Assessment of Shoot Priming Efficiency to Counteract Complex Metal Stress in Halotolerant Lobularia maritima
Abstract
:1. Introduction
2. Results and Discussion
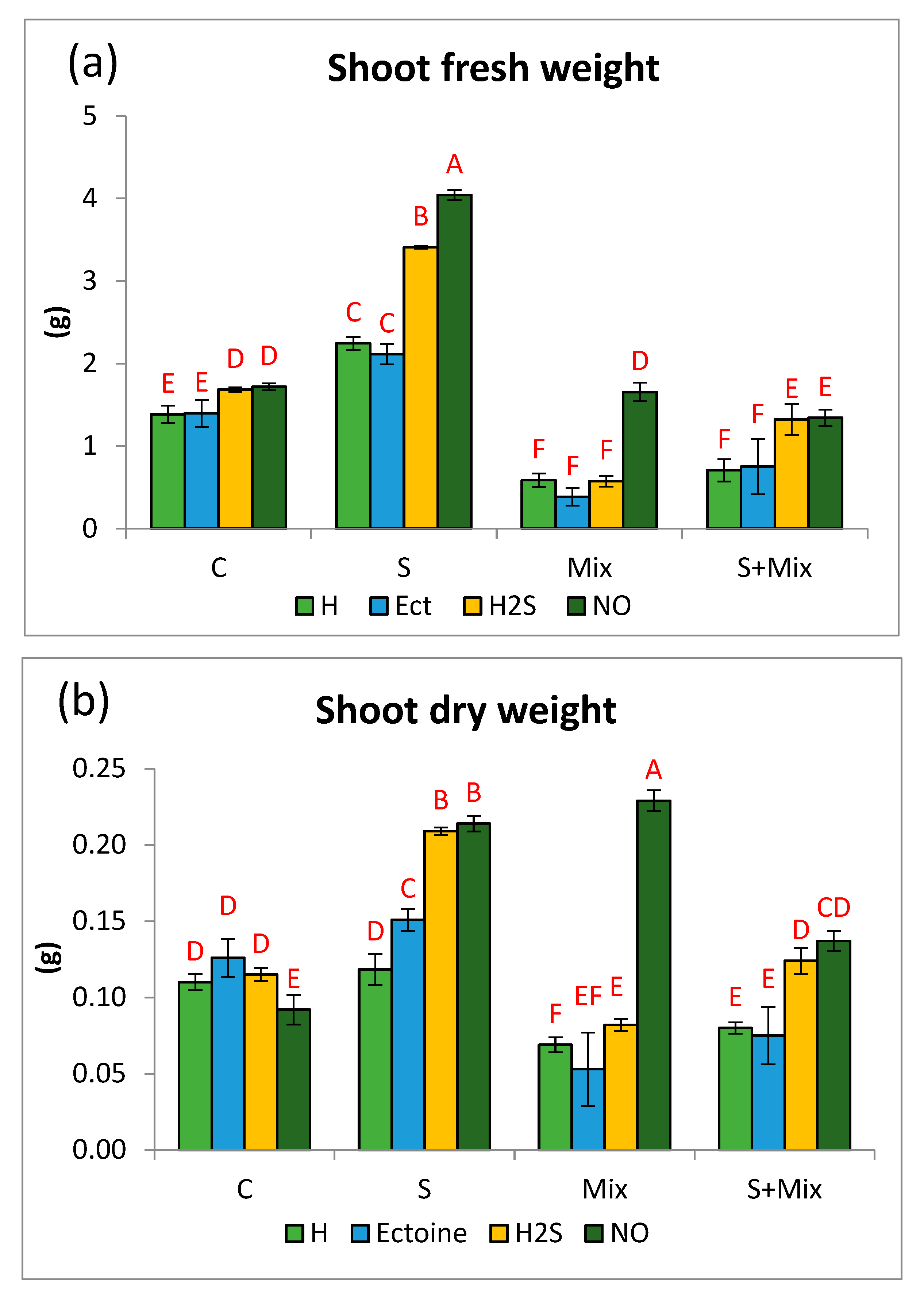
2.1. Priming Effect on Growth Response of L. maritima Varies in Different Stress Conditions
2.2. Detoxification Machinery in L. maritima Is Promoted Mostly by NO Donor
2.2.1. Glutathione S-Transferase Activity
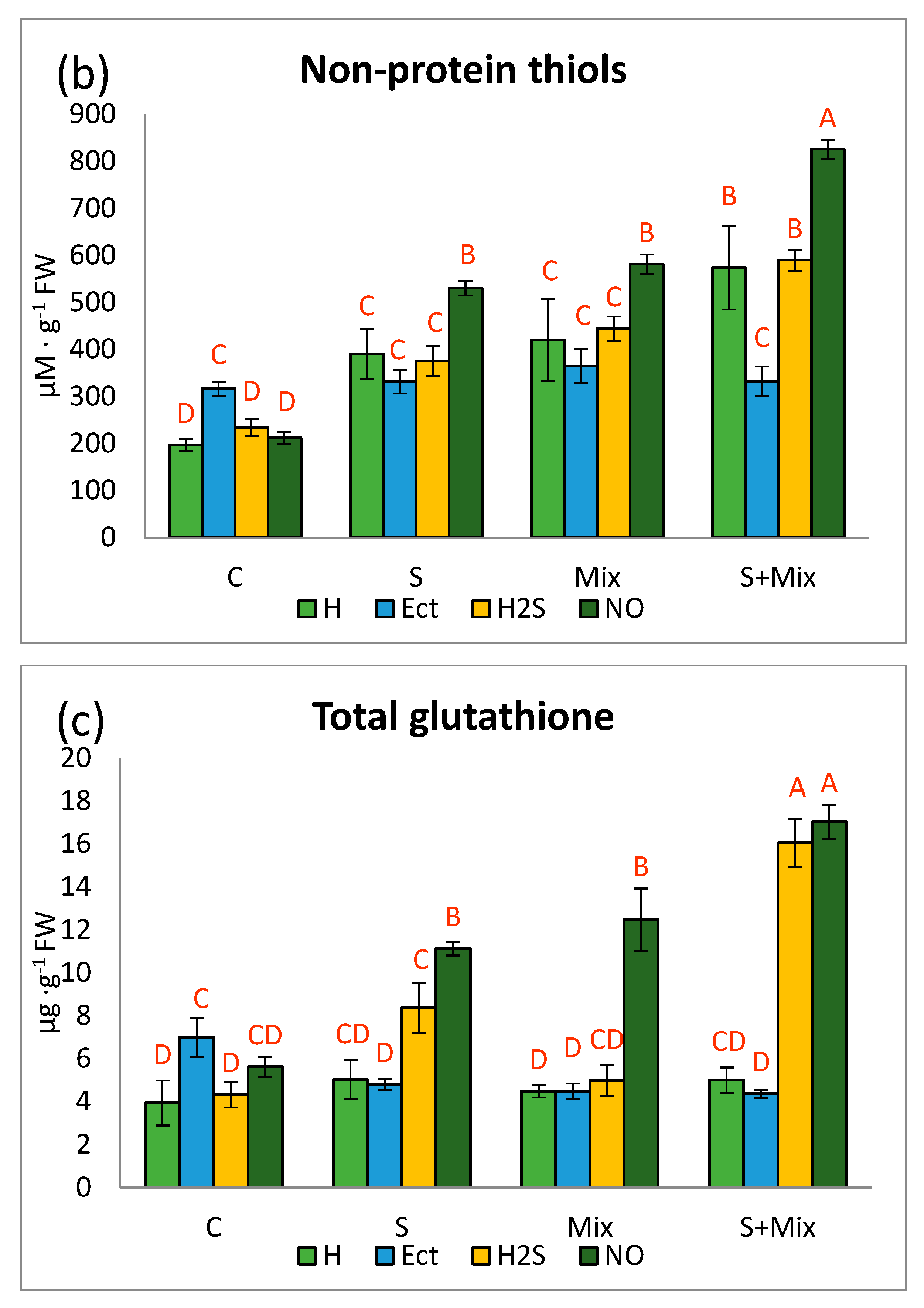
2.2.2. The Content of Non-Protein Thiols (NPT)
2.2.3. Glutathione Content
2.3. Contribution of Phenolic Compounds as Non-Enzymatic Antioxidants in Priming-Affected Stress Response
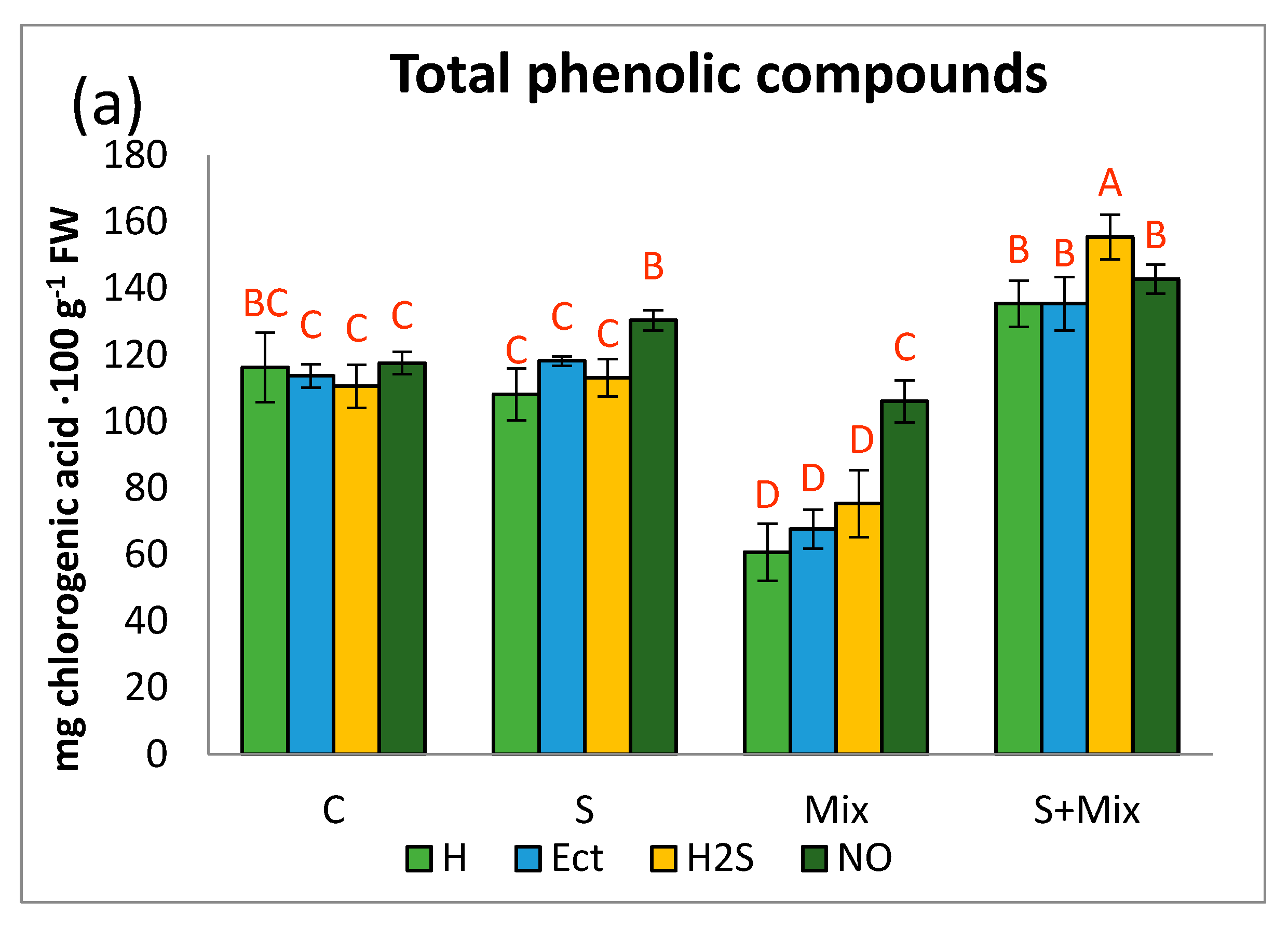
2.3.1. Phenolic Profile
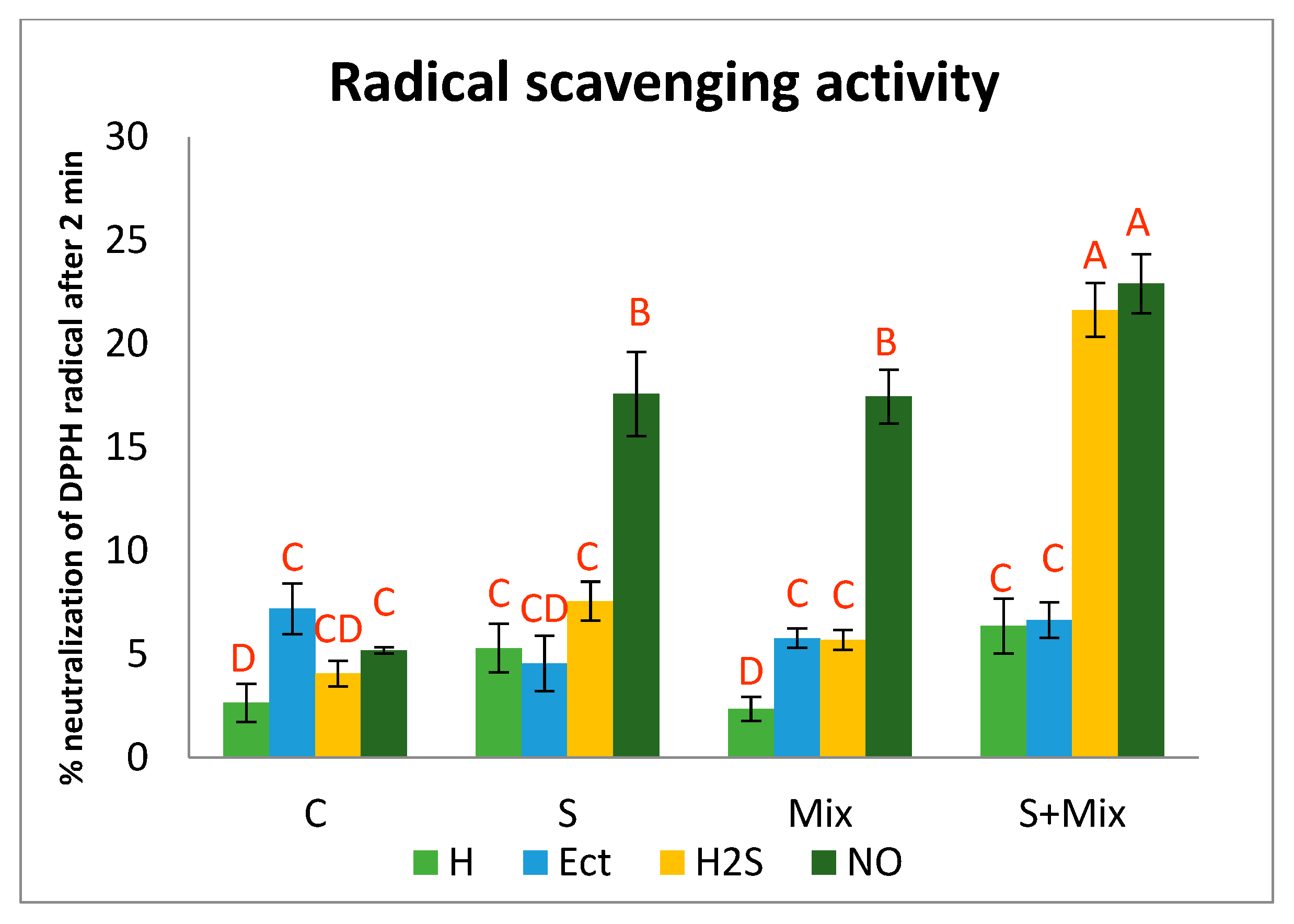
2.3.2. Radical Scavenging Activity
3. Materials and Methods
3.1. Plant Material and Experimental Conditions
3.2. Growth Parameters
3.3. Glutathione-S-Transferase Activity
3.4. Determination of Non-Protein Thiols
3.5. Glutathione Determination
3.6. Radical Scavenging Activity
3.7. Phenolic Profile
3.8. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galhaut, L.; de Lespinay, A.; Walker, D.J.; Bernal, M.P.; Correal, E.; Lutts, S. Seed Priming of Trifolium repens L. Improved Germination and Early Seedling Growth on Heavy Metal-Contaminated Soil. Water Air Soil Pollut. 2014, 225, 1905. [Google Scholar] [CrossRef]
- Pérez-Esteban, J.; Escolástico, C.; Ruiz-Fernández, J.; Masaguer, A.; Moliner, A. Bioavailability and extraction of heavy metals from contaminated soil by Atriplex halimus. EEB 2013, 88, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dresler, S.; Wójciak-Kosior, M.; Sowa, I.; Stanisławski, G.; Bany, I.; Wójcik, M. Effect of short-term Zn/Pb or long-term multi-metal stress on physiological and morphological parameters of metallicolous and nonmetallicolous Echium vulgare L. populations. Plant Physiol. Biochem. 2017, 115, 380–389. [Google Scholar] [CrossRef]
- Diarra, I.; Kotra, K.K.; Prasad, S. Assessment of biodegradable chelating agents in the phytoextraction of heavy metals from multi–metal contaminated soil. Chemosphere 2021, 273, 128483. [Google Scholar] [CrossRef] [PubMed]
- Sirguey, C.; Ouvrard, S. Contaminated soils salinity, a threat for phytoextraction? Chemosphere 2013, 91, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Ouvrard, S.; Barnier, C.; Bauda, P.; Beguiristain, T.; Biache, C.; Bonnard, M.; Leyval, C. In situ assessment of phytotechnologies for multicontaminated soil management. Int. J. Phytoremediat. 2011, 13, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, I.; Iqbal, M.; Bliek, M.; Schat, H. Salt and heavy metal tolerance and expression levels of candidate tolerance genes among four extremophile Cochlearia species with contrasting habitat preferences. Sci. Total Environ. 2017, 584, 731–741. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M.; Hanus-Fajerska, E. Changes in proteolytic activity and protein carbonylation in shoots of Alyssum montanum ecotypes under multi-metal stress. J. Plant Physiol. 2019, 232, 61–64. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M.; Różańska, E.; Hanus-Fajerska, E.; Znojek, E. Heavy metal tolerance in contrasting ecotypes of Alyssum montanum. Ecotoxicol. Environ. Saf. 2018, 161, 305–317. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Kamińska, I.; Hanus-Fajerska, E.; Sliwinska, E.; Koźmińska, A. Distinct co-tolerance responses to combined salinity and cadmium exposure in metallicolous and non-metallicolous ecotypes of Silene vulgaris. Ecotoxicol. Environ. Saf. 2020, 201, 110823. [Google Scholar] [CrossRef] [PubMed]
- Manquián-Cerda, K.; Cruces, E.; Escudey, M.; Zúñiga, G.; Calderón, R. Interactive effects of aluminum and cadmium on phenolic compounds, antioxidant enzyme activity and oxidative stress in blueberry (Vaccinium corymbosum L.) plantlets cultivated in vitro. Ecotoxicol. Environ. Saf. 2018, 150, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Samiei, L.; Davoudi Pahnehkolayi, M.; Karimian, Z.; Nabati, J. Morpho-Physiological Responses of Halophyte Climacoptera crassa to Salinity and Heavy Metal Stresses in In Vitro Condition. S. Afr. J. Bot. 2020, 131, 468–474. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Koźmińska, A.; Wiszniewska, A.; Hanus-Fajerska, E.; Boscaiu, M.; Al Hassan, M. Identification of Salt and Drought Biochemical Stress Markers in Several Silene vulgaris Populations. Sustainability 2019, 11, 800. [Google Scholar] [CrossRef] [Green Version]
- Nikalje, G.C.; Suprasanna, P. Coping with metal toxicity—Cues from halophytes. Front. Plant Sci. 2018, 9, 777. [Google Scholar] [CrossRef]
- Manousaki, E.; Kalogerakis, N. Halophytes Present New Opportunities in Phytoremediation of Heavy Metals and Saline Soils. Ind. Eng. Chem. Res. 2011, 50, 656–660. [Google Scholar] [CrossRef]
- Koźmińska, A.; Al Hassan, M.; Wiszniewska, A.; Hanus-Fajerska, E.; Boscaiu, M.; Vicente, O. Responses of succulents to drought: Comparative analysis of four Sedum (Crassulaceae) species. Sci. Hort. 2019, 243, 235–242. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Maggio, A. Functional biology of halophytes in the phytoremediation of heavy metal contaminated soils. Environ. Exp. Bot. 2015, 111, 135–146. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Kamińska, I.; Koźmińska, A.; Hanus-Fajerska, E. Aspects of co-tolerance towards salt and heavy metal stresses in halophytic plant species. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzamanm, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 477–498. ISBN 978-981-10-9043-1. [Google Scholar]
- Wiszniewska, A.; Koźmińska, A.; Hanus-Fajerska, E.; Dziurka, M.; Dziurka, K. Insight into mechanisms of multiple stresses tolerance in a halophyte Aster tripolium subjected to salinity and heavy metal stress. Ecotoxicol. Environ. Saf. 2019, 180, 12–22. [Google Scholar] [CrossRef]
- Liang, L.; Liu, W.; Sun, Y.; Huo, X.; Li, S.; Zhou, Q. Phytoremediation of heavy metal contaminated saline soils using halophytes: Current progress and future perspectives. Environ. Rev. 2017, 25, 269–281. [Google Scholar] [CrossRef] [Green Version]
- Marrelli, M.; Argentieri, M.P.; Avato, P.; Conforti, F. Lobularia maritima (L.) Desv. Aerial Parts Methanolic Extract: In Vitro Screening of Biological Activity. Plants 2020, 9, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.W.; Liu, D.F.; Zhao, M.; Li, Z.N.; Li, M.Y.; Sue, S.Z. Artificially induced polyploidization in Lobularia maritima (L.) Desv. and its effect on morphological traits. HortSci 2015, 50, 636–639. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zeng, C.; Wang, D.; yan Yang, J. Phytoextraction of cobalt (Co)-contaminated soils by sweet alyssum (Lobularia maritima (L.) Desv.) is enhanced by biodegradable chelating agents. J. Soils Sediments 2020, 20, 1931–1942. [Google Scholar] [CrossRef]
- Popova, O.V.; Yang, O.; Dietz, K.J.; Golldack, D. Differential transcript regulation in Arabidopsis thaliana and the halotolerant Lobularia maritima indicates genes with potential function in plant salt adaptation. Gene 2008, 423, 142–148. [Google Scholar] [CrossRef]
- Ben Saad, R.; Ben Romdhane, W.; Zouari, N.; Ben Hsouna, A.; Harbaoui, M.; Brini, F.; Ghneim-Herrera, T. Characterization of a novel LmSAP gene promoter from Lobularia maritima: Tissue specificity and environmental stress responsiveness. PLoS ONE 2020, 15, e0236943. [Google Scholar] [CrossRef]
- Ben Saad, R.; Ben Romdhane, W.; Bouteraa, M.T.; Jrad, O.; Ben Hsouna, A. Lobularia maritima thioredoxin-h2 gene mitigates salt and osmotic stress damage in tobacco by modeling plant antioxidant system. Plant Growth Regul. 2022, 97, 101–115. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Michalak, M.; Kukula-Koch, W.; Ben Saad, R.; ben Romdhane, W.; Zeljković, S.Ć.; Mnif, W. Evaluation of Halophyte Biopotential as an Unused Natural Resource: The Case of Lobularia maritima. Biomolecules 2022, 12, 1583. [Google Scholar] [CrossRef]
- Bouteraa, M.T.; Mishra, A.; Romdhane, W.B.; Hsouna, A.B.; Siddique, K.H.M.; Saad, R.B. Bio-Stimulating Effect of Natural Polysaccharides from Lobularia maritima on Durum Wheat Seedlings: Improved Plant Growth, Salt Stress Tolerance by Modulating Biochemical Responses and Ion Homeostasis. Plants 2022, 11, 1991. [Google Scholar] [CrossRef]
- Zammali, I.; Dabbous, A.; Youssef, S.; Ben Hamed, K. Effects of Chemical Priming on the Germination of the Ornamental Halophyte Lobularia maritima under NaCl Salinity. Seeds 2022, 1, 99–109. [Google Scholar] [CrossRef]
- Wiszniewska, A. Priming Strategies for Benefiting Plant Performance under Toxic Trace Metal Exposure. Plants 2021, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- Balmer, A.; Pastor, V.; Gamir, J.; Flors, V.; Mauch-Mani, B. The ‘prime-ome’: Towards a holistic approach to priming. Trends Plant Sci. 2015, 20, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.; Savvides, A.; Christou, A.; Fotopoulos, V. Unravelling chemical priming machinery in plants: The role of reactive oxygen–nitrogen–sulfur species in abiotic stress tolerance enhancement. Curr. Opin. Plant Biol. 2016, 33, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kabała, K.; Zboińska, M.; Głowiak, D.; Reda, M.; Jakubowska, D.; Janicka, M. Interaction between the signaling molecules hydrogen sulfide and hydrogen peroxide and their role in vacuolar H+-ATPase regulation in cadmium-stressed cucumber roots. Physiol. Plant. 2019, 166, 688–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, S.; Calderon-Urrea, A.; Jihua, Y.U.; Liao, W.; Xie, J.; Lv, J.; Tang, Z. The role of hydrogen sulfide in plant alleviates heavy metal stress. Plant Soil 2020, 449, 1–10. [Google Scholar] [CrossRef]
- Karalija, E.; Selović, A.; Dahija, S.; Demir, A.; Samardžić, J.; Vrobel, O.; Ćavar-Zeljković, S.; Parić, A. Use of seed priming to improve Cd accumulation and tolerance in Silene sendtneri, novel Cd hyper-accumulator. Ecotoxicol. Environ. Saf. 2021, 210, 111882. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Abbas, Z.; Seleiman, M.F.; Rizwan, M.; YAVAŞ, İ.; Alhammad, B.A.; Shami, A.; Hasanuzzaman, M.; Kalderis, D. Glycine Betaine Accumulation, Significance and Interests for Heavy Metal Tolerance in Plants. Plants 2020, 9, 896. [Google Scholar] [CrossRef]
- Czech, L.; Hermann, L.; Stöveken, N.; Richter, A.A.; Höppner, A.; Smits, S.H.J.; Heider, J.; Bremer, E. Role of the Extremolytes Ectoine and Hydroxyectoine as Stress Protectants and Nutrients: Genetics, Phylogenomics, Biochemistry, and Structural Analysis. Genes 2018, 9, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.H.; Yuan, F.W.; Chen, W.C.; Chen, S.Y. Production and characterization of ectoine by Marinococcus sp. ECT1 isolated from a high-salinity environment. J. Biosci. Bioeng. 2011, 111, 336–342. [Google Scholar] [CrossRef]
- Westhofen, P.; Ismail, G.; Zoglauer, K.; Boehm, R. Protease activity in the medium of larch (Larix spec.) embryogenic suspension cultures and medium-protein stabilization by compatible solutes. JABFQ 2007, 81, 56–61. [Google Scholar]
- Ozfidan-Konakci, C.; Elbasan, F.; Arikan, B.; Nur Alp, F.; Yildiztugay, E.; Keles, R.; Kucukoduk, M. Ex-foliar applied extremolyte ectoine improves water management, photosystem, antioxidant system and redox homeostasis in Zea mays under cadmium toxicity. S. Afr. J. Bot. 2022, 147, 130–141. [Google Scholar] [CrossRef]
- Hou, M.; Li, M.; Yang, X.; Pan, R. Responses of nonprotein thiols to stress of vanadium and mercury in maize (Zea mays L.) seedlings. Bull. Environ. Contam. Toxicol. 2019, 102, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, M.; Dresler, S.; Plak, A.; Tukiendorf, A. Naturally evolved enhanced Cd tolerance of Dianthus carthusianorum L. is not related to accumulation of thiol peptides and organic acids. Environ. Sci. Pollut. Res. 2015, 22, 7906–7917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taji, T.; Komatsu, K.; Katori, T.; Kawasaki, Y.; Sakata, Y.; Tanaka, S.; Kobayashi, M.; Toyoda, A.; Seki, M.; Shinozaki, K. Comparative genomic analysis of 1047 completely sequenced cDNA’s from an Arabidopsis-related model halophyte, Thellungiella halophila. BMC Plant Biol. 2010, 10, 261. [Google Scholar] [CrossRef] [Green Version]
- Cong, M.; Zhao, J.; Lü, J.; Ren, Z.; Wu, H. Homologous cloning, characterization and expression of a new halophyte phytochelatin synthase gene in Suaeda salsa. Chin. J. Oceanol. Limnol. 2016, 34, 1034–1043. [Google Scholar] [CrossRef]
- Makowski, W.; Tokarz, K.M.; Tokarz, B.; Banasiuk, R.; Witek, K.; Królicka, A. Elicitation-Based Method for Increasing the Production of Antioxidant and Bactericidal Phenolic Compounds in Dionaea muscipula J. Ellis Tissue. Molecules 2020, 25, 1794. [Google Scholar] [CrossRef]
- Jakovljević, K.; Durović, S.; Antušević, M.; Mihailović, N.; Buzurović, U.; Tomović, G. Heavy metal tolerance of Pontechium maculatum (Boraginaceae) from several ultramafic localities in Serbia. Bot. Serbica 2019, 43, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Cummins, I.; Cole, D.J.; Edwards, R. A role for glutathione transferases functioning as glutathione peroxidases in resistance to multiple herbicides in black-grass. Plant J. 1999, 18, 285–292. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Glutathione S-transferases: Role in combating abiotic stresses including arsenic detoxification in plants. Front. Plant Sci. 2018, 9, 751. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Arora, R. Priming memory invokes seed stress-tolerance. Environ. Exp. Bot. 2013, 94, 33–45. [Google Scholar] [CrossRef]
- Carter, J.M.; Brown, E.M.; Grace, J.P.; Salem, A.K.; Irish, E.E.; Bowden, N.B. Improved growth of pea, lettuce, and radish plants using the slow release of hydrogen sulfide from GYY-4137. PLoS ONE 2018, 13, e0208732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Yang, H.; Duan, K.; Zhang, X.; Zhao, H.; You, S.; Jiang, Q. Sodium nitroprusside promotes multiplication and regeneration of Malus hupehensis in vitro plantlets. Plant Cell Tissue Organ Cult. 2009, 96, 29–34. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Maloupa, E. Effect of the NO donor “sodium nitroprusside” (SNP), the ethylene inhibitor “cobalt chloride” (CoCl2) and the antioxidant vitamin E “α-tocopherol” on in vitro shoot proliferation of Sideritis raeseri Boiss. & Heldr. subsp. raeseri. Plant Cell Tissue Organ Cult. 2017, 128, 619–629. [Google Scholar]
- Subiramani, S.; Sundararajan, S.; Sivakumar, H.P.; Rajendran, V.; Ramalingam, S. Sodium nitroprusside enhances callus induction and shoot regeneration in high value medicinal plant Canscora diffusa. Plant Cell Tissue Organ Cult. 2019, 139, 65–75. [Google Scholar] [CrossRef]
- Liu, S.; Yang, R.; Pan, Y.; Ma, M.; Pan, J.; Zhao, Y.; Cheng, Q.; Wu, M.; Wang, M.; Zhang, L. Nitric oxide contributes to minerals absorption, proton pumps and hormone equilibrium under cadmium excess in Trifolium repens L. plants. Ecotoxicol. Environ. Saf. 2015, 119, 35–46. [Google Scholar] [CrossRef]
- Xiong, J.; An, L.; Lu, H.; Zhu, C. Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 2009, 230, 755–765. [Google Scholar] [CrossRef]
- Xiong, Y.; Liang, H.; Yan, H.; Guo, B.; Niu, M.; Chen, S.; Jian, S.; Ren, H.; Zhang, X.; Li, Y.; et al. NaCl-induced stress: Physiological responses of six halophytic species in in vitro and in vivo culture. Plant Cell Tissue Organ Cult. 2019, 139, 531–546. [Google Scholar] [CrossRef]
- Sato, R.; Tran, D.Q.; Yoshida, K.; Dang, J. NaCl-promoted Respiration and Cell Division in Halophilism of a Halophyte, the Common Ice Plant Mesembryanthemum crystallinum L. J. Fac. Agric. 2022, 67, 153–164. [Google Scholar] [CrossRef]
- Fang, P.; Sun, T.; Wang, Y.; Ding, Y.; Pandey, A.K.; Zhu, C.; Xu, P. Plant gasotransmitters: Light molecules interplaying with heavy metals. In Reviews in Environmental Science and Bio-Technology; Springer Science and Business Media B.V.: New York, NY, USA, 2021; pp. 1–23. [Google Scholar]
- Kharbech, O.; Ben Massoud, M.; Sakouhi, L.; Djebali, W.; Jose Mur, L.A.; Chaoui, A. Exogenous application of hydrogen sulfide reduces chromium toxicity in maize seedlings by suppressing NADPH oxidase activities and methylglyoxal accumulation. Plant Physiol. Biochem. 2020, 154, 646–656. [Google Scholar] [CrossRef]
- Ali, B.; Mwamba, T.M.; Gill, R.A.; Yang, C.; Ali, S.; Daud, M.K.; Wu, Y.; Zhou, W. Improvement of element uptake and antioxidative defense in Brassica napus under lead stress by application of hydrogen sulfide. Plant Growth Regul. 2014, 74, 261–273. [Google Scholar] [CrossRef]
- Yang, L.; Ji, J.; Harris-Shultz, K.R.; Wang, H.; Wang, H.; Abd-Allah, E.F.; Luo, Y.; Hu, X. The dynamic changes of the plasma membrane proteins and the protective roles of nitric oxide in rice subjected to heavy metal cadmium stress. Front. Plant Sci. 2016, 7, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.-G.; Min, X.; Zhou, Z.-H. Hydrogen Sulfide: A Signal Molecule in Plant Cross-Adaptation. Front. Plant Sci. 2016, 7, 621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corpas, F.J.; González-Gordo, S.; Cañas, A.; Palma, J.M. Nitric oxide and hydrogen sulfide in plants: Which comes first? J. Exp. Bot. 2019, 70, 4391–4404. [Google Scholar] [CrossRef]
- Remme, R.N. Effect of Salinity Stress on Glutathione–S-Transferases (GSTs) of Maize. IOSR J. Agric. Vet. Sci. 2013, 4, 42–52. [Google Scholar] [CrossRef]
- Tiwari, V.; Patel, M.K.; Chaturvedi, A.K.; Mishra, A.; Jha, B. Functional characterization of the tau class glutathione-S-transferases gene (SbGSTU) promoter of Salicornia brachiata under salinity and osmotic stress. PLoS ONE 2016, 11, 2. [Google Scholar]
- Hasanuzzaman, M.; Fujita, M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 2013, 22, 584–596. [Google Scholar] [CrossRef]
- Méndez, A.A.; Pena, L.B.; Benavides, M.P.; Gallego, S.M. Priming with NO controls redox state and prevents cadmium-induced general up-regulation of methionine sulfoxide reductase gene family in Arabidopsis. Biochimie 2016, 131, 128–136. [Google Scholar] [CrossRef]
- Fu, M.-M.; Dawood, M.; Wang, N.-H.; Wu, F. Exogenous hydrogen sulfide reduces cadmium uptake and alleviates cadmium toxicity in barley. Plant Growth Regul. 2019, 89, 227–237. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, L.; Wang, L.; Zhou, C.; Shangguan, Y.; Yang, Y. Antioxidative enzymes activity and thiol metabolism in three leafy vegetables under Cd stress. Ecotoxicol. Environ. Saf. 2019, 173, 214–224. [Google Scholar] [CrossRef]
- Mishra, S.; Tripathi, R.; Srivastava, S.; Dwivedi, S.; Trivedi, P.K.; Dhankher, O.; Khare, A. Thiol metabolism play significant role during cadmium detoxification by Ceratophyllum demersum L. Bioresour. Technol. 2009, 100, 2155–2161. [Google Scholar] [CrossRef]
- Praveen, A.; Pandey, A.; Gupta, M. Protective role of nitric oxide on nitrogen-thiol metabolism and amino acids profiling during arsenic exposure in Oryza sativa L. Ecotoxicology 2020, 29, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Bankaji, I.; Caçador, I.; Sleimi, N. Physiological and biochemical response of Sueda fruticosa to cadmium and copper stress: Growth, nutrient uptake, antioxidant enzymes, phytochelatin, and glutathione levels. Environ. Sci. Pollut. Res. 2015, 22, 13058–13069. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Khan, M.I.R.; Asgher, M.; Fatma, M.; Masood, A.; Syeed, S. Salinity Tolerance in Plants: Revisiting the Role of Sulfur Metabolites. J. Plant Biochem. Physiol. 2014, 2, 2. [Google Scholar]
- Wiszniewska, A.; Muszyńska, E.; Kołton, A.; Kamińska, I.; Hanus-Fajerska, E. In vitro acclimation to prolonged metallic stress is associated with modulation of antioxidant responses in a woody shrub Daphne jasminea. Plant Cell Tissue Organ Cult. 2019, 139, 339–357. [Google Scholar] [CrossRef] [Green Version]
- Schutzendubel, A.; Polle, A. Plant responses to abiotic stresses: Heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef]
- Saad, R.B.; Hsouna, A.B.; Saibi, W.; Hamed, K.B.; Brini, F.; Ghneim-Herrera, T. A stress-associated protein, LmSAP, from the halophyte Lobularia maritima provides tolerance to heavy metals in tobacco through increased ROS scavenging and metal detoxification processes. J. Plant. Physiol. 2018, 231, 234–243. [Google Scholar] [CrossRef]
- Verma, K.; Mehta, S.K.; Shekhawat, G.S. Nitric oxide (NO) counteracts cadmium induced cytotoxic processes mediated by reactive oxygen species (ROS) in Brassica juncea: Cross-talk between ROS, NO and antioxidant responses. Biometals 2013, 26, 255–269. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, Y.; Li, C.; Zhao, J.; Wei, M.; Li, X.; Yang, S.; Mi, Y. Effect of sodium nitroprusside treatment on shikimate and phenylpropanoid pathways of apple fruit. Food Chem. 2019, 290, 263–269. [Google Scholar] [CrossRef]
- Montesinos-Pereira, D.; Barrameda-Medina, Y.; Baenas, N.; Moreno, D.A.; Sanchez-Rodriguez, E.; Blasco, B.; Ruiz, J.M. Evaluation of hydrogen sulfide supply to biostimulate the nutritive and phytochemical quality and the antioxidant capacity of Cabbage (Brassica oleracea L. ‘Bronco’). J. Appl. Bot Food Qual. 2016, 89, 290–298. [Google Scholar]
- Chen, P.; Yang, W.; Jin, S.; Liu, Y. Hydrogen sulfide alleviates salinity stress in Cyclocarya paliurus by maintaining chlorophyll fluorescence and regulating nitric oxide level and antioxidant capacity. Plant Physiol. Biochem. 2021, 167, 738–747. [Google Scholar] [CrossRef]
- Tokarz, K.M.; Makowski, W.; Tokarz, B.; Hanula, M.; Sitek, E.; Muszyńska, E.; Jędrzejczyk, R.; Banasiuk, R.; Chajec, Ł.; Mazur, S. Can Ceylon Leadwort (Plumbago zeylanica L.) Acclimate to Lead Toxicity?—Studies of Photosynthetic Apparatus Efficiency. Int. J. Mol. Sci. 2020, 21, 1866. [Google Scholar] [CrossRef] [Green Version]
- Kahveci, H.; Bilginer, N.; Diraz-Yildirim, E.; Kulak, M.; Yazar, E.; Kocacinar, F.; Karaman, S. Priming with salicylic acid, β-carotene and tryptophan modulates growth, phenolics and essential oil components of Ocimum basilicum L. grown under salinity. Sci. Hortic. 2021, 281, 109964. [Google Scholar] [CrossRef]
- Gohari, G.; Alavi, Z.; Esfandiari, E.; Panahirad, S.; Hajihoseinlou, S.; Fotopoulos, V. Interaction between hydrogen peroxide and sodium nitroprusside following chemical priming of Ocimum basilicum L. against salt stress. Physiol. Plant. 2020, 168, 361–373. [Google Scholar] [PubMed] [Green Version]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sherin, G.; Aswathi, K.R.; Puthur, J.T. Photosynthetic functions in plants subjected to stresses are positively influenced by priming. Plant Stress 2022, 4, 100079. [Google Scholar] [CrossRef]
- Mueller, L.A.; Goodman, C.D.; Silady, R.A.; Walbot, V. AN9, a petunia glutathione S-transferase required for anthocyanin sequestration, is a flavonoid-binding protein. Plant Physiol. 2000, 123, 1561–1570. [Google Scholar] [CrossRef] [Green Version]
- Bownik, A.; Stępniewska, Z.; Skowroński, T. Protective effects of ectoine on heat-stressed Daphnia magna. J. Comp. Physiol. B 2014, 184, 961–976. [Google Scholar]
- Mohsenzadeh, S.; Zohrabi, M. Auxin and Sodium Nitroprusside Effects on Wheat Antioxidants in Salinity. Russ. J. Plant Physiol. 2018, 65, 651–657. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kaur, G.; Arora, K.; Kohli, R.K. Nitric oxide (as sodium nitroprusside) supplementation ameliorates Cd toxicity in hydroponically grown wheat roots. EEB 2008, 63, 158–167. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Seraj, Z.I.; Fujita, M. Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma 2014, 251, 1373–1386. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Int. Plant Propagators Soc. 1982, 30, 421–427. [Google Scholar]
- Habig, W.H.; Jakoby, W.B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981, 77, 398–405. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- de Vos, C.H.R.; Vonk, M.J.; Vooijs, R.; Schat, H. Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol. 1992, 98, 853–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queval, G.; Noctor, G. A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: Application to redox profiling during Arabidopsis rosette development. Anal. Biochem. 2007, 363, 58–69. [Google Scholar] [CrossRef]
- Makowski, W.; Królicka, A.; Tokarz, B.; Miernicka, K.; Kołton, A.; Pięta, Ł.; Malek, K.; Ekiert, H.; Szopa, A.; Tokarz, K.M. Response of Physiological Parameters in Dionaea muscipula J. Ellis Teratomas Transformed with RolB Oncogene. BMC Plant Biol. 2021, 21, 564. [Google Scholar] [CrossRef] [PubMed]
- Pekkarinen, S.S.; Stöckmann, H.; Schwarz, K.; Heinonen, I.M.; Hopia, A.I. Antioxidant activity and partitioning of phenolic acids in bulk and emulsified methyl linoleate. J. Agric. Food Chem. 1999, 47, 3036–3043. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiszniewska, A.; Makowski, W. Assessment of Shoot Priming Efficiency to Counteract Complex Metal Stress in Halotolerant Lobularia maritima. Plants 2023, 12, 1440. https://doi.org/10.3390/plants12071440
Wiszniewska A, Makowski W. Assessment of Shoot Priming Efficiency to Counteract Complex Metal Stress in Halotolerant Lobularia maritima. Plants. 2023; 12(7):1440. https://doi.org/10.3390/plants12071440
Chicago/Turabian StyleWiszniewska, Alina, and Wojciech Makowski. 2023. "Assessment of Shoot Priming Efficiency to Counteract Complex Metal Stress in Halotolerant Lobularia maritima" Plants 12, no. 7: 1440. https://doi.org/10.3390/plants12071440





