A Look at Plant-Growth-Promoting Bacteria
Abstract
:1. Plant-Growth-Promoting Rhizobacterias (PGPRs)
2. Direct and Indirect Mechanisms of Microorganisms in Plants
3. Plant Strategies to Select Beneficial Microorganisms
4. Mechanisms of Plant–Bacteria Interaction: A Transcriptomics View
5. Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R.A.; Del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Gupta, S.; Dubey, N.K.; Singh, S.P.; Kheni, J.K.; Varshney, A. Plant Growth-Promoting Rhizobacteria (PGPR): Current and Future Prospects for Crop Improvement. Current Trends in Microbial Biotechnology for Sustainable Agriculture. In Environmental and Microbial Biotechnology; Springer: Singapore, 2021; pp. 203–226. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant—Fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- Haichar, F.E.Z.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008, 2, 1221–1230. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef]
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, V. Exploring the Potential and Opportunities of Current Tools for Removal of Hazardous Materials From Environments. Phytomanagement Polluted Sites Mark. Oppor. Sustain. Phytoremediation 2019, 1, 501–516. [Google Scholar] [CrossRef]
- Raimi, A.; Roopnarain, A.; Adeleke, R. Biofertilizer production in Africa: Current status, factors impeding adoption and strategies for success. Sci. Afr. 2021, 11, e00694. [Google Scholar] [CrossRef]
- Maitra, P.; Al-Rashid, J.; Mandal, D.; Azam, M.S.; Rasul, N.M. Polyvinylpyrrolidone (PVP) and Na-Alginate Addition Enhances the Survival and Agronomic Performances of a Liquid Inoculant of Bradyrhizobium japonicum for Soybean (Glycine max (L.) Merr.). Agronomy 2021, 11, 1009. [Google Scholar] [CrossRef]
- Brahmaprakash, G.P.; Sahu, P.K.; Lavanya, G.; Gupta, A.; Nair, S.S.; Gangaraddi, V. Role of Additives in Improving Efficiency of Bioformulation for Plant Growth and Development. In Frontiers in Soil and Environmental Microbiology; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–10. [Google Scholar] [CrossRef]
- Berninger, T.; González López, Ó.; Bejarano, A.; Preininger, C.; Sessitsch, A. Maintenance and assessment of cell viability in formulation of non-sporulating bacterial inoculants. Microb. Biotechnol. 2018, 11, 277. [Google Scholar] [CrossRef]
- Ray, P.; Lakshmanan, V.; Labbé, J.L.; Craven, K.D. Microbe to Microbiome: A Paradigm Shift in the Application of Microorganisms for Sustainable Agriculture. Front. Microbiol. 2020, 11, 3323. [Google Scholar] [CrossRef]
- Koskey, G.; Mburu, S.W.; Awino, R.; Njeru, E.M.; Maingi, J.M. Potential Use of Beneficial Microorganisms for Soil Amelioration, Phytopathogen Biocontrol, and Sustainable Crop Production in Smallholder Agroecosystems. Front. Sustain. Food Syst. 2021, 5, 130. [Google Scholar] [CrossRef]
- Andrews, M.; Cripps, M.; Edwards, G.; Andrews, C.M. The potential of beneficial microorganisms in agricultural systems. Ann. Appl. Biol. 2012, 160, 1–5. [Google Scholar] [CrossRef]
- Admassie, M.; Woldehawariat, Y.; Alemu, T.; Gonzalez, E.; Jimenez, J.F. The role of plant growth-promoting bacteria in alleviating drought stress on pepper plants. Agric. Water Manag. 2022, 272, 107831. [Google Scholar] [CrossRef]
- Farhaoui, A.; Adadi, A.; Tahiri, A.; El Alami, N.; Khayi, S.; Mentag, R.; Ezrari, S.; Radouane, N.; Mokrini, F.; Belabess, Z.; et al. Biocontrol potential of plant growth-promoting rhizobacteria (PGPR) against Sclerotiorum rolfsii diseases on sugar beet (Beta vulgaris L.). Physiol. Mol. Plant Pathol. 2022, 119, 101829. [Google Scholar] [CrossRef]
- Ngalimat, M.S.; Hata, E.M.; Zulperi, D.; Ismail, S.I.; Ismail, M.R.; Zainudin, N.A.I.M.; Saidi, N.B.; Yusof, M.T. Streptomyces-mediated growth enhancement and Bacterial Panicle Blight disease suppression in rice plants under greenhouse conditions. J. Biotechnol. 2022, 359, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, Q.; Huang, L.; Xu, S.; Fu, Y.; Hou, D.; Feng, Y.; Yang, X. Cadmium phytoextraction through Brassica juncea L. under different consortia of plant growth-promoting bacteria from different ecological niches. Ecotoxicol. Environ. Saf. 2022, 237, 113541. [Google Scholar] [CrossRef]
- Costa Júnior, P.S.P.; Cardoso, F.P.; Martins, A.D.; Teixeira Buttrós, V.H.; Pasqual, M.; Dias, D.R.; Schwan, R.F.; Dória, J. Endophytic bacteria of garlic roots promote growth of micropropagated meristems. Microbiol. Res. 2020, 241, 126585. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Bhatia, A.; Kaushik, R. Inoculation of plant growth promoting-methane utilizing bacteria in different N-fertilizer regime influences methane emission and crop growth of flooded paddy. Sci. Total Environ. 2021, 775, 145826. [Google Scholar] [CrossRef]
- Andrade, G.V.S.; Rodrigues, F.A.; Nadal, M.C.; Caroline, M.d.S.D.; Martins, A.D.; Rodrigues, V.A.; dos Reis Ferreira, G.M.; Pasqual, M.; Buttros, V.H.; Dória, J. Plant-endophytic bacteria interactions associated with root and leaf microbiomes of Cattleya walkeriana and their effect on plant growth. Sci. Hortic. (Amst.) 2023, 309, 111656. [Google Scholar] [CrossRef]
- Devi, R.; Kaur, T.; Kour, D.; Yadav, A.N. Microbial consortium of mineral solubilizing and nitrogen fixing bacteria for plant growth promotion of amaranth (Amaranthus hypochondrius L.). Biocatal. Agric. Biotechnol. 2022, 43, 102404. [Google Scholar] [CrossRef]
- Khairina, Y.; Jog, R.; Boonmak, C.; Toyama, T.; Oyama, T.; Morikawa, M. Indigenous bacteria, an excellent reservoir of functional plant growth promoters for enhancing duckweed biomass yield on site. Chemosphere 2021, 268, 129247. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.; Furtana, G.B.; Ellialtioglu, Ş. Physiological and biochemical assay of drought stress responses in eggplant (Solanum melongena L.) inoculated with commercial inoculant of Azotobacter chroococum and Azotobacter vinelandii. Sci. Hortic. (Amst.) 2022, 305, 111394. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35 (Suppl. 4), 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 871, 1473. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [PubMed]
- Chandran, H.; Meena, M.; Swapnil, P. Plant Growth-Promoting Rhizobacteria as a Green Alternative for Sustainable Agriculture. Sustainability 2021, 13, 10986. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J. Auxin and Plant-Microbe Interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Basu, P.S. Production and metabolism of indole acetic acid in roots and root nodules of Phaseolus mungo. Microbiol. Res. 2006, 161, 362–366. [Google Scholar] [CrossRef]
- Dodd, I.C.; Zinovkina, N.Y.; Safronova, V.I.; Belimov, A.A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- Zaidi, A.; Ahmad, E.; Khan, M.S.; Saif, S.; Rizvi, A. Role of plant growth promoting rhizobacteria in sustainable production of vegetables: Current perspective. Sci. Hortic. (Amst.) 2015, 193, 231–239. [Google Scholar] [CrossRef]
- Khan, A.L.; Waqas, M.; Hussain, J.; Al-Harrasi, A.; Hamayun, M.; Lee, I.J. Phytohormones enabled endophytic fungal symbiosis improve aluminum phytoextraction in tolerant Solanum lycopersicum: An examples of Penicillium janthinellum LK5 and comparison with exogenous GA3. J. Hazard. Mater. 2015, 295, 70–78. [Google Scholar] [CrossRef] [PubMed]
- You, Y.H.; Yoon, H.; Kang, S.M.; Shin, J.H.; Choo, Y.S.; Lee, I.J.; Lee, J.M.; Kim, J.G. Fungal Diversity and Plant Growth Promotion of Endophytic Fungi from Six Halophytes in Suncheon Bay. J. Microbiol. Biotechnol. 2012, 22, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Egamberdieva, D.; Varma, A. Plant Growth-Promoting Rhizobacteria (PGPR) and Medicinal Plants: The State of the Art. In Plant-Growth-Promoting Rhizobacteria (PGPR) and Medicinal Plants; Springer: Cham, Switzerland, 2015; pp. 1–16. [Google Scholar] [CrossRef]
- De Rybel, B.; Mähönen, A.P.; Helariutta, Y.; Weijers, D. Plant vascular development: From early specification to differentiation. Nat. Rev. Mol. Cell Biol. 2015, 17, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, H. CYTOKININS: Activity, Biosynthesis, and Translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Veselov, S.U.; Melentiev, A.I.; Martynenko, E.V.; Kudoyarova, G.R. Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 2005, 272, 201–209. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.L.; Luo, L. Effects of Engineered Sinorhizobium meliloti on Cytokinin Synthesis and Tolerance of Alfalfa to Extreme Drought Stress. Appl. Environ. Microbiol. 2012, 78, 8056. [Google Scholar] [CrossRef] [PubMed]
- Turan, M.; Kıtır, N.; Alkaya, Ü.; Günes, A.; Tüfenkçi, Ş.; Yıldırım, E.; Nikerel, E.; Turan, M.; Kıtır, N.; Alkaya, Ü.; et al. Making Soil More Accessible to Plants: The Case of Plant Growth Promoting Rhizobacteria. Plant Growth 2016, 1, 61–69. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2011, 28, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem. Biophys. Rep. 2020, 22, 100737. [Google Scholar] [CrossRef]
- Meena, M.; Zehra, A.; Swapnil, P.; Harish; Marwal, A.; Yadav, G.; Sonigra, P. Endophytic Nanotechnology: An Approach to Study Scope and Potential Applications. Front. Chem. 2021, 9, 613343. [Google Scholar] [CrossRef]
- De Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef]
- Mohammadi, K.; Sohrabi, Y. BACTERIAL BIOFERTILIZERS FOR SUSTAINABLE CROP PRODUCTION: A REVIEW. ARPN J. Agric. Biol. Sci. 2012, 7, 307–316. [Google Scholar]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Ansari, R.A.; Mahmood, I.; Rizvi, R.; Sumbul, A. Safiuddin Siderophores: Augmentation of soil health and crop productivity. In Probiotics in Agroecosystem; Springer: Singapore, 2017; pp. 291–312. [Google Scholar] [CrossRef]
- Almoneafy, A.A.; Moustafa-Farag, M.; Mohamed, H.I.; Almoneafy, A.A.; Moustafa-Farag, M.; Mohamed, H.I. The Auspicious Role of Plant Growth-Promoting Rhizobacteria in the Sustainable Management of Plant Diseases. In Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management; Springer: Cham, Switzerland, 2021; pp. 251–283. [Google Scholar] [CrossRef]
- Ngalimat, M.S.; Hata, E.M.; Zulperi, D.; Ismail, S.I.; Ismail, M.R.; Zainudin, N.A.I.M.; Saidi, N.B.; Yusof, M.T. Plant Growth-Promoting Bacteria as an Emerging Tool to Manage Bacterial Rice Pathogens. Microorganisms 2021, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Husson, E.; Hadad, C.; Huet, G.; Laclef, S.; Lesur, D.; Lambertyn, V.; Jamali, A.; Gottis, S.; Sarazin, C.; Nguyen Van Nhien, A. The effect of room temperature ionic liquids on the selective biocatalytic hydrolysis of chitin via sequential or simultaneous strategies. Green Chem. 2017, 19, 4122–4131. [Google Scholar] [CrossRef]
- Vaddepalli, P.; Fulton, L.; Wieland, J.; Wassmer, K.; Schaeffer, M.; Ranf, S.; Schneitz, K. The cell wall-localized atypical β-1,3 glucanase ZERZAUST controls tissue morphogenesis in Arabidopsis thaliana. Development 2017, 144, 2259–2269. [Google Scholar] [CrossRef]
- Friedrich, N.; Hagedorn, M.; Soldati-Favre, D.; Soldati, T. Prison Break: Pathogens’ Strategies To Egress from Host Cells. Microbiol. Mol. Biol. Rev. 2012, 76, 707. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, L.; Hao, R.; Bai, X.; Wang, Y.; Yu, X. Drought-tolerant plant growth-promoting rhizobacteria isolated from jujube (Ziziphus jujuba) and their potential to enhance drought tolerance. Plant Soil 2020, 452, 423–440. [Google Scholar] [CrossRef]
- Chiappero, J.; Cappellari, L.d.R.; Sosa Alderete, L.G.; Palermo, T.B.; Banchio, E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind. Crops Prod. 2019, 139, 111553. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Amby, D.B.; Hegelund, J.N.; Fimognari, L.; Großkinsky, D.K.; Westergaard, J.C.; Müller, R.; Moelbak, L.; Liu, F.; Roitsch, T. Bacillus licheniformis FMCH001 Increases Water Use Efficiency via Growth Stimulation in Both Normal and Drought Conditions. Front. Plant Sci. 2020, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Shi, Y.; Zeng, G.; Gu, Y.; Chen, G.; Shi, L.; Hu, Y.; Tang, B.; Zhou, J. Acyl-homoserine lactone-based quorum sensing and quorum quenching hold promise to determine the performance of biological wastewater treatments: An overview. Chemosphere 2016, 157, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Beneficial plant-bacterial interactions. Benef. Plant-Bact. Interact. 2015, 2, 1–243. [Google Scholar] [CrossRef]
- Chen, F.; Gao, Y.; Chen, X.; Yu, Z.; Li, X. Quorum Quenching Enzymes and Their Application in Degrading Signal Molecules to Block Quorum Sensing-Dependent Infection. Int. J. Mol. Sci. 2013, 14, 17477. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.; Zaidi, A.; Khan, M.S.; Saif, S.; Ahmed, B.; Shahid, M. Growth improvement and management of vegetable diseases by plant growth-promoting rhizobacteria. In Microbial Strategies for Vegetable Production; Springer: Cham, Switzerland, 2017; pp. 99–123. [Google Scholar] [CrossRef]
- Toral, L.; Rodríguez, M.; Béjar, V.; Sampedro, I. Crop Protection against Botrytis cinerea by Rhizhosphere Biological Control Agent Bacillus velezensis XT1. Microorganisms 2020, 8, 992. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2008, 321, 341–361. [Google Scholar] [CrossRef]
- Rudrappa, T.; Czymmek, K.J.; Paré, P.W.; Bais, H.P. Root-Secreted Malic Acid Recruits Beneficial Soil Bacteria. Plant Physiol. 2008, 148, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Brettell, L.E.; Singh, B. Linking the Phyllosphere Microbiome to Plant Health. Trends Plant Sci. 2020, 25, 841–844. [Google Scholar] [CrossRef]
- Andrews, J.H.; Harris, R.F. The Ecology and Biogeography of Microorganisms on Plant Surfaces. Annu. Rev. Phytopathol. 2003, 38, 145–180. [Google Scholar] [CrossRef]
- Legein, M.; Smets, W.; Vandenheuvel, D.; Eilers, T.; Muyshondt, B.; Prinsen, E.; Samson, R.; Lebeer, S. Modes of Action of Microbial Biocontrol in the Phyllosphere. Front. Microbiol. 2020, 11, 1619. [Google Scholar] [CrossRef]
- Bashir, I.; War, A.F.; Rafiq, I.; Reshi, Z.A.; Rashid, I.; Shouche, Y.S. Phyllosphere microbiome: Diversity and functions. Microbiol. Res. 2022, 254, 126888. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Xiong, C.; Wei, Z.; Chen, Q.L.; Ma, B.; Zhou, S.Y.; Tan, J.; Zhang, L.M.; Cui, H.L.; Duan, G.L. Impacts of global change on the phyllosphere microbiome. New Phytol. 2022, 234, 1977–1986. [Google Scholar] [CrossRef]
- Compant, S.; Van Der Heijden, M.G.A.; Sessitsch, A. Climate change effects on beneficial plant–microorganism interactions. FEMS Microbiol. Ecol. 2010, 73, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Wille, L.; Messmer, M.M.; Studer, B.; Hohmann, P. Insights to plant-microbe interactions provide opportunities to improve resistance breeding against root diseases in grain legumes. Plant. Cell Environ. 2019, 42, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.A.; Sloan, D.B. The Hologenome Concept: Helpful or Hollow? PLoS Biol. 2015, 13, e1002311. [Google Scholar] [CrossRef]
- Douglas, A.E.; Werren, J.H. Holes in the Hologenome: Why Host-Microbe Symbioses Are Not Holobionts. MBio 2016, 7, e02099-15. [Google Scholar] [CrossRef]
- Rosenberg, E.; Zilber-Rosenberg, I. The hologenome concept of evolution after 10 years. Microbiome 2018, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.V.; Bull, J.J.; Lau, J.A. Symbiosis and stress: How plant microbiomes affect host evolution. Philos. Trans. R. Soc. B 2020, 375, 20190590. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Grube, M.; Köberl, M. The plant microbiome explored: Implications for experimental botany. J. Exp. Bot. 2016, 67, 995. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.P.; Grillo, M.A.; Podowski, J.C.; Heath, K.D. Soil origin and plant genotype structure distinct microbiome compartments in the model legume Medicago truncatula. Microbiome 2020, 8, 20190590. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.T.; Zhang, L.; He, S.Y. Plant-Microbe Interactions Facing Environmental Challenge. Cell Host Microbe 2019, 26, 183–192. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef]
- Plett, J.M.; Martin, F.M. Know your enemy, embrace your friend: Using omics to understand how plants respond differently to pathogenic and mutualistic microorganisms. Plant J. 2018, 93, 729–746. [Google Scholar] [CrossRef] [PubMed]
- Rey, T.; Chatterjee, A.; Buttay, M.; Toulotte, J.; Schornack, S. Medicago truncatula symbiosis mutants affected in the interaction with a biotrophic root pathogen. New Phytol. 2015, 206, 497–500. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Q.; Jiang, X.; Li, R.; Dhar, N. Cotton CC-NBS-LRR Gene GbCNL130 Confers Resistance to Verticillium Wilt Across Different Species. Front. Plant Sci. 2021, 12, 1904. [Google Scholar] [CrossRef]
- Nobori, T.; Velásquez, A.C.; Wu, J.; Kvitko, B.H.; Kremer, J.M.; Wang, Y.; He, S.Y.; Tsuda, K. Transcriptome landscape of a bacterial pathogen under plant immunity. Proc. Natl. Acad. Sci. USA 2018, 115, E3055–E3064. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, N.R.; Parys, K.; Lee, H.S.; Conway, J.M.; Kim, N.H.; Edelbacher, N.; Mucyn, T.S.; Madalinski, M.; Law, T.F.; Jones, C.D.; et al. A complex immune response to flagellin epitope variation in commensal communities. Cell Host Microbe 2021, 29, 635–649.e9. [Google Scholar] [CrossRef] [PubMed]
- Rosier, A.; Medeiros, F.H.V.; Bais, H.P. Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil 2018, 428, 35–55. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef]
- Ramachandran, V.K.; East, A.K.; Karunakaran, R.; Downie, J.A.; Poole, P.S. Adaptation of Rhizobium leguminosarum to pea, alfalfa and sugar beet rhizospheres investigated by comparative transcriptomics. Genome Biol. 2011, 12, R106. [Google Scholar] [CrossRef] [PubMed]
- Chagas, F.O.; Pessotti, R.D.C.; Caraballo-Rodríguez, A.M.; Pupo, M.T. Chemical signaling involved in plant–microbe interactions. Chem. Soc. Rev. 2018, 47, 1652–1704. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Godínez, L.J.; Fernandez-Valverde, S.L.; Martinez Romero, J.C.; Martínez-Romero, E. Metatranscriptomics and nitrogen fixation from the rhizoplane of maize plantlets inoculated with a group of PGPRs. Syst. Appl. Microbiol. 2019, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Schenk, P.M.; Carvalhais, L.C.; Kazan, K. Unraveling plant–microbe interactions: Can multi-species transcriptomics help? Trends Biotechnol. 2012, 30, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lardi, M.; Pessi, G. Functional Genomics Approaches to Studying Symbioses between Legumes and Nitrogen-Fixing Rhizobia. High-Throughput 2018, 7, R106. [Google Scholar] [CrossRef] [PubMed]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2019, 39, 3–17. [Google Scholar] [CrossRef]
- Xie, S.; Wu, H.; Chen, L.; Zang, H.; Xie, Y.; Gao, X. Transcriptome profiling of Bacillus subtilis OKB105 in response to rice seedlings. BMC Microbiol. 2015, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, D.; Wang, D.; Miao, Y.; Shao, J.; Zhou, X.; Xu, Z.; Li, Q.; Feng, H.; Li, S.; et al. Whole transcriptomic analysis of the plant-beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 during enhanced biofilm formation regulated by maize root exudates. BMC Genom. 2015, 16, 685. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, R.; Ramachandran, V.K.; Seaman, J.C.; East, A.K.; Mouhsine, B.; Mauchline, T.H.; Prell, J.; Skeffington, A.; Poole, P.S. Transcriptomic analysis of Rhizobium leguminosarum biovar viciae in symbiosis with host plants Pisum sativum and Vicia cracca. J. Bacteriol. 2009, 191, 4002–4014. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Noyola, J.L.; Rosenblueth, M.; Santiago-Martínez, M.G.; Martínez-Romero, E. Transcriptomic Responses of Rhizobium phaseoli to Root Exudates Reflect Its Capacity to Colonize Maize and Common Bean in an Intercropping System. Front. Microbiol. 2021, 12, 3087. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Sun, H.; Han, X.; Peng, Y.; Liu, J.; Liu, K.; Ding, Y.; Wang, C.; Du, B. Transcriptome Profiles Reveal the Growth-Promoting Mechanisms of Paenibacillus polymyxa YC0136 on Tobacco (Nicotiana tabacum L.). Front. Microbiol. 2020, 11, 2384. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef]
- Kandel, S.L.; Joubert, P.M.; Doty, S.L. Bacterial Endophyte Colonization and Distribution within Plants. Microorganisms 2017, 5, 77. [Google Scholar] [CrossRef]
- Green, E.R.; Mecsas, J. Bacterial Secretion Systems: An Overview. Microbiol. Spectr. 2016, 4, 213–239. [Google Scholar] [CrossRef]
- Jiménez-Guerrero, I.; Acosta-Jurado, S.; del Cerro, P.; Navarro-Gómez, P.; López-Baena, F.J.; Ollero, F.J.; Vinardell, J.M.; Pérez-Montaño, F. Transcriptomic Studies of the Effect of nod Gene-Inducing Molecules in Rhizobia: Different Weapons, One Purpose. Genes 2017, 9, 1. [Google Scholar] [CrossRef]
- Salinero-Lanzarote, A.; Pacheco-Moreno, A.; Domingo-Serrano, L.; Durán, D.; Ormeño-Orrillo, E.; Martínez-Romero, E.; Albareda, M.; Palacios, J.M.; Rey, L. The Type VI secretion system of Rhizobium etli Mim1 has a positive effect in symbiosis. FEMS Microbiol. Ecol. 2019, 95, fiz054. [Google Scholar] [CrossRef]
- Shidore, T.; Dinse, T.; Öhrlein, J.; Becker, A.; Reinhold-Hurek, B. Transcriptomic analysis of responses to exudates reveal genes required for rhizosphere competence of the endophyte Azoarcus sp. strain BH72. Environ. Microbiol. 2012, 14, 2775–2787. [Google Scholar] [CrossRef]
- López-Guerrero, M.G.; Ormeño-Orrillo, E.; Acosta, J.L.; Mendoza-Vargas, A.; Rogel, M.A.; Ramírez, M.A.; Rosenblueth, M.; Martínez-Romero, J.; Martínez-Romero, E. Rhizobial extrachromosomal replicon variability, stability and expression in natural niches. Plasmid 2012, 68, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Terra, L.A.; de Soares, C.P.; Meneses, C.H.S.G.; Tadra Sfeir, M.Z.; de Souza, E.M.; Silveira, V.; Vidal, M.S.; Baldani, J.I.; Schwab, S. Transcriptome and proteome profiles of the diazotroph Nitrospirillum amazonense strain CBAmC in response to the sugarcane apoplast fluid. Plant Soil 2020, 451, 145–168. [Google Scholar] [CrossRef]
- Cadot, S.; Guan, H.; Bigalke, M.; Walser, J.C.; Jander, G.; Erb, M.; van der Heijden, M.G.A.; Schlaeppi, K. Specific and conserved patterns of microbiota-structuring by maize benzoxazinoids in the field. Microbiome 2021, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Mavrodi, O.V.; McWilliams, J.R.; Peter, J.O.; Berim, A.; Hassan, K.A.; Elbourne, L.D.H.; LeTourneau, M.K.; Gang, D.R.; Paulsen, I.T.; Weller, D.M.; et al. Root Exudates Alter the Expression of Diverse Metabolic, Transport, Regulatory, and Stress Response Genes in Rhizosphere Pseudomonas. Front. Microbiol. 2021, 12, 698. [Google Scholar] [CrossRef]
- Mark, G.L.; Dow, J.M.; Kiely, P.D.; Higgins, H.; Haynes, J.; Baysse, C.; Abbas, A.; Foley, T.; Franks, A.; Morrissey, J.; et al. Transcriptome profiling of bacterial responses to root exudates identifies genes involved in microbe-plant interactions. Proc. Natl. Acad. Sci. USA 2005, 102, 17454. [Google Scholar] [CrossRef] [PubMed]
- González-Pasayo, R.; Martínez-Romero, E. Multiresistance genes of Rhizobium etli CFN42. Mol. Plant. Microbe Interact. 2000, 13, 572–577. [Google Scholar] [CrossRef]
- Vargas, P.; Felipe, A.; Michán, C.; Gallegos, M.T. Induction of Pseudomonas syringae pv. tomato DC3000 MexAB-OprM multidrug efflux pump by flavonoids is mediated by the repressor PmeR. Mol. Plant. Microbe Interact. 2011, 24, 1207–1219. [Google Scholar] [CrossRef]
- Fan, B.; Carvalhais, L.C.; Becker, A.; Fedoseyenko, D.; Von Wirén, N.; Borriss, R. Transcriptomic profiling of Bacillus amyloliquefaciens FZB42 in response to maize root exudates. BMC Microbiol. 2012, 12, 116. [Google Scholar] [CrossRef]
- Tu, P.W.; Chiu, J.S.; Lin, C.; Chien, C.C.; Hsieh, F.C.; Shih, M.C.; Yang, Y.L. Evaluation of the antifungal activities of Photorhabdus akhurstii and its secondary metabolites against phytopathogenic Colletotrichum gloeosporioides. J. Fungi 2022, 8, 403. [Google Scholar] [CrossRef] [PubMed]
- Báez-Astorga, P.A.; Cázares-Álvarez, J.E.; Cruz-Mendívil, A.; Quiroz-Figueroa, F.R.; Sánchez-Valle, V.I.; Maldonado-Mendoza, I.E. Molecular and biochemical characterisation of antagonistic mechanisms of the biocontrol agent Bacillus cereus B25 inhibiting the growth of the phytopathogen Fusarium verticillioides P03 during their direct interaction in vitro. Biocontrol Sci. Technol. 2022, 32, 1074–1094. [Google Scholar] [CrossRef]
- Park, G.; Nam, J.; Kim, J.; Song, J.; Kim, P.I.; Min, H.J.; Lee, C.W. Structure and Mechanism of Surfactin Peptide from Bacillus velezensis Antagonistic to Fungi Plant Pathogens. Bull. Korean Chem. Soc. 2019, 40, 704–709. [Google Scholar] [CrossRef]
- Maitra, S.; Brestic, M.; Bhadra, P.; Shankar, T.; Praharaj, S.; Palai, J.B.; Shah, M.M.R.; Barek, V.; Ondrisik, P.; Skalický, M.; et al. Bioinoculants-Natural Biological Resources for Sustainable Plant Production. Microorganisms 2021, 10, 51. [Google Scholar] [CrossRef]
- Gómez-Godínez, L.J.; Martínez-Romero, E.; Banuelos, J.; Arteaga-Garibay, R.I. Tools and challenges to exploit microbial communities in agriculture. Curr. Res. Microb. Sci. 2021, 2, 100062. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.A.; Sundelin, T.; Nielsen, J.T.; Erbs, G. MAMP (microbe-associated molecular pattern) triggered immunity in plants. Front. Plant Sci. 2013, 4, 139. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Loo, E.P.; Yasuda, S. Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 2018, 93, 592–613. [Google Scholar] [CrossRef]
- Tonelli, M.L.; Figueredo, M.S.; Rodríguez, J.; Fabra, A.; Ibañez, F. Induced systemic resistance—Like responses elicited by rhizobia. Plant Soil 2020, 448, 1–14. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Höfte, M. Chapter 6 Rhizobacteria-Induced Systemic Resistance. Adv. Bot. Res. 2009, 51, 223–281. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.M.; Zhang, S. Induced Systemic Resistance and Promotion of Plant Growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef]
- Takishita, Y.; Charron, J.B.; Smith, D.L. Biocontrol Rhizobacterium Pseudomonas sp. 23S Induces Systemic Resistance in Tomato (Solanum lycopersicum L.) Against Bacterial Canker Clavibacter michiganensis subsp. michiganensis. Front. Microbiol. 2018, 9, 2119. [Google Scholar] [CrossRef]
- Raacke, I.C.; Von Rad, U.; Mueller, M.J.; Berger, S. Yeast Increases Resistance in Arabidopsis Against Pseudomonas syringae and Botrytis cinerea by Salicylic Acid-Dependent as Well as -Independent Mechanisms. Mol. Plant Microbe Interact. 2007, 19, 1138–1146. [Google Scholar] [CrossRef]
- Dreischhoff, S.; Das, I.S.; Jakobi, M.; Kasper, K.; Polle, A. Local Responses and Systemic Induced Resistance Mediated by Ectomycorrhizal Fungi. Front. Plant Sci. 2020, 11, 1908. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Bodenhausen, N.; Gruissem, W.; Vorholt, J.A. The Arabidopsis leaf transcriptome reveals distinct but also overlapping responses to colonization by phyllosphere commensals and pathogen infection with impact on plant health. New Phytol. 2016, 212, 192–207. [Google Scholar] [CrossRef]
- Feng, D.X.; Tasset, C.; Hanemian, M.; Barlet, X.; Hu, J.; Trémousaygue, D.; Deslandes, L.; Marco, Y. Biological control of bacterial wilt in Arabidopsis thaliana involves abscissic acid signalling. New Phytol. 2012, 194, 1035–1045. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd-Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; de Carvalho, T.L.G.; Ballesteros, H.G.F.; Bellieny-Rabelo, D.; Rojas, C.A.; Venancio, T.M.; Ferreira, P.C.G.; Hemerly, A.S. Genome-wide transcriptome profiling provides insights into the responses of maize (Zea mays L.) to diazotrophic bacteria. Plant Soil 2020, 451, 121–143. [Google Scholar] [CrossRef]
- Jatan, R.; Tiwari, S.; Asif, M.H.; Lata, C. Genome-wide profiling reveals extensive alterations in Pseudomonas putida-mediated miRNAs expression during drought stress in chickpea (Cicer arietinum L.). Environ. Exp. Bot. 2019, 157, 217–227. [Google Scholar] [CrossRef]
- Halim, V.A.; Vess, A.; Scheel, D.; Rosahl, S. The role of salicylic acid and jasmonic acid in pathogen defence. Plant Biol. 2006, 8, 307–313. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, J.; Wu, J.; Wang, K.; Wu, S.; Liu, H.; Jiang, M. Transcriptome analysis of the growth-promoting effect of volatile organic compounds produced by Microbacterium aurantiacum GX14001 on tobacco (Nicotiana benthamiana). BMC Plant Biol. 2022, 22, 4927–4932. [Google Scholar] [CrossRef]
- Ryu, C.M.; Faragt, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef]
- Yasmin, H.; Rashid, U.; Hassan, M.N.; Nosheen, A.; Naz, R.; Ilyas, N.; Sajjad, M.; Azmat, A.; Alyemeni, M.N. Volatile organic compounds produced by Pseudomonas pseudoalcaligenes alleviated drought stress by modulating defense system in maize (Zea mays L.). Physiol. Plant. 2021, 172, 896–911. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.J.; López-Lara, I.M.; Geiger, O.; Romero-Puertas, M.C.; van Dillewijn, P. Rhizobial Volatiles: Potential New Players in the Complex Interkingdom Signaling With Legumes. Front. Plant Sci. 2021, 12, 1219. [Google Scholar] [CrossRef] [PubMed]

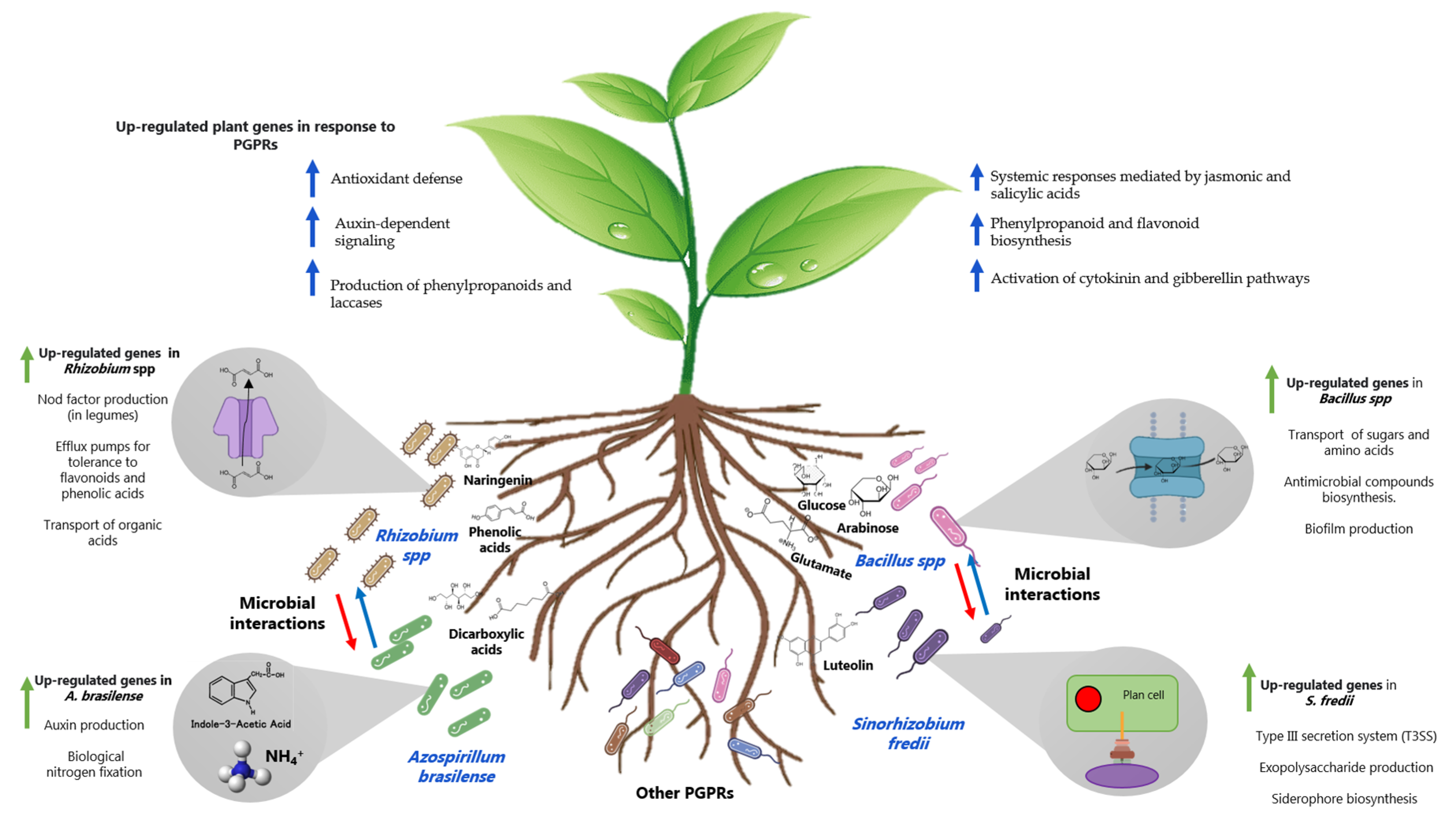
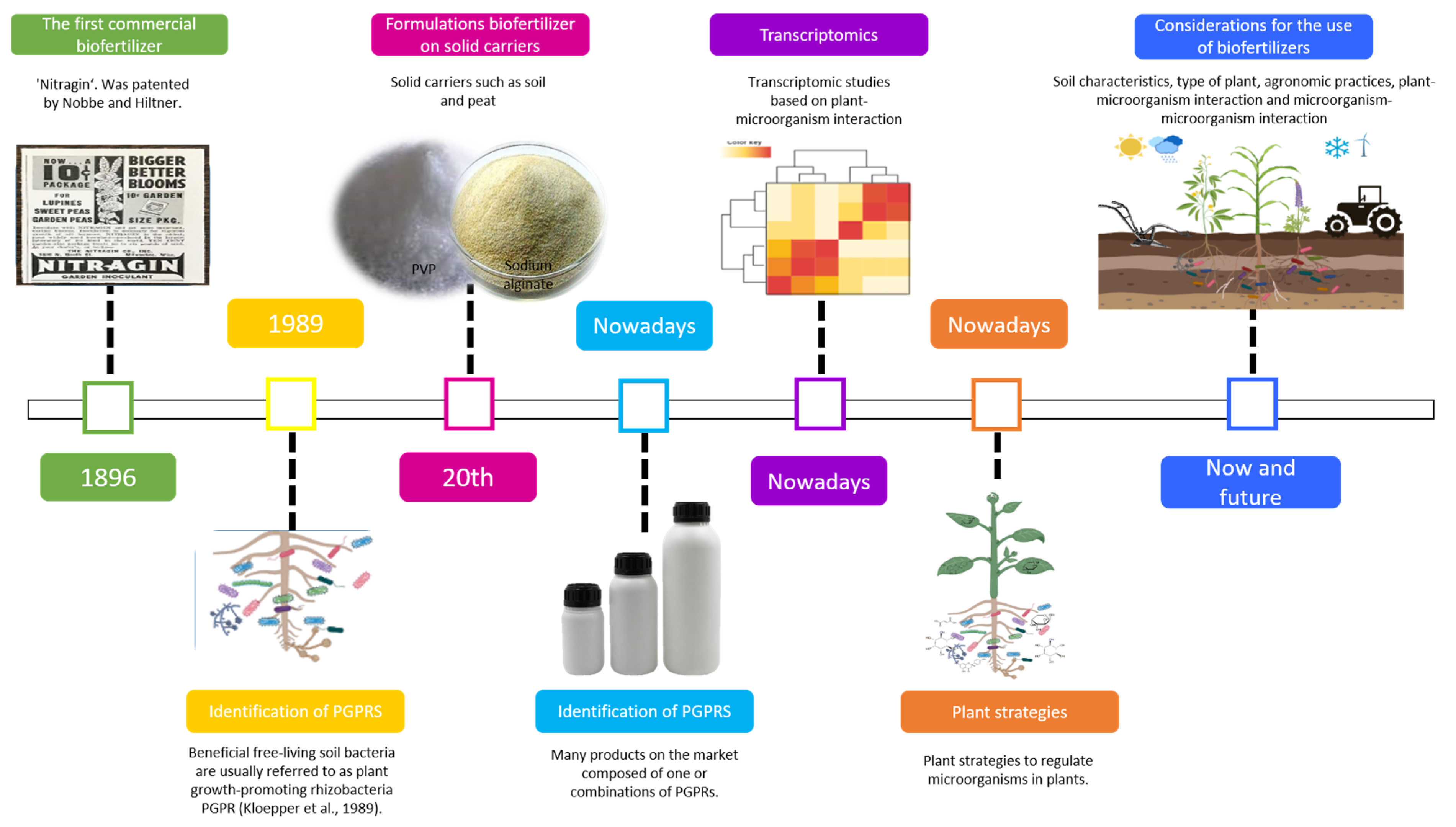
| Bacterial Strains | Crop | Evaluation Conditions | Highlights | Reference |
|---|---|---|---|---|
| Enterobacter hormaechei, Rhizobium spp., Pseudomonas fluorescence, and AAULE51 (undetermined) | Pepper | Greenhouse using inoculated seeds. | Plants produced from inoculated seeds exhibited higher shoot and root lengths in addition to showing resistance to drought stress. | Admassie et al., 2022 [15] |
| Bacillus subtilis (MW644678, MW644686, MW644650, MW644649, MH845220, MZ488941, MZ488846), Bacillus amyloliquefaciens MW644651, Bacillus safensis MK212368, and Bacillus halotolerans MW644679. | Sugar beet | Under greenhouse conditions, using sugar beet seeds treated with each bacterium. | Antifungal activity against Sclerotium rolfsii Sacc and a reduction in the severity and incidence of root rot disease. Furthermore, increases in length of shoots and roots and plant fresh and dry weight were recorded. | Farhaoui et al., 2022 [16] |
| Streptomyces corchorusii TKR8, Streptomyces corchorusii JAS2 and Streptomyces misionensis TBS5 | Rice | Greenhouse conditions using inoculated seeds. | Streptomyces-treated plants exhibited improvement in rice plants’ growth and grain yield. Additionally, a reduction in the disease severity of bacterial panicle blight (BPB) was observed in treated plants. | Ngalimat et al., 2022 [17] |
| Plant Growth-Promoting Bacteria (PGPB) consortia | Oilseed rape | Pot experiment using Cd naturally polluted soil. | PGPB-based consortia promoted plant growth, increased Cd uptake of oilseed rape, Cd phytoextraction, and Cd removal from soil. Further, consortia increased microbial carbon, urease and sucrase activities, and the relative abundance of selected bacteria genera in soil. | Wang et al., 2022 [18] |
| Enterobacter cloacae and Burkholderia cepacia | Garlic | In vitro growth. | Both growth and physiological attributes of garlic were increased when their meristems were inoculated with the PGPB. | Costa Júnior et al., 2020 [19] |
| Methylobacterium oryzae MNL7 and Paenibacillus polymyxa MaAL70 | Flooded paddy | 100 g of field soil deposited into cork sealed beakers and filled up to 1.5 cm of water. | Grain yield and grain nutrient quality were improved by the co-inoculation; meanwhile, methane emission was reduced in comparison with uninoculated treatments. | Rani et al., 2021 [20] |
| Paenibacillus taichungensis, Enterobacter sp., Rhizobium sp., Paenibacillus sp., Pseudomonas sp., and Paenibacillus pabuli | Walker’s cattleya orchid | In vitro inoculation of seedlings obtained by micropropagation of Cattleya walkeriana. | Potential effect on improved nutrient acquisition and overall growth. Antioxidant enzyme activity and non-enzymatic antioxidants were increased. | Andrade et al., 2023 [21] |
| Pseudomanas gessardi EU LWNA-25 and Erwinia rhapontici EU-B1SP1 | Amaranth | Controlled (pot) and natural (experimental farm) conditions. | Bacteria used as microbial consortia enhanced the growth of Amaranthus crops, expressed as the growth, grain, and yield. | Devi et al., 2022 [22] |
| Acinetobacter calcoaceticus P23, Pseudomonas fulva Ps6 and Chryseobacterium strains | Duckweed | Biomass production using a low nitrogen content and high salt food factory effluent (WW). | PGPRs promoted the growth of the crop under standard conditions but not when WW was used. | Khairina et al., 2020 [23] |
| Azotobacter chroococum and A. vinelandii | Eggplant | Root inoculation in plants exposed to different levels of drought stress. | Inoculated plants under drought stress exhibited higher dry matter production, leaf relative water, ions (K, Ca, and Mg), protein in roots, phenolic compounds, and proline. | Kiran et al., 2022 [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Godínez, L.J.; Aguirre-Noyola, J.L.; Martínez-Romero, E.; Arteaga-Garibay, R.I.; Ireta-Moreno, J.; Ruvalcaba-Gómez, J.M. A Look at Plant-Growth-Promoting Bacteria. Plants 2023, 12, 1668. https://doi.org/10.3390/plants12081668
Gómez-Godínez LJ, Aguirre-Noyola JL, Martínez-Romero E, Arteaga-Garibay RI, Ireta-Moreno J, Ruvalcaba-Gómez JM. A Look at Plant-Growth-Promoting Bacteria. Plants. 2023; 12(8):1668. https://doi.org/10.3390/plants12081668
Chicago/Turabian StyleGómez-Godínez, Lorena Jacqueline, José Luis Aguirre-Noyola, Esperanza Martínez-Romero, Ramón Ignacio Arteaga-Garibay, Javier Ireta-Moreno, and José Martín Ruvalcaba-Gómez. 2023. "A Look at Plant-Growth-Promoting Bacteria" Plants 12, no. 8: 1668. https://doi.org/10.3390/plants12081668






