A Nanotechnological Approach to Exploit and Enhance the Bioactivity of an Extract from Onopordum illyricum L. Leaves
Abstract
:1. Introduction
2. Results
2.1. Quali-Quantitative Determination of Phenolic Compounds in O. illyricum Extract
2.2. Vesicle Characterization
2.3. Antioxidant Assays
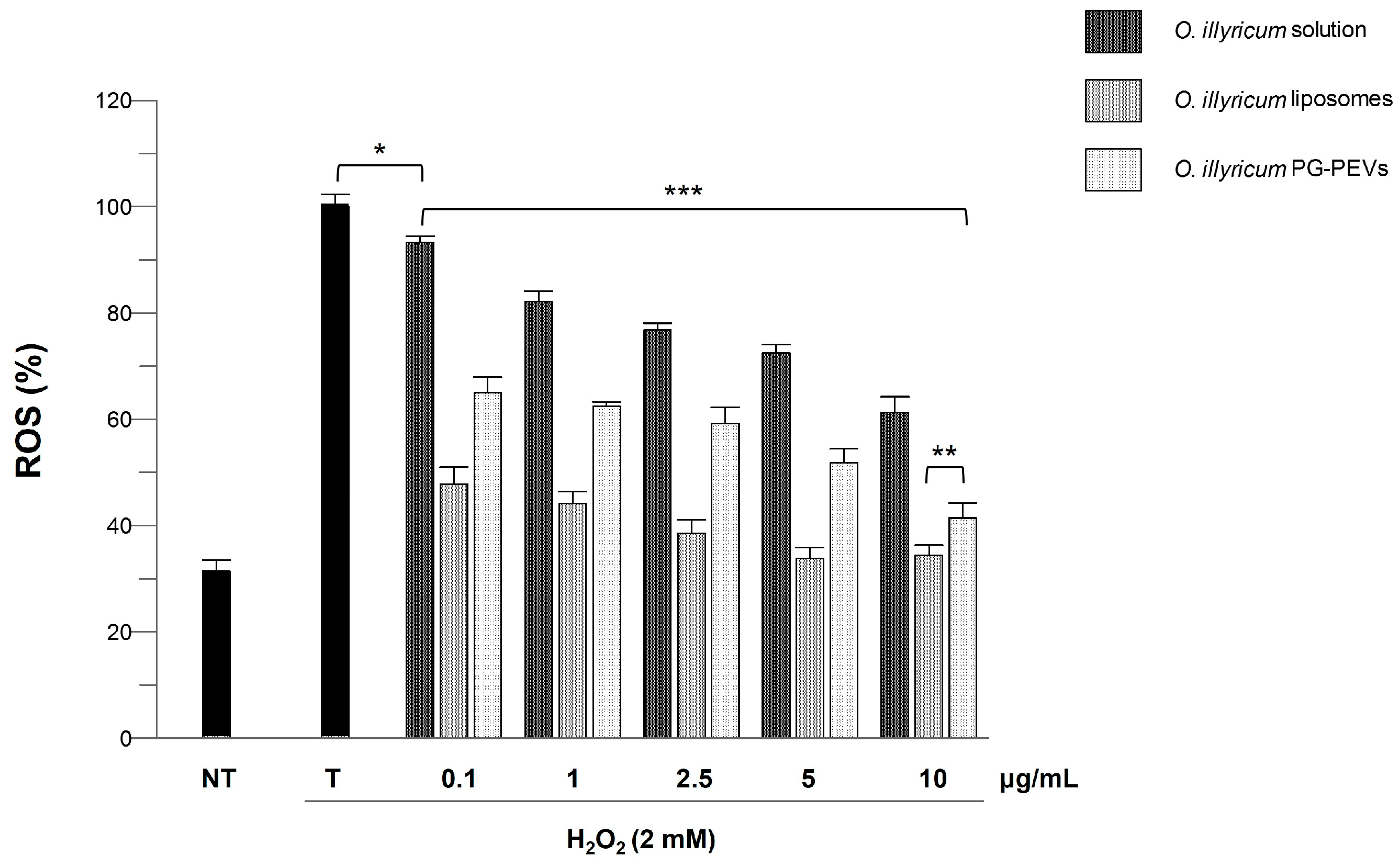
2.4. Cell Viability and Anti-ROS Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Extract Preparation
4.4. High-Resolution LC-ESI-QTOF-MS-MS Analysis
4.5. HPLC-DAD Analysis
4.6. Vesicle Preparation and Characterization
4.7. Antioxidant Assays
4.8. Cell Viability
4.9. Antioxidant Activity in Cells
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atzei, A.D. Le Piante Nella Tradizione Popolare della Sardegna; Carlo Delfino Editore: Sassari, Italy, 2003. [Google Scholar]
- Ballero, M.; Bruni, A.; Sacchetti, G.; Poli, F. Le piante utilizzate nella medicina popolare nel Comune di Tempio Pausania (Sardegna settentrionale). Acta Phytother. 1997, 1, 23–29. [Google Scholar]
- Braca, A.; De Tommasi, N.; Morelli, I.I.; Pizza, C. New metabolites from Onopordum illyricum. J. Nat. Prod. 1999, 62, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Verotta, L.; Belvisi, L.; Bertacche, V.; Loi, M. Complete characterization of extracts of Onopordum illyricum L. (Asteraceae) by HPLC/PDA/ESIMS and NMR. Nat. Prod. Commun. 2008, 3, 2037–2042. [Google Scholar] [CrossRef] [Green Version]
- Bruno, M.; Maggio, A.; Rosselli, S.; Safder, M.; Bancheva, S. The metabolites of the genus Onopordum (Asteraceae): Chemistry and biological properties. Curr. Org. Chem. 2011, 15, 888–927. [Google Scholar] [CrossRef]
- Rosselli, S.; Maggio, A.M.; Canzoneri, M.; Simmonds, M.S.; Bruno, M. Sesquiterpenes from Onopordum illyricum and their antifeedant activity. Nat. Prod. Commun. 2012, 7, 1131–1132. [Google Scholar] [CrossRef] [Green Version]
- Formisano, C.; Sanna, C.; Ballero, M.; Chianese, G.; Sirignano, C.; Rigano, D.; Millán, E.; Muñoz, E.; Taglialatela-Scafati, O. Anti-inflammatory sesquiterpene lactones from Onopordum illyricum L. (Asteraceae), an Italian medicinal plant. Fitoterapia 2017, 116, 61–65. [Google Scholar] [CrossRef]
- Sanna, C.; Rigano, D.; Cortis, P.; Corona, A.; Ballero, M.; Parolin, C.; Del Vecchio, C.; Chianese, G.; Saccon, E.; Formisano, C.; et al. Onopordum illyricum L., a Mediterranean plant, as a source of anti HIV-1 compounds. Plant Biosyst. 2018, 152, 1274–1281. [Google Scholar] [CrossRef]
- Marengo, A.; Fumagalli, M.; Sanna, C.; Maxia, A.; Piazza, S.; Cagliero, C.; Rubiolo, P.; Sangiovanni, E.; Dell’Agli, M. The hydro-alcoholic extracts of Sardinian wild thistles (Onopordum spp.) inhibit TNFα-induced IL-8 secretion and NF-κB pathway in human gastric epithelial AGS cells. J. Ethnopharmacol. 2018, 210, 469–476. [Google Scholar] [CrossRef] [Green Version]
- Kyriakoudi, A.; Spanidi, E.; Mourtzinos, I.; Gardikis, K. Innovative Delivery Systems Loaded with Plant Bioactive Ingredients: Formulation Approaches and Applications. Plants 2021, 10, 1238. [Google Scholar] [CrossRef]
- Gupta, M.K.; Sansare, V.; Shrivastava, B.; Jadhav, S.; Gurav, P. Comprehensive review on use of phospholipid based vesicles for phytoactive delivery. J. Liposome Res. 2022, 32, 211–223. [Google Scholar] [CrossRef]
- Sahu, A.N.; Mohapatra, D. Nanovesicular transferosomes for the topical delivery of plant bioactives. Nanomedicine 2021, 16, 2491–2495. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yan, H.; Pang, S.; Ya, M.; Qiu, F.; Qin, P.; Zeng, C.; Lu, Y. Liposomes as multifunctional nano-carriers for medicinal natural products. Front. Chem. 2022, 10, 963004. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.S.; Othman, H.H.; Hammadi, N.I.; Yeap, S.K.; Amin, K.M.; Abdul Samad, N.; Alitheen, N.B. Novel drug delivery systems for loading of natural plant extracts and their biomedical applications. Int. J. Nanomed. 2020, 15, 2439–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- KNApSAcK Core System. Available online: http://www.knapsackfamily.com/knapsack_core/top.php (accessed on 19 December 2022).
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.A.; Nothias, L.F.; Ludwig, M.; Fleischauer, M.; Gentry, E.C.; Witting, M.; Dorrestein, P.C.; Dührkop, K.; Böcker, S. High-confidence structural annotation of metabolites absent from spectral libraries. Nat. Biotechnol. 2022, 40, 411–421. [Google Scholar] [CrossRef]
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software tools and approaches for compound identification of LC-MS/MS data in metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, R.; Kiprotich, J.; Kuhnert, N. Determination of the hydroxycinnamate profile of 12 members of the Asteraceae family. Phytochemistry 2011, 72, 781–790. [Google Scholar] [CrossRef]
- Jaiswal, R.; Kuhnert, N. Identification and characterization of five new classes of chlorogenic acids in burdock (Arctium lappa L.) roots by liquid chromatography/tandem mass spectrometry. Food Funct. 2011, 2, 63–71. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Lamuela-Raventós, R.M.; Bastida, J.; Viladomat, F.; Codina, C. Identification of phenolic compounds in artichoke waste by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2003, 1008, 57–72. [Google Scholar] [CrossRef]
- Kubik, J.; Waszak, Ł.; Adamczuk, G.; Humeniuk, E.; Iwan, M.; Adamczuk, K.; Michalczuk, M.; Korga-Plewko, A.; Józefczyk, A. Phytochemical analysis and anti-cancer properties of extracts of Centaurea castriferrei Borbás & Waisb Genus of Centaurea L. Molecules 2022, 27, 7537. [Google Scholar] [CrossRef]
- Abrankó, L.; Clifford, M.N. An unambiguous nomenclature for the acyl-quinic acids commonly known as chlorogenic acids. J. Agric. Food Chem. 2017, 65, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- Benedek, B.; Geisz, N.; Jäger, W.; Thalhammer, T.; Kopp, B. Choleretic effects of yarrow (Achillea millefolium s.l.) in the isolated perfused rat liver. Phytomedicine 2006, 13, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Sarawek, S.; Feistel, B.; Pischel, I.; Butterweck, V. Flavonoids of Cynara scolymus possess potent xanthinoxidase inhibitory activity in vitro but are devoid of hypouricemic effects in rats after oral application. Planta Med. 2008, 74, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orčić, D.Z.; Mimica-Dukić, N.M.; Francišković, M.M.; Petrović, S.S.; Jovin, E.D. Antioxidant activity relationship of phenolic compounds in Hypericum perforatum L. Chem. Cent. J. 2011, 5, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Riaguas, A.; Fernández-de Córdova, M.L.; Llorent-Martínez, E.J. Phenolic profile and antioxidant activity of Euonymus japonicus thunb. Nat. Prod. Res. 2022, 36, 3445–3449. [Google Scholar] [CrossRef] [PubMed]
- Pagano, I.; Piccinelli, A.L.; Celano, R.; Campone, L.; Gazzerro, P.; De Falco, E.; Rastrelli, L. Chemical profile and cellular antioxidant activity of artichoke by-products. Food Funct. 2016, 7, 4841–4850. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-Antioxidant Activity Relationships of Luteolin and Catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef]
- Kut, K.; Bartosz, G.; Soszyński, M.; Sadowska-Bartosz, I. Antioxidant properties of hispidulin. Nat. Prod. Res. 2022, 36, 6401–6404. [Google Scholar] [CrossRef]
- Aisha, A.F.; Majid, A.M.; Ismail, Z. Preparation and characterization of nano liposomes of Orthosiphon stamineus ethanolic extract in soybean phospholipids. BMC Biotechnol. 2014, 14, 23. [Google Scholar] [CrossRef] [Green Version]
- Păvăloiu, R.-D.; Sha’at, F.; Neagu, G.; Deaconu, M.; Bubueanu, C.; Albulescu, A.; Sha’at, M.; Hlevca, C. Encapsulation of polyphenols from Lycium barbarum leaves into liposomes as a strategy to improve their delivery. Nanomaterials 2021, 11, 1938. [Google Scholar] [CrossRef]
- Shariare, M.H.; Pinky, N.J.K.; Abedin, J.; Kazi, M.; Aldughaim, M.S.; Uddin, M.N. Liposomal drug delivery of Blumea lacera leaf extract: In-Vivo hepatoprotective effects. Nanomaterials 2022, 12, 2262. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.; Lucchesi, D.; Tuberoso, C.I.G.; Fernàndez-Busquets, X.; Vassallo, A.; Martelli, G.; Fadda, A.M.; Pucci, L.; Caddeo, C. Liposomal formulations to improve antioxidant power of myrtle berry extract for potential skin application. Pharmaceutics 2022, 14, 910. [Google Scholar] [CrossRef] [PubMed]


| Compound No. | Rt min | Identity | UV λmax nm | [M-H]− m/z | Molecular Formula | Δ ppm | MS/MS * m/z | References | Level # |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 8.96 | 5-O-caffeoylquinic acid | 218, 326 | 353.0884 | C16H18O9 | 0.39 | 191.0559(100) | [4,19] | 1 |
| 2 | 12.16 | 1,3-Dicaffeoylquinic acid | 244, 322 | 515.1197 | C25H24O12 | 0.18 | 353.0884(12)/191.0567(100) | [4,19,20] | 1 |
| 3 | 13.14 | Feruloylquinic acid | 244, 328 | 367.1038 | C17H20O9 | 0.68 | 193.0482(11)/191.0560(100) | [19] | 2 |
| 4 | 17.10 | Luteolin glucuronide | 256, 267, 348 | 461.0725 | C21H18O12 | −0.04 | 285.0404(100) | [21] | 2 |
| 5 | 17.73 | 3,4-Dicaffeoylquinic acid | 244, 328 | 515.1197 | C25H24O12 | 0.15 | 353.0883(9)/191.0567(100) | [4,19,20] | 1 |
| 6 | 17.99 | 3,5-Dicaffeoylquinic acid | 242, 328 | 515.1197 | C25H24O12 | 0.26 | 353.0882(15)/191.0567(100) | [4,19,20] | 1 |
| 7 | 18.56 | Dicaffeoylquinic acid | 242, 328 | 515.1197 | C25H24O12 | 0.44 | 353.0863(12)/191.0562(100)/179.0339(29) | [4,19,20] | 2 |
| 8 | 18.77 | 1,5-Dicaffeoylquinic acid | 245, 330 | 515.1197 | C25H24O12 | 0.37 | 353.0863(7)/191.0562(100)/179.0339(8) | [4,19,20] | 2 |
| 9 | 19.32 | Apigenin rutinoside | 48, 338 | 577.1564 | C27H30O14 | 0.02 | 269.0452(100) | [4] | 2 |
| 10 | 19.76 | Dicaffeoylsuccunoylquinic acid I | 243, 330 | 615.1361 | C29H28O15 | 0.52 | 515.06924(9)/353.0855(9)/191.0598(98) | [4,9,20] | 2 |
| 11 | 19.91 | 4,5-Dicaffeoylquinic acid | 244, 328 | 515.1197 | C25H24O12 | −0.06 | 353.0883(14)/191.0567(100) | [4,19,20] | 1 |
| 12 | 20.24 | Dicaffeoylsuccunoylquinic acid II | 244, 330 | 615.1361 | C29H28O15 | 0.52 | 515.0774(20)/353.0821(11)/191.0564(100) | [4,9,20] | 2 |
| 13 | 20.73 | Hispidulin glucuronide | 270, 334 | 475.0880 | C22H20O12 | −0.41 | 299.0535(100)/284.0334(57) | [22] | 2 |
| 14 | 22.61 | Feruloylcaffeoylquinic acid | 244, 330 | 529.1354 | C26H26O12 | 0.68 | 367.1038(15)/353.0867(7)/191.0560(100) | [19] | 2 |
| 15 | 24.39 | Luteolin | 256, 268, 346 | 285.0398 | C15H10O6 | −0.04 | 212.0463(25)/171.0460(12) | [4,8] | 1 |
| 16 | 30.02 | Hispidulin | 274, 337 | 299.0558 | C16H12O6 | −1.05 | 284.0334(100) | [4,8] | 1 |
| Compound | No. | O. illyricum Extract (mg/g dm) |
|---|---|---|
| Mean ± SD | ||
| Total Hydroxycinnamic acids | 9.59 ± 0.07 | |
| 5-O-caffeoylquinic acid | 1 | 1.30 ± 0.02 |
| 1,3-di-O-caffeoylquinic acid | 2 | 0.1 ± 0.00 |
| Feruloyl quinic acid A | 3 | 0.13 ± 0.00 |
| 3,4-di-O-caffeoylquinic acid | 5 | 0.35 ± 0.00 |
| 3,5-di-O-caffeoylquinic acid | 6 | 0.44 ± 0.01 |
| Dicaffeoylquinic acid B | 7 | 0.67 ± 0.00 |
| 1,5-di-O-caffeoylquinic acid B | 8 | 3.40 ± 0.07 |
| Dicaffeoyl succinoyl quinic acid I B | 10 | 0.30 ± 0.01 |
| 4,5-di-O-caffeoylquinic acid | 11 | 0.77 ± 0.02 |
| Dicaffeoyl succinoyl quinic acid II B | 12 | 1.70 ± 0.00 |
| Feruloyl caffeoyl quinic acid A | 14 | 0.44 ± 0.01 |
| Total Flavonols | 7.32 ± 0.05 | |
| Luteolin-glucuronide C | 4 | 4.37 ± 0.00 |
| Apigenin-rutinoside D | 9 | 0.24 ± 0.00 |
| Hispidulin-glucuronide E | 13 | 0.28 ± 0.00 |
| Luteolin | 15 | 1.14 ± 0.01 |
| Hispidulin | 16 | 1.30 ± 0.02 |
| Total polyphenols | 16.91 ± 0.83 |
| Formulation | MD nm ± SD | PI ± SD | ZP mV ± SD |
|---|---|---|---|
| Empty liposomes | 80 ± 3.6 | 0.31 ± 0.04 | −14 ± 1.4 |
| O. illyricum liposomes | *** 96 ± 3.3 | *** 0.24 ± 0.01 | *** −6 ± 1.6 |
| Empty PG-PEVs | 77 ± 10.1 | 0.36 ± 0.08 | −15 ± 2.8 |
| O. illyricum PG-PEVs | §§§ 77 ± 3.1 | §§§, °°° 0.22 ± 0.01 | °°° −6 ± 1.2 |
| Formulation | Time | MD nm ± SD | PI ± SD | ZP mV ± SD |
|---|---|---|---|---|
| Empty liposomes | t0 | 80 ± 3.6 | 0.31 ± 0.04 | −14 ± 1.4 |
| t2 | * 91 ± 4.7 | 0.39 ± 0.08 | −15 ± 2.8 | |
| O. illyricum liposomes | t0 | 96 ± 3.3 | 0.24 ± 0.01 | −6 ± 1.6 |
| t2 | 103 ± 2.4 | § 0.28 ± 0.02 | −10 ± 2.2 | |
| Empty PG-PEVs | t0 | 77 ± 10.1 | 0.36 ± 0.08 | −15 ± 2.8 |
| t2 | 90 ± 6.3 | 0.42 ± 0.07 | −17 ± 3.8 | |
| O. illyricum PG-PEVs | t0 | 77 ± 3.1 | 0.22 ± 0.01 | −6 ± 1.2 |
| t2 | 84 ± 4.1 | 0.25 ± 0.03 | −10 ± 3.5 |
| Peak No. | Compound | EE% ± SD | |
|---|---|---|---|
| PG-PEVs | Liposomes | ||
| Hydroxycinnamic acids | |||
| 1 | 5-O-caffeoylquinic acid | 38.8 ± 5.4 a | 60.0 ± 2.5 b |
| 2 | 1,3-Dicaffeoylquinic acid | 43.0 ± 6.7 a | 66.7 ± 3.4 b |
| 3 | Feruloyl quinic acid A | 45.8 ± 4.8 a | 57.1 ± 7.8 a |
| 5 | 3,4-Dicaffeoylquinic acid | 97.6 ± 5.0 a | 95.6 ± 9.8 a |
| 6 | 3,5-Dicaffeoylquinic acid | 93.5 ± 4.3 a | 93.1 ± 6.1 a |
| 7 | Dicaffeoylquinic acid B | 83.0 ± 5.6 a | 96.0 ± 7.8 a |
| 8 | 1,5-Dicaffeoylquinic acid B | 88.2 ± 8.7 a | 77.8 ± 8.8 a |
| 10 | Dicaffeoylsuccunoylquinic acid I B | 82.6 ± 3.5 a | 99.3 ± 5.5 b |
| 11 | 4,5-Dicaffeoylquinic acid | 80.7 ± 4.9 a | 94.5 ± 4.8 b |
| 12 | Dicaffeoylsuccunoylquinic acid II B | 77.3 ± 9.8 a | 69.6 ± 6.8 a |
| 14 | Feruloyl caffeoyl quinic acid A | 89.2 ± 7.2 a | 100.3 ± 7.2 a |
| Flavonols | |||
| 4 | Luteolin glucuronide C | 82.5 ± 6.1 a | 97.3 ± 2.7 b |
| 13 | Hispidulin glucuronide D | 77.5 ± 5.1 a | 94.9 ± 5.5 b |
| Formulation | DPPH Assay | FRAP Assay | |
|---|---|---|---|
| AA (%) | TE (µg Trolox Equivalents/mL) | FE (µg Fe2+ Equivalents/mL) | |
| O. illyricum solution | 94 ± 4.4 | 673 ± 23 | 445 ± 15 |
| Empty liposomes | 39 ± 9.9 | 262 ± 28 | 186 ± 9 |
| O. illyricum liposomes | 99 ± 0.3 | 715 ± 3 | ** 590 ± 19 |
| Empty PG-PEVs | 39 ± 6.9 | 265 ± 32 | 186 ± 15 |
| O. illyricum PG-PEVs | 99 ± 0.2 | 716 ± 2 | ** 560 ± 6 |
| Formulation | P90G 1 | O. illyricum 2 | PG 3 | H2O |
|---|---|---|---|---|
| Empty liposomes | 90 mg | -- | -- | 1 mL |
| O. Illyricum liposomes | 90 mg | 2 mg | -- | 1 mL |
| Empty PG-PEVs | 90 mg | -- | 0.1 mL | 0.9 mL |
| O. illyricum PG-PEVs | 90 mg | 2 mg | 0.1 mL | 0.9 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caddeo, C.; Tuberoso, C.I.G.; Floris, S.; Masala, V.; Sanna, C.; Pintus, F. A Nanotechnological Approach to Exploit and Enhance the Bioactivity of an Extract from Onopordum illyricum L. Leaves. Plants 2023, 12, 1453. https://doi.org/10.3390/plants12071453
Caddeo C, Tuberoso CIG, Floris S, Masala V, Sanna C, Pintus F. A Nanotechnological Approach to Exploit and Enhance the Bioactivity of an Extract from Onopordum illyricum L. Leaves. Plants. 2023; 12(7):1453. https://doi.org/10.3390/plants12071453
Chicago/Turabian StyleCaddeo, Carla, Carlo Ignazio Giovanni Tuberoso, Sonia Floris, Valentina Masala, Cinzia Sanna, and Francesca Pintus. 2023. "A Nanotechnological Approach to Exploit and Enhance the Bioactivity of an Extract from Onopordum illyricum L. Leaves" Plants 12, no. 7: 1453. https://doi.org/10.3390/plants12071453






