Soil Amendments and Foliar Melatonin Reduced Pb Uptake, and Oxidative Stress, and Improved Spinach Quality in Pb-Contaminated Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Soil
2.2. Foliar Sprays and Soil Amendment
2.3. Experimental Setup
2.4. Experiment Termination
2.5. Soil Analysis
2.5.1. Lead Bioavailability in Soil
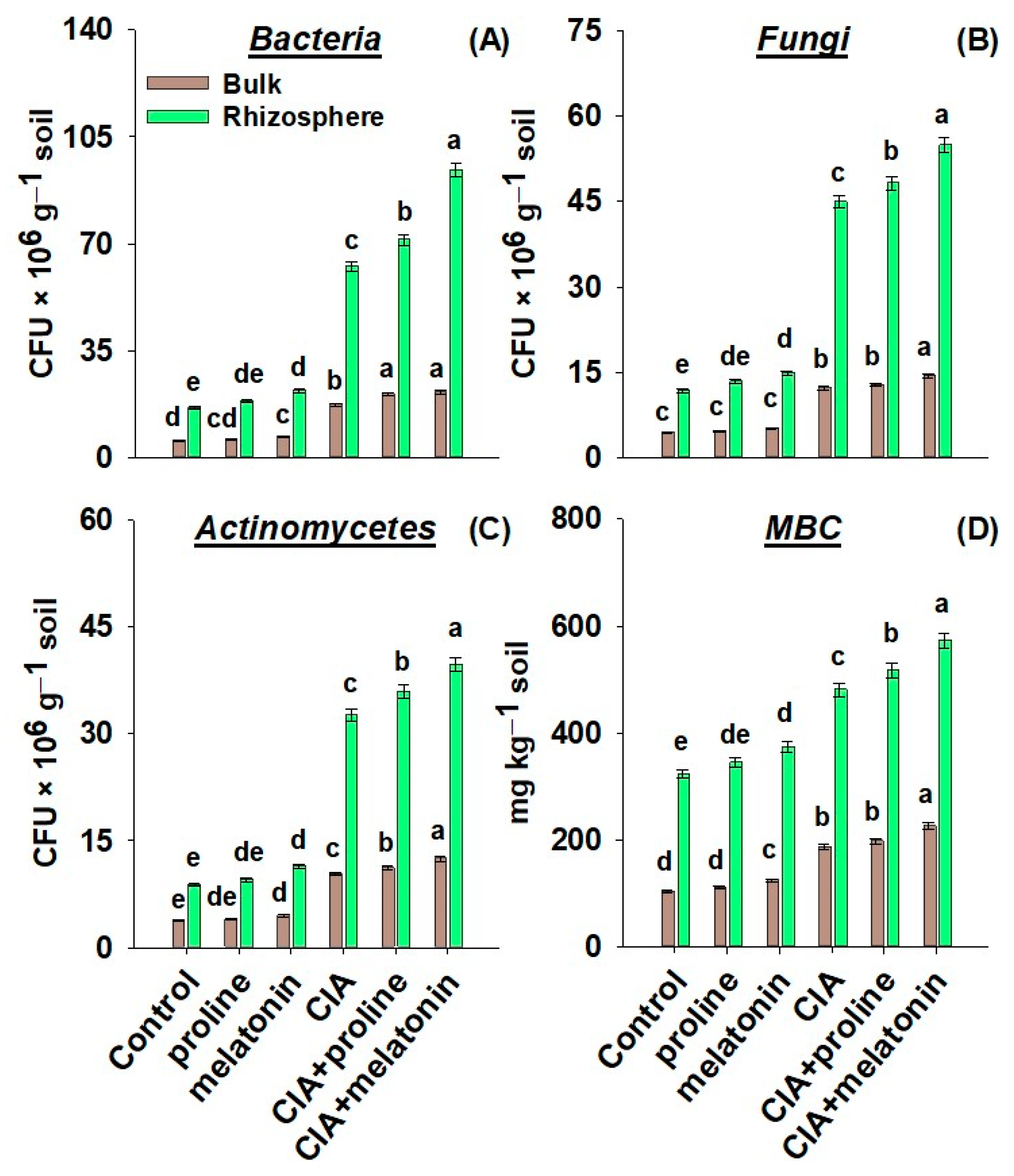
2.5.2. Microbial Numbers and Microbial Biomass Carbon
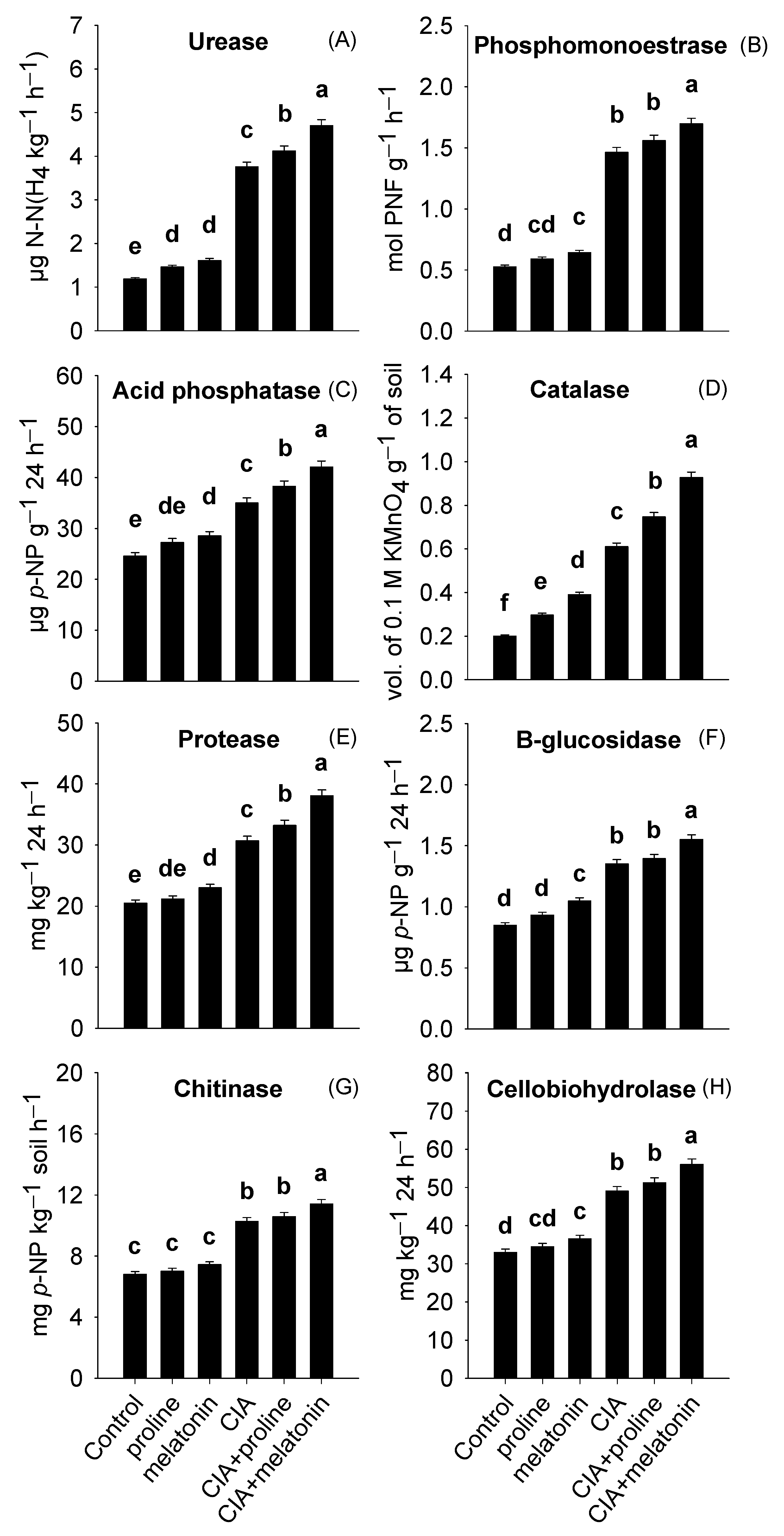
2.5.3. Soil Enzymatic Activities
2.6. Plant Analysis
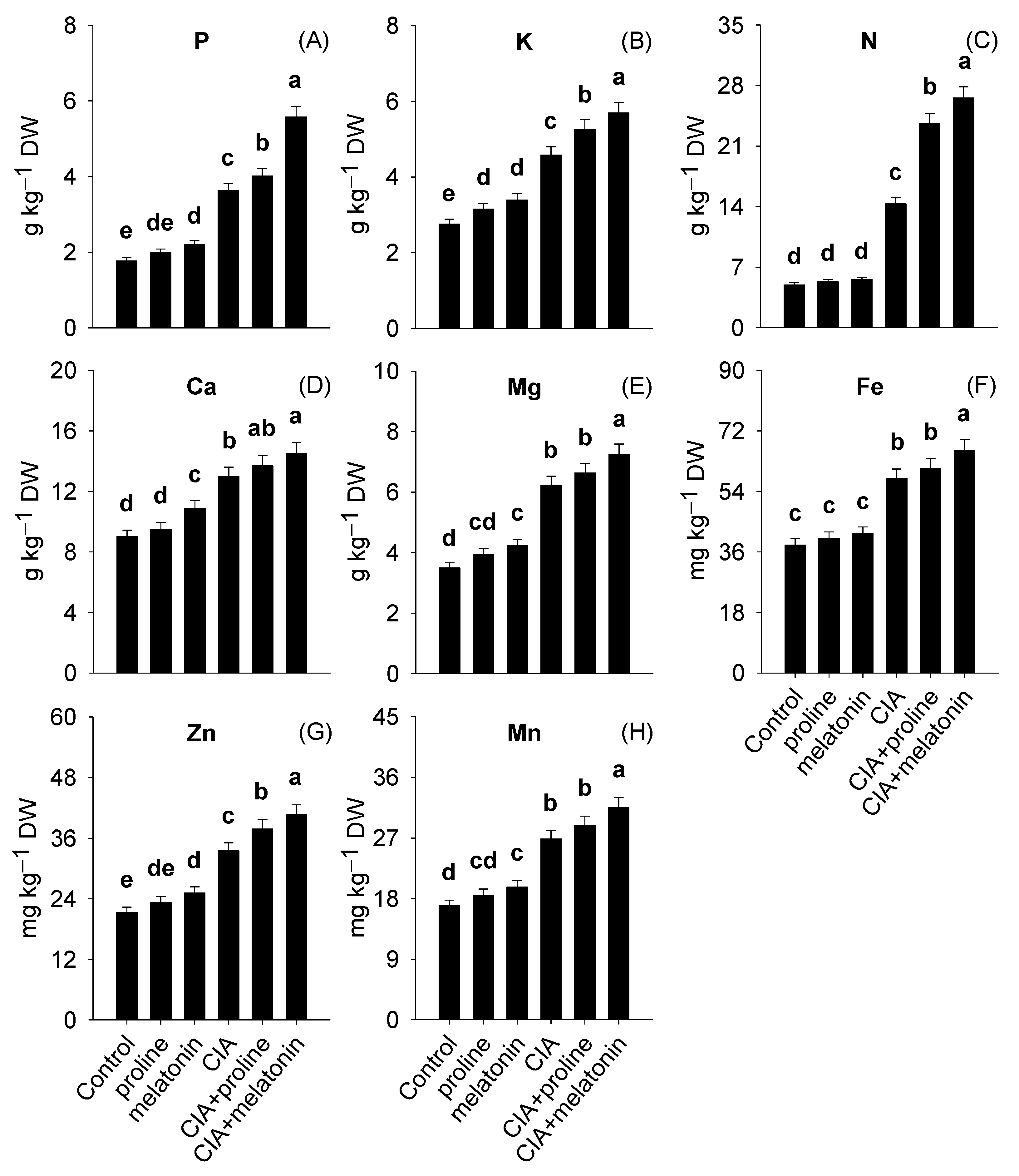
2.6.1. Lead Concentrations in Plants and Leaf Nutrients
2.6.2. Calculating BAF(ST), BAF(RT), and TF(ST)
2.6.3. Photosynthetic Pigments and Relative Water Content
2.6.4. Antioxidant Defense System
2.6.5. Leaf Biochemistry
2.7. Quality Assurance and Quality Control
2.8. Statistical Analysis
3. Results
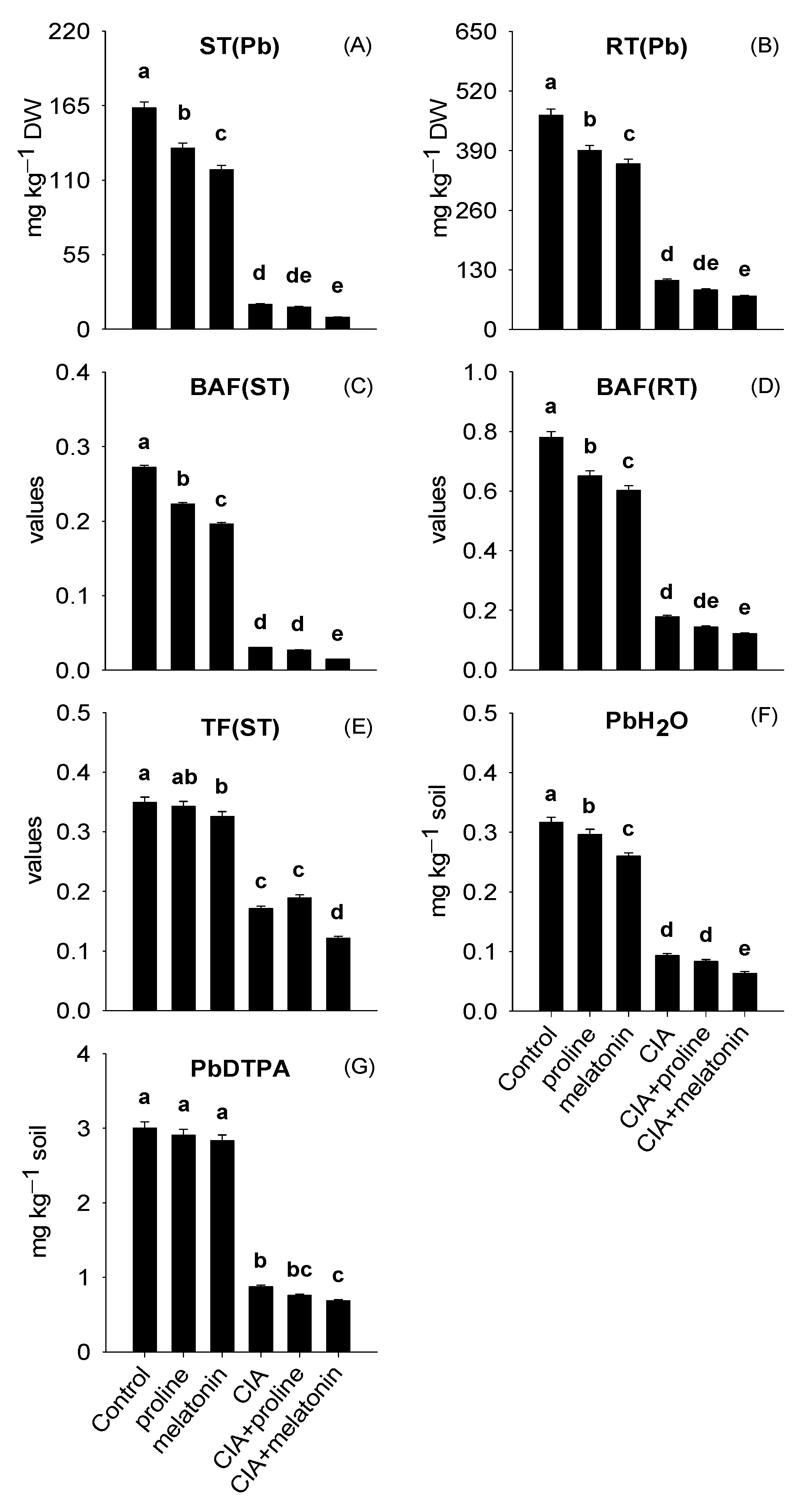
3.1. Lead Concentrations in Plant Parts and Plant-Accessible Pb
3.2. Growth, Yield, Photosynthetic Pigments, and Moisture Content of Spinach
3.3. Leaf Dietary Value
3.4. Stress Response and Antioxidant Defense System
3.5. Enzymes, Microbial Numbers, and MBC in the Soil
4. Discussion
4.1. Lead Concentrations in Plant Parts and Plant-Accessible Pb
4.2. Growth, Yield, Photosynthetic Pigments, and Moisture Content of Spinach
4.3. Leaf Dietary Value
4.4. Stress Response and Antioxidant Defense System
4.5. Enzymatic Activities, Microbial Numbers, and MBC in the Soil
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, D.K.; Chatterjee, S.; Walther, C. (Eds.) Lead in Plants and the Environment; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Verma, F.; Singh, S.; Dhaliwal, S.S.; Kumar, V.; Kumar, R.; Singh, J.; Parkash, C. Appraisal of pollution of potentially toxic elements in different soils collected around the industrial area. Heliyon 2021, 7, e08122. [Google Scholar] [CrossRef]
- Popa, C.L.; Bretcan, P.; Radulescu, C.; Carstea, E.M.; Tanislav, D.; Dontu, S.I.; Dulama, I.D. Spatial distribution of groundwater quality in connection with the surrounding land use and anthropogenic activity in rural areas. Acta Montan. Slovaca 2019, 24, 73–87. [Google Scholar]
- Huang, Y.N.; Dang, F.; Li, M.; Zhou, D.M.; Song, Y.; Wang, J.B. Environmental and human health risks from metal exposures nearby a Pb-Zn-Ag mine, China. Sci. Total Environ. 2020, 698, 134326. [Google Scholar] [CrossRef] [PubMed]
- Šimková, Z.; Očenášová, M.; Tudoš, D.; Róth, B. The political frame of the European Union for mining of non-energetic raw materials. Acta Montan. Slovaca 2019, 24, 35–43. [Google Scholar]
- Naeem, I.; Masood, N.; Turan, V.; Iqbal, M. Prospective usage of magnesium potassium phosphate cement combined with Bougainvillea alba derived biochar to reduce Pb bioavailability in soil and its uptake by Spinacia oleracea L. Ecotoxicol. Environ. Saf. 2021, 208, 111723. [Google Scholar] [CrossRef]
- Salavati, J.; Fallah, H.; Niknejad, Y.; Tari, D.B. Methyl jasmonate ameliorates lead toxicity in Oryza sativa by modulating chlorophyll metabolism, antioxidative capacity and metal translocation. Physiol. Mol. Biol. Plants 2021, 27, 1089–1104. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Kaur, N.; Pati, P.K. Phytohormones: Key players in the modulation of heavy metal stress tolerance in plants. Ecotoxicol. Environ. Saf. 2021, 223, 112578. [Google Scholar] [CrossRef]
- Lamhamdi, M.; El Galiou, O.; Bakrim, A.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Aarab, A.; Lafont, R. Effect of lead stress on mineral content and growth of wheat (Triticum aestivum) and spinach (Spinacia oleracea) seedlings. Saudi J. Biol. Sci. 2013, 20, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Turan, V. Potential of pistachio shell biochar and dicalcium phosphate combination to reduce Pb speciation in spinach, improved soil enzymatic activities, plant nutritional quality, and antioxidant defense system. Chemosphere 2020, 245, 125611. [Google Scholar] [CrossRef]
- WHO/FAO. Joint FAO/WHO Food Standard Programme Codex Alimentarius Commission 13th Session. In Proceedings of the 38th Session of the Codex Committee on Food Hygiene, Houston, TX, USA, 2007. [Google Scholar]
- Hussain, N.; Shafiq Ahmed, M.; Hussain, S.M. Potential health risks assessment cognate with selected heavy metals contents in some vegetables grown with four different irrigation sources near Lahore, Pakistan. Saudi J. Biol. Sci. 2022, 29, 1813–1824. [Google Scholar] [CrossRef]
- Boostani, H.R.; Najafi-Ghiri, M.; Safizadeh, M. Can Addition of Biochar and Zeolite to a Contaminated Calcareous Soil Mitigate the Pb-toxicity Effects on Spinach (Spinacia oleracea L.) Growth? Commun. Soil Sci. Plant Anal. 2021, 52, 136–148. [Google Scholar] [CrossRef]
- Maroušek, J.; Minofar, B.; Maroušková, A.; Strunecký, O.; Gavurová, B. Environmental and economic advantages of production and application of digestate biochar. Environ. Technol. Innov. 2023, 30, 103109. [Google Scholar] [CrossRef]
- Hao, N.; Cao, J.; Ye, J.; Zhang, C.; Li, C.; Bate, B. Content and morphology of lead remediated by activated carbon and biochar: A spectral induced polarization study. J. Hazard. Mater. 2021, 411, 124605. [Google Scholar] [CrossRef]
- Zulqurnain Haider, M.; Hussain, S.; Muhammad Adnan Ramzani, P.; Iqbal, M.; Iqbal, M.; Shahzad, T.; Fatima, M.; Ali Khan, S.; Khan, I.; Shahid, M.; et al. Bentonite and Biochar Mitigate Pb Toxicity in Pisum sativum by Reducing Plant Oxidative Stress and Pb Translocation. Plants 2019, 8, 571. [Google Scholar] [CrossRef]
- Namdjoyan, S.; Soorki, A.A.; Elyasi, N.; Kazemi, N.; Simaei, M. Melatonin alleviates lead-induced oxidative damage in safflower (Carthamus tinctorius L.) seedlings. Ecotoxicology 2020, 29, 108–118. [Google Scholar] [CrossRef]
- Siddique, A.; Kandpal, G.; Kumar, P. Proline accumulation and its defensive role under Diverse Stress condition in Plants: An Overview. J. Pure Appl. Microbiol. 2018, 12, 1655–1659. [Google Scholar] [CrossRef]
- Sofy, M.R.; Seleiman, M.F.; Alhammad, B.A.; Alharbi, B.M.; Mohamed, H.I. Minimizing adverse effects of pb on maize plants by combined treatment with jasmonic, salicylic acids and proline. Agronomy 2020, 10, 699. [Google Scholar] [CrossRef]
- Shahbaz, A.K.; Ramzani, P.M.A.; Saeed, R.; Turan, V.; Iqbal, M.; Lewińska, K.; Abbas, F.; Saqib, M.; Tauqeer, H.M.; Iqbal, M.; et al. Effects of biochar and zeolite soil amendments with foliar proline spray on nickel immobilization, nutritional quality and nickel concentrations in wheat. Ecotoxicol. Environ. Saf. 2019, 173, 182–191. [Google Scholar] [CrossRef]
- Okant, M.; Kaya, C. The role of endogenous nitric oxide in melatonin-improved tolerance to lead toxicity in maize plants. Environ. Sci. Pollut. Res. 2019, 26, 11864–11874. [Google Scholar] [CrossRef]
- Chen, M.; Ma, L.Q. Comparison of three aqua regia digestion methods for twenty Florida soils. Soil Sci. Soc. Am. J. 2001, 65, 491–499. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Helal, A.A.; El Aziz, N.A.; Gamal, R.; Shaker, N.O.; Helal, A.A. Influence of some organic ligands on the adsorption of lead by agricultural soil. Arab. J. Chem. 2019, 12, 2540–2547. [Google Scholar] [CrossRef]
- Saminathan, S.K.; Sarkar, D.; Andra, S.S.; Datta, R. Lead fractionation and bioaccessibility in contaminated soils with variable chemical properties. Chem. Speciat. Bioavailab. 2010, 22, 215–225. [Google Scholar] [CrossRef]
- Miretzky, P.; Fernandez-Cirelli, A. Phosphates for Pb immobilization in soils: A review. Environ. Chem. Lett. 2008, 6, 121–133. [Google Scholar] [CrossRef]
- Stavkova, J.; Maroušek, J. Novel sorbent shows promising financial results on P recovery from sludge water. Chemosphere 2021, 276, 130097. [Google Scholar] [CrossRef]
- Monsant, A.C.; Tang, C.; Baker, A.J.M. The effect of nitrogen form on rhizosphere soil pH and zinc phytoextraction by Thlaspi caerulescens. Chemosphere 2008, 73, 635–642. [Google Scholar] [CrossRef]
- Tagliavini, M.; Masia, A.; Quartieri, M. Bulk soil pH and rhizosphere pH of peach trees in calcareous and alkaline soils as affected by the form of nitrogen fertilizers. Plant Soil 1995, 176, 263–271. [Google Scholar] [CrossRef]
- Li, X.; Dong, S.; Yao, Y.; Shi, W.; Wu, M.; Xu, H. Inoculation of bacteria for the bioremediation of heavy metals contaminated soil by Agrocybe aegerita. RSC Adv. 2016, 6, 65816–65824. [Google Scholar] [CrossRef]
- Wu, B.; Cheng, G.; Jiao, K.; Shi, W.; Wang, C.; Xu, H. Mycoextraction by Clitocybe maxima combined with metal immobilization by biochar and activated carbon in an aged soil. Sci. Total Environ. 2016, 562, 732–739. [Google Scholar] [CrossRef]
- Voroney, R.P.; Brookes, P.C.; Beyaert, R.P. Soil microbial biomass C, N, P, and S. Soil Sampl. Methods Anal. 2008, 2, 637–652. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soil 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Xu, G.H.; Zheng, H.Y. Handbook of Analysis of Soil Microorganisms; Agriculture Press: Beijing, China, 1986. (In Chinese) [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Fu, S.; Méndez, A.; Gascó, G. Interactive effects of biochar and the earthworm Pontoscolex corethrurus on plant productivity and soil enzyme activities. J. Soils Sediments 2014, 14, 483–494. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Ladd, J.N.; Butler, J.H.A. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Šnajdr, J.; Valášková, V.; Merhautová, V.; Herinková, J.; Cajthaml, T.; Baldrian, P. Spatial variability of enzyme activities and microbial biomass in the upper layers of Quercus petraea forest soil. Soil Biol. Biochem. 2008, 40, 2068–2075. [Google Scholar] [CrossRef]
- Hayano, K. A method for determination of b-glucosidase activity in soil. Soil Sci. Plant Nutr. 1973, 19, 103–108. [Google Scholar] [CrossRef]
- Jones, J.R.J.; Case, V.W. Sampling, handling, and analyzing plant tissue samples. In Soil Testing and Plant Analysis; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1990; Volume 3, pp. 389–428. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Methods of Soil, Plants and Water Analysis; University of California Division of Agricultural Sciences: Berkeley, CA, USA, 1961; pp. 60–69. [Google Scholar]
- Buresh, R.J.; Austin, E.R.; Craswell, E.T. Analytical methods in 15 N research. Fertil. Res. 1982, 3, 37–62. [Google Scholar] [CrossRef]
- Munir, M.A.M.; Liu, G.; Yousaf, B.; Mian, M.M.; Ali, M.U.; Ahmed, R.; Cheema, A.I.; Naushad, M. Contrasting effects of biochar and hydrothermally treated coal gangue on leachability, bioavailability, speciation and accumulation of heavy metals by rapeseed in copper mine tailings. Ecotoxicol. Environ. Saf. 2020, 191, 110244. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Mullan, D.; Pietragalla, J. Leaf relative water content. In Physiological Breeding II: A Field Guide to Wheat Phenotyping; CIMMYT: Mexico City, Mexico, 2012; pp. 25–27. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- El-Shabrawi, H.; Kumar, B.; Kaul, T.; Reddy, M.K.; Singla-Pareek, S.L.; Sopory, S.K. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 2010, 245, 85–96. [Google Scholar] [CrossRef]
- Abi, H. Catalase in vitro. Method Enzymol. 1984, 105, 121–126. [Google Scholar]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Yang, H.; Wu, F.; Cheng, J. Reduced chilling injury in cucumber by nitric oxide and the antioxidant response. Food Chem. 2011, 127, 1237–1242. [Google Scholar] [CrossRef]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. In Plant Stress Tolerance; Humana Press: Totowa, NJ, USA, 2010; pp. 291–297. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Van Slyke, D.D.; Lemish, S. The gasometric determination of free amino acids in blood filtrates by the ninhydrin-carbon dioxide method. J. Biol. Chem. 1943, 150, 231–250. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official’s Analytical Chemists, 17th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2003. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official’s Analytical Chemists, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Steelm, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics; A Biometrical Approach, 3rd ed.; McGraw-Hill: Boston, MA, USA, 1997. [Google Scholar]
- Ramana, S.; Tripathi, A.K.; Bharati, K.; Singh, A.B.; Kumar, A.; Sahu, A.; Rajput, P.S.; Dey, P.; Saha, J.K.; Patra, A.K. Tolerance of cotton to elevated levels of Pb and its potential for phytoremediation. Environ. Sci. Pollut. Res. 2021, 28, 32299–32309. [Google Scholar] [CrossRef]
- Vannini, A.; Bianchi, E.; Avi, D.; Damaggio, N.; Di Lella, L.A.; Nannoni, F.; Protano, G.; Loppi, S. Biochar Amendment Reduces the Availability of Pb in the Soil and Its Uptake in Lettuce. Toxics 2021, 9, 268. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Guo, B.; Tsang, D.C.; Huang, L.; Ok, Y.S.; Mechtcherine, V. Red mud-enhanced magnesium phosphate cement for remediation of Pb and as contaminated soil. J. Hazard. Mater. 2020, 400, 123317. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.; Pignata, M.L.; Lascano, H.R.; Rodriguez, J.H. Assessment of lead tolerance on Glycine max (L.) Merr. At early growth stages. Environ. Sci. Pollut. Res. 2021, 28, 22843–22852. [Google Scholar] [CrossRef]
- Alamri, S.A.; Siddiqui, M.H.; Al-Khaishany, M.Y.; Nasir Khan, M.; Ali, H.M.; Alaraidh, I.A.; Alsahli, A.A.; Al-Rabiah, H.; Mateen, M. Ascorbic acid improves the tolerance of wheat plants to lead toxicity. J. Plant Interact. 2018, 13, 409–419. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.; Yu, H.; Zhan, J.; Ye, D.; Zheng, Z.; Zhang, X.; Wang, Y.; Li, T. Nitrilotriacetic acid enhanced lead accumulation in Athyrium wardii (Hook.) Makino by modifying rhizosphere characteristics. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Maroušek, J.; Maroušková, A.; Periakaruppan, R.; Gokul, G.M.; Anbukumaran, A.; Bohatá, A.; Kříž, P.; Bárta, J.; Černý, P.; Olšan, P. Silica nanoparticles from coir pith synthesized by acidic sol-gel method improve germination economics. Polymers 2022, 14, 266. [Google Scholar] [CrossRef]
- Liang, C.; Li, A.; Yu, H.; Li, W.; Liang, C.; Guo, S.; Zhang, R.; Chu, C. Melatonin regulates root architecture by modulating auxin response in rice. Front. Plant Sci. 2017, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Nabaei, M.; Amooaghaie, R. Melatonin and nitric oxide enhance cadmium tolerance and phytoremediation efficiency in Catharanthus roseus (L.) G. Don. Environ. Sci. Pollut. Res. 2020, 27, 6981–6994. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H. Rangeland application of biochar and rotational grazing interact to influence soil and plant nutrient dynamics. Geoderma 2022, 408, 115572. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Roy, R. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ. Saf. 2020, 197, 110593. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Su, T.; Huo, L.; Wei, H.; Jiang, Y.; Xu, L.; Ma, F. Unveiling the mechanism of melatonin impacts on maize seedling growth: Sugar metabolism as a case. J. Pineal Res. 2015, 59, 255–266. [Google Scholar] [CrossRef]
- Liang, D.; Shen, Y.; Ni, Z.; Wang, Q.; Lei, Z.; Xu, N.; Deng, Q.; Lin, L.; Wang, J.; Lv, X.; et al. Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front. Plant Sci. 2018, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, Y.; Zhang, J.; Gong, X.; Zhang, Z.; Sun, J.; Chen, X.; Wang, Y. Exogenous melatonin improves physiological characteristics and promotes growth of strawberry seedlings under cadmium stress. Hort. Plant J. 2021, 7, 13–22. [Google Scholar] [CrossRef]
- Navabpour, S.; Yamchi, A.; Bagherikia, S.; Kafi, H. Lead-induced oxidative stress and role of antioxidant defense in wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2020, 26, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Tousi, S.; Zoufan, P.; Ghahfarrokhie, A.R. Alleviation of cadmium-induced phytotoxicity and growth improvement by exogenous melatonin pretreatment in mallow (Malva parviflora) plants. Ecotoxicol. Environ. Saf. 2020, 206, 111403. [Google Scholar] [CrossRef]
- Cui, L.; Yan, J.; Yang, Y.; Li, L.; Quan, G.; Ding, C.; Chen, T.; Fu, Q.; Chang, A. Influence of biochar on microbial activities of heavy metals contaminated paddy fields. BioResources 2013, 8, 5536–5548. [Google Scholar] [CrossRef]
- Liu, H.; Xu, F.; Xie, Y.; Wang, C.; Zhang, A.; Li, L.; Xu, H. Effect of modified coconut shell biochar on availability of heavy metals and biochemical characteristics of soil in multiple heavy metals contaminated soil. Sci. Total Environ. 2018, 645, 702–709. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Razavi, B.S. Rhizosphere size and shape: Temporal dynamics and spatial stationarity. Soil Biol. Biochem. 2019, 135, 343–360. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; Abu El-Ezz, A.; Almoneafy, A.; Mahmoud, A.; Awad, M.; et al. Role of melatonin in plant tolerance to soil stressors: Salinity, pH and heavy metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef] [PubMed]
- Rostami, S.; Azhdarpoor, A.; Baghapour, M.A.; Dehghani, M.; Samaei, M.R.; Jaskulak, M.; Jafarpour, S.; Samare-Najaf, M. The effects of exogenous application of melatonin on the degradation of polycyclic aromatic hydrocarbons in the rhizosphere of Festuca. Environ. Pollut. 2021, 274, 116559. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cao, Y.; Li, Z.; Zhukova, A.; Yang, S.; Wang, J.; Tang, Z.; Cao, Y.; Zhang, Y.; Wang, D. Interactive effects of exogenous melatonin and Rhizophagus intraradices on saline-alkaline stress tolerance in Leymus chinensis. Mycorrhiza 2020, 30, 357–371. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Units | Values |
|---|---|---|
| Soil texture | - | Clay loam |
| Clay | g kg−1 | 301 ± 3.51 |
| Silt | g kg−1 | 286 ± 2.90 |
| Sand | g kg−1 | 413 ± 4.82 |
| pH (H2O) | − | 7.10 ± 0.02 |
| EC a | dSm−1 | 3.7 ± 0.03 |
| CEC b | cmolc kg−1 | 24.9 ± 0.52 |
| OM c | g kg−1 | 7.11 ± 0.04 |
| CaCO3 | g kg−1 | 27.8 ± 0.82 |
| DTPA–extractable Pb | mg kg−1 | 0.89 ± 0.07 |
| Total Pb | mg kg−1 | 21.24 ± 1.06 |
| Exchangeable K | mg kg−1 | 87.0 ± 2.97 |
| Available P | mg kg−1 | 91.0 ± 3.21 |
| Treatments | Acronyms | The Dose of CIA Added to the Soil | Concentrations of Foliar Spray | |
|---|---|---|---|---|
| (%) | PL (mM L−1) | ML (µM L−1) | ||
| Control | Control | - | - | - |
| Proline | Proline | - | 20 | - |
| Melatonin | Melatonin | - | - | 100 |
| Combo immobilizing amendment | CIA | 3 | - | - |
| Combo immobilizing amendment+proline | CIA+proline | 3 | 20 | - |
| Combo immobilizing amendment+melatonin | CIA+melatonin | 3 | - | 100 |
| Treatments | APX | SOD | CAT | AsA | DHAR | H2O2 | O2•− | MDA |
|---|---|---|---|---|---|---|---|---|
| (µmol min−1 Protein) | (U min−1 mg−1 Protein) | (µmol min−1 Protein) | (nmol g−1 FW) | (µmol min−1 Protein) | (nmol g−1 FW) | (nmol min−1 g−1 FW) | (nmol g−1 FW) | |
| Control | 0.39 ± 0.11 e | 42.9 ± 1.22 d | 25.7 ± 0.73 e | 559.3 ± 15.6 d | 26.5 ± 0.75 d | 66.9 ± 1.89 a | 39.6 ± 1.10 a | 60.3 ± 1.71 a |
| Proline | 0.46 ± 0.01 d | 50.5 ± 1.43 cd | 30.5 ± 0.87 de | 714.3 ± 20.0 c | 32.4 ± 0.90 c | 58.1 ± 1.63 b | 35.7 ± 0.99 b | 52.8 ± 1.48 b |
| Melatonin | 0.52 ± 0.01 d | 56.6 ± 1.59 c | 33.1 ± 0.93 d | 778.7 ± 21.8 c | 37.0 ± 1.04 c | 53.3 ± 1.48 c | 31.7 ± 0.87 c | 48.1 ± 1.36 c |
| CIA | 0.96 ± 0.03 c | 142.3 ± 4.00 b | 78.6 ± 2.21 c | 1297.3 ± 36.5 b | 69.4 ± 1.94 b | 27.8 ± 0.78 d | 17.1 ± 0.46 d | 22.0 ± 0.61 d |
| CIA+proline | 1.15 ± 0.03 b | 148.6 ± 4.18 ab | 85.6 ± 2.41 b | 1386.7 ± 38.9 ab | 74.1 ± 2.09 b | 25.2 ± 0.70 d | 15.7 ± 0.43 d | 18.7 ± 0.52 de |
| CIA+melatonin | 1.27 ± 0.03 a | 157.0 ± 4.41 a | 93.7 ± 2.64 a | 1474.3 ± 41.5 a | 81.9 ± 2.29 a | 21.2 ± 0.58 e | 12.7 ± 0.35 e | 15.9 ± 0.44 e |
| References of the protocols | [47] | [48] | [49] | [50,51] | [47] | [52] | [53] | [54] |
| Treatments | Plant Height (cm) | Shoots DW (g pot−1) | Roots DW (g pot−1) | CLR–a (mg g−1 FW) | CLR–b (mg g−1 FW) | RTW (%) |
|---|---|---|---|---|---|---|
| Control | 12.1 ± 0.35 e | 1.02 ± 0.03 e | 0.56 ± 0.01 e | 0.98 ± 0.03 e | 0.67 ± 0.02 d | 83.3 ± 1.19 b |
| Proline | 13.6 ± 0.38 de | 1.19 ± 0.03 d | 0.65 ± 0.02 d | 1.20 ± 0.03 d | 0.79 ± 0.02 c | 84.9 ± 1.19 b |
| Melatonin | 15.4 ± 0.44 d | 1.29 ± 0.03 d | 0.80 ± 0.02 c | 1.33 ± 0.04 d | 0.89 ± 0.03 c | 86.1 ± 1.22 b |
| CIA | 26.5 ± 0.75 c | 1.72 ± 0.05 c | 1.23 ± 0.03 b | 1.57 ± 0.04 c | 1.38 ± 0.04 b | 94.5 ± 1.33 a |
| CIA+proline | 28.6 ± 0.78 b | 1.85 ± 0.05 b | 1.31 ± 0.04 ab | 1.76 ± 0.05 b | 1.47 ± 0.04 b | 95.8 ± 1.37 a |
| CIA+melatonin | 31.2 ± 0.87 a | 2.02 ± 0.06 a | 1.39 ± 0.04 a | 1.92 ± 0.05 a | 1.59 ± 0.04 a | 96.4 ± 1.37 a |
| LSD0.05 value | 1.95 | 0.13 | 0.09 | 0.13 | 0.10 | 3.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, N.; Tanzeem-ul-Haq, H.S.; Gull-e-Faran; Turan, V.; Iqbal, M. Soil Amendments and Foliar Melatonin Reduced Pb Uptake, and Oxidative Stress, and Improved Spinach Quality in Pb-Contaminated Soil. Plants 2023, 12, 1829. https://doi.org/10.3390/plants12091829
Iqbal N, Tanzeem-ul-Haq HS, Gull-e-Faran, Turan V, Iqbal M. Soil Amendments and Foliar Melatonin Reduced Pb Uptake, and Oxidative Stress, and Improved Spinach Quality in Pb-Contaminated Soil. Plants. 2023; 12(9):1829. https://doi.org/10.3390/plants12091829
Chicago/Turabian StyleIqbal, Naeem, Hafiz Syed Tanzeem-ul-Haq, Gull-e-Faran, Veysel Turan, and Muhammad Iqbal. 2023. "Soil Amendments and Foliar Melatonin Reduced Pb Uptake, and Oxidative Stress, and Improved Spinach Quality in Pb-Contaminated Soil" Plants 12, no. 9: 1829. https://doi.org/10.3390/plants12091829







