bulkholderia vietnamiensis TUR04-01a | 3.76 (± 0.04) B | 3.94(± 0.04) B |
| Bacillus siamensis TUR07-02b | 2.99 (± 0.12) C | 3.23 (± 0.01) D |
| Bulkholderia vietnamiensis la1a4 (Control) | 3.58 (± 0.03) B | 3.99 (± 0.01) B |
| Uninoculated Control | 2.22 (± 0.08) C | 2.99 (± 0.03) E |
| CV (%) | 5.24 | 1.47 |
| Strains | ppm (mg L−1) | |||
|---|---|---|---|---|
| 0 | 150 | 300 | 600 | |
| P. megaterium TUT05-02 | 45.43 (± 1.52) Bc | 77.69 (± 11.75) Ab | 144.78 (± 1.45) Aa | 85.75 (± 3.93) Cb |
| Bacillus siamensis TUR07-02b | 40.27 (± 6.27) Bc | 62.31 (± 11.58) ABbc | 123.17 (± 5.59) Ba | 98.44 (± 8.77) Bab |
| B. vietnamiensis TUR04-01a | 26.94 (± 1.04) CDb | 12.10 (± 3.00) Db | 50.48 (± 7.39) Ea | 28.44 (± 2.79) Fb |
| p. megaterium SMBH14-02 | 58.33 (± 4.30) Ab | 51.45 (± 5.14) Bb | 71.56 (± 2.73) Db | 335.65 (± 6.45) Aa |
| B. vietnamiensis TUR04-03 | 36.08 (± 2.08) BCb | 40.38 (± 4.72) BCb | 75.97 (± 6.06) Da | 43.28 (± 3.36) Eb |
| Bacillus tequilensis SMNCT17-02 | 19.73 (± 1.37) CDb | 25.00 (± 5.29) CDEb | 111.67 (± 4.57) Ba | 2.20 (± 0.47) Gc |
| P. aryabhattai SMNCH17-07 | 18.01 (± 0.88) Db | ND | 57.69 (± 2.17) DEa | 10.27 (± 1.51) Gc |
| B. vietnamiensis la3c3 (Control) | 18.55 (± 3.57) Db | 21.02 (± 5.27) CDb | ND | 64.57 (± 5.28) Da |
| CV (%) | 16.19 | 27.17 | 10.29 | 9.27 |
| Strains | Phosphate Solubilization (µg mL−1) | Fixation of Nitrogen | Production of Extracellular Enzymes | |||||
|---|---|---|---|---|---|---|---|---|
| Ca-P | Fe-P | Al-P | Burk | JMV | Cellulases | Pectinases | Chitinases | |
| P. megaterium TUT05-02 | 0 | 0 | 0 | + | + | - | - | - |
| Bacillus siamensis TUR07-02b | 0 | 0 | 0 | + | + | - | + | - |
| B. vietnamiensis TUR04-01a | 0 | 0 | 0 | + | + | - | - | - |
| P. megaterium SMBh14-02 | 20.94 A | 0 | 0 | + | + | + | + | - |
| B. vietnamiensis TUR04-03 | 0 | 0 | 0 | + | + | - | - | - |
| Bacillus tequilensis SMNCT17-02 | 0 | 0 | 7.15 A | + | + | - | - | - |
| P. aryabhattai SMNCH17-07 | 6.84 B | 0 | 0 | + | + | + | + | - |
| R. tropici CIAT 899 (Control) | 2.56 B | 4.56 A | 3.42 B | ND | ND | ND | ND | ND |
| B. vietnamiensis la3c3 (Control) | ND | ND | ND | + | + | ND | ND | ND |
| CV (%) | 10.41 | 3.49 | 4.21 | - | - | - | - | - |
| Strains | Germination (%) |
|---|---|
| P. megaterium SMBH14-02 | 93.00 (± 1.00) A |
| Bacillus siamensis TUR07-02b | 92.00 (± 1.63) A |
| B. vietnamiensis TUR04-01a | 91.00 (± 1.00) A |
| B. vietnamiensis TUR04-03 | 91.00 (± 1.00) A |
| P. megaterium TUT05-02 | 90.00 (± 2.00) A |
| Bacillus tequilensis SMNCT17-02 | 85.37 (± 2.13) B |
| P. aryabhattai SMNCH17-07 | 84.00 (± 3.27) B |
| Uninoculated Control | 82.00 (± 5.29) B |
| CV (%) | 5.77 |
| Nitrogen Dose (%) * | B. siamensis TUR07-02b | P. megaterium SMBH14-02 | B. siamensis TUR07-02b + P. megaterium SMBH14-02 | Chemical Control | CV (%) |
|---|---|---|---|---|---|
| Panicle length (mm) | |||||
| 0 | 29.43 (± 0.21)Aa | 29.58 (± 0.46) Aa | 28.68 (± 0.26) Ba | 24.87 (± 1.06) Ab | 6.78 |
| 50 | 29.42 (± 1.03) Aa | 28.36 (± 0.60) Aa | 30.38 (± 0.52) Aa | 25.01 (± 0.61) Ab | 8.01 |
| 75 | 28.56 (± 0.33) Ab | 28.28 (± 0.57) Ab | 30.90 (± 0.67) Aa | 24.72 (± 0.32) Ac | 5.57 |
| 100 | 26.82 (± 1.10) Ab | 28.69 (± 0.43) Ab | 32.27 (± 0.59) Aa | 24.41 (± 0.76) Ac | 8.60 |
| CV (%) | 8.64 | 5.70 | 5.52 | 9.43 | 7.34 |
| Grains per panicle | |||||
| 0 | 89.30 (± 3.12) Aa | 87.50 (± 3.86) Aa | 83.20 (± 3.34) Ca | 80.20 (± 5.58) Ba | 13.82 |
| 50 | 83.40 (± 2.45) Ab | 85.70 (± 3.88) Aa | 115.70 (± 6.43) Ba | 88.60 (± 3.33) Bb | 14.28 |
| 75 | 87.80 (± 2.53) Ac | 77.90 (± 3.04) Bc | 150.80 (± 4.78) Aa | 115.40 (± 4.93) Ab | 16.48 |
| 100 | 70.20 (± 5.38) Bb | 73.80 (± 4.20) Bb | 129.20 (± 4.40) Ba | 67.50 (± 9.47) Bb | 16.06 |
| CV (%) | 13.67 | 14.67 | 12.85 | 20.12 | 15.41 |
| Panicles per plant | |||||
| 0 | 2.40 (± 0.24) Aa | 2.20 (± 0.20) Aa | 2.20 (± 0.20) Aa | 2.37 (± 0.00) Aa | 19.02 |
| 50 | 2.20 (± 0.20) Aa | 2.40 (± 0.24) Aa | 2.60 (± 0.24) Aa | 2.00 (± 0.00) Aa | 19.44 |
| 75 | 2.20 (± 0.20) Aa | 2.00 (± 0.00) Aa | 2.20 (± 0.20) Aa | 3.17 (± 0.24) Aa | 19.02 |
| 100 | 1.80 (± 0.20) Aa | 2.20 (± 0.20) Aa | 1.60 (± 0.24) Aa | 3.63 (± 0.24) Aa | 17.78 |
| CV (%) | 22.06 | 19.02 | 23.26 | 19.36 | 21.05 |
| Grain yield (g plant−1) | |||||
| 0 | 3.69 (± 0.36) Aa | 3.16 (± 0.36) Aa | 2.78 (± 0.28) Ba | 2.71 (± 0.33) Aa | 24.15 |
| 50 | 2.67 (± 0.42) Aa | 3.50 (± 0.39) Aa | 4.06 (± 0.12) Aa | 3.09 (± 0.26) Aa | 21.55 |
| 75 | 2.70 (± 0.36) Ab | 2.64 (± 0.18) Ab | 4.34 (± 0.16) Aa | 3.11 (± 0.36) Ab | 19.67 |
| 100 | 1.90 (± 0.21) Ab | 2.72 (± 0.32) Aa | 3.42 (± 0.34) Ba | 1.56 (± 0.17) Bb | 25.06 |
| CV (%) | 28.35 | 24.00 | 14.72 | 24.64 | 22.51 |
| Straw yield (g plant−1) | |||||
| 0 | 16.45 (± 0.59) Ab | 21.72 (± 3.06) Aa | 13.91 (± 0.89) Ab | 10.87 (± 0.80) Ab | 23.72 |
| 50 | 16.66 (± 1.14) Aa | 17.84 (± 0.80) Aa | 15.10 (± 1.44) Aa | 14.02 (± 1.39) Aa | 17.14 |
| 75 | 18.15 (± 1.79) Aa | 15.25 (± 1.15) Aa | 15.28 (± 0.57) Aa | 12.85 (± 0.70) Aa | 18.57 |
| 100 | 10.77 (± 1.60) Ba | 16.17 (± 2.51) Aa | 12.67 (± 0.79) Aa | 10.74 (± 1.32) Aa | 19.80 |
| CV (%) | 19.64 | 26.48 | 15.33 | 20.21 | 21.77 |
| Harvest index (%) | |||||
| 0 | 18.23 (± 1.37) Aa | 13.08 (± 1.08) Aa | 16.69 (± 1.40) Ba | 20.01 (± 0.50) Aa | 21.06 |
| 50 | 13.75 (± 1.71) Aa | 16.46 (± 1.90) Aa | 21.66 (± 1.61) Aa | 18.48 (± 0.38) Aa | 23.10 |
| 75 | 12.89 (± 1.01) Ab | 14.95 (± 1.23) Ab | 22.16 (± 0.56) Aa | 19.70 (± 0.06) Aa | 19.53 |
| 100 | 16.04 (± 2.72) Aa | 15.07 (± 2.05) Aa | 21.20 (± 1.53) Aa | 13.34 (± 0.90) Aa | 18.93 |
| CV (%) | 26.65 | 24.36 | 14.69 | 17.86 | 23.28 |
| Species | Place of Origin | Isolation Organ | Similar Type Strain | Similarity Percentage (%) | Number of Nucleotides |
|---|---|---|---|---|---|
| P. aryabhattai SMNCH17-07 | Nueva Cajamarca, San Martín | leaf | B8W22T | 100.00 | 1416 |
| P. megaterium SMBH14-02 | Bellavista, San Martín | Leaf | NBRC15308T | 99.79 | 1438 |
| B. tequilensis SMNCT17-02 | Nueva Cajamarca, San Martín | Stem | 10bT | 99.65 | 1437 |
| P. megaterium TUT05-02 | Tumbes | Stem | NBRC15308T | 100.00 | 1403 |
| B. vietnamiensis TUR04-03 | Tumbes | Root | LMG10929T | 99.93 | 1403 |
| B. vietnamiensis TUR04-01a | Tumbes | Root | LMG10929T | 99.93 | 1416 |
| B. siamensis TUR07-02b | Tumbes | Root | KCTC13613T | 99.78 | 1389 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rios-Ruiz, W.F.; Tuanama-Reátegui, C.; Huamán-Córdova, G.; Valdez-Nuñez, R.A. Co-Inoculation of Endophytes Bacillus siamensis TUR07-02b and Priestia megaterium SMBH14-02 Promotes Growth in Rice with Low Doses of Nitrogen Fertilizer. Plants 2023, 12, 524. https://doi.org/10.3390/plants12030524
Rios-Ruiz WF, Tuanama-Reátegui C, Huamán-Córdova G, Valdez-Nuñez RA. Co-Inoculation of Endophytes Bacillus siamensis TUR07-02b and Priestia megaterium SMBH14-02 Promotes Growth in Rice with Low Doses of Nitrogen Fertilizer. Plants. 2023; 12(3):524. https://doi.org/10.3390/plants12030524
Chicago/Turabian StyleRios-Ruiz, Winston Franz, Ciceron Tuanama-Reátegui, Gamaniel Huamán-Córdova, and Renzo Alfredo Valdez-Nuñez. 2023. "Co-Inoculation of Endophytes Bacillus siamensis TUR07-02b and Priestia megaterium SMBH14-02 Promotes Growth in Rice with Low Doses of Nitrogen Fertilizer" Plants 12, no. 3: 524. https://doi.org/10.3390/plants12030524






