Rosemary as a Potential Source of Natural Antioxidants and Anticancer Agents: A Molecular Docking Study
Abstract
:1. Introduction
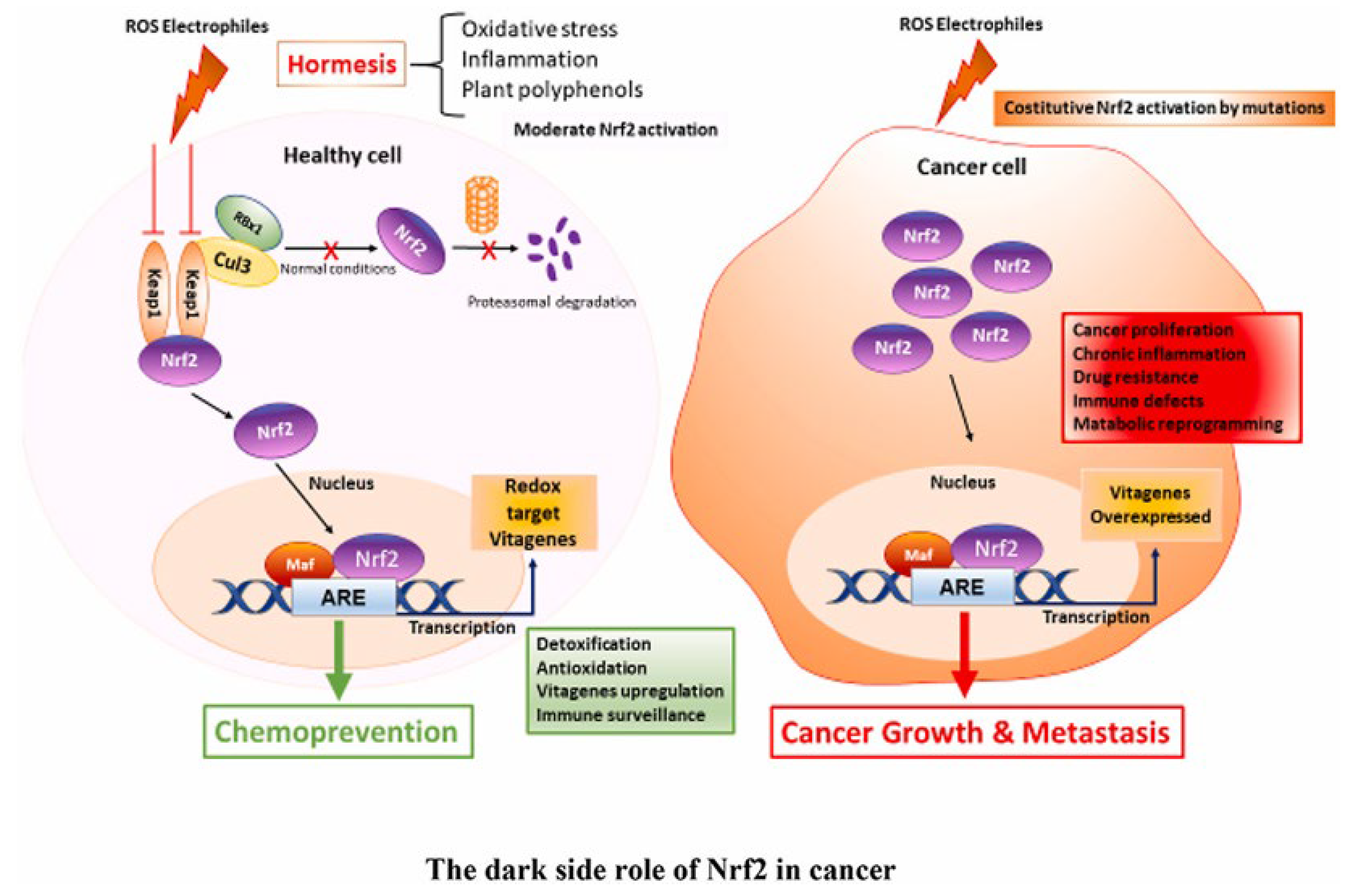
2. Potential Antioxidant and Anticancer Effects of Res
2.1. Polyphenols: Diverse Roles in Plants and Humans
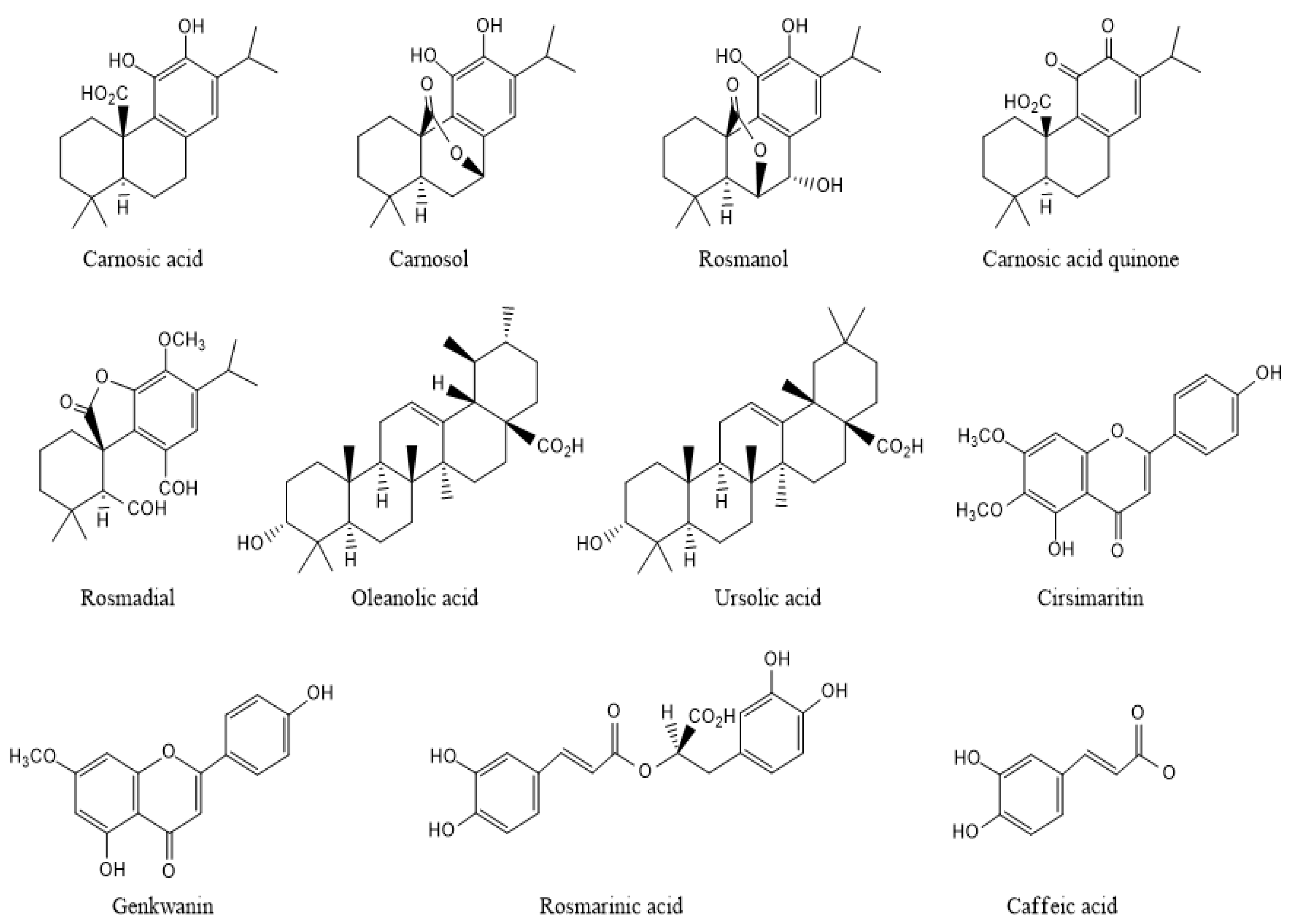
2.2. Phenolic Powerhouses in Rosemary: Carnosic and Rosmarinic Acids
3. Charting the Antioxidant Odyssey: Carnosic Acid and Rosmarinic Acid in the Fight against Different Type of Cancer
3.1. Oral and Ovarian Cancers
3.2. Kidney Cancer
3.3. Pancreatic Cancer
3.4. Colon Cancer
3.5. Prostate Cancer
4. Theoretical Study
4.1. Compounds Preparation
4.2. Sequence Alignment and Molecular Docking
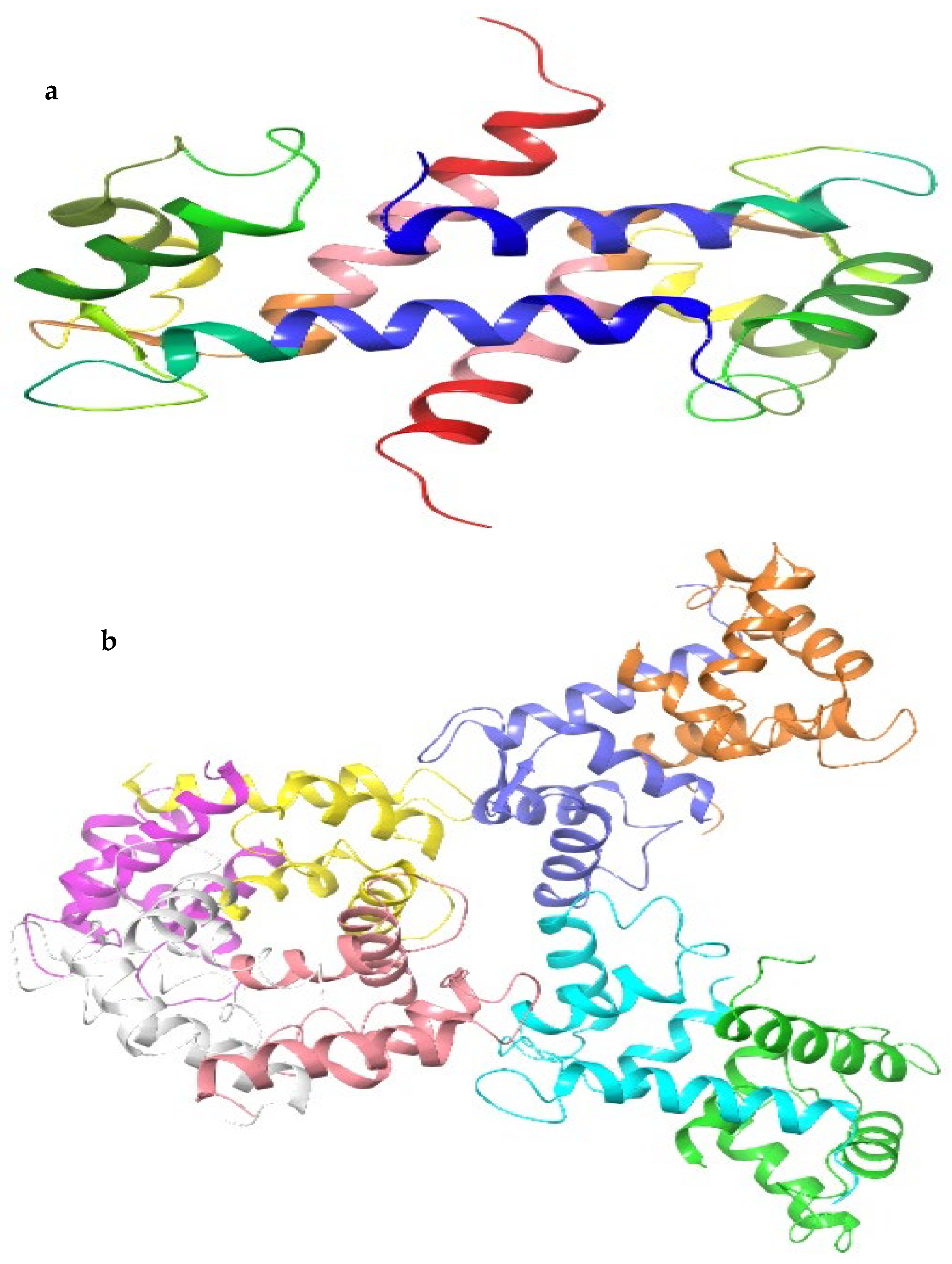
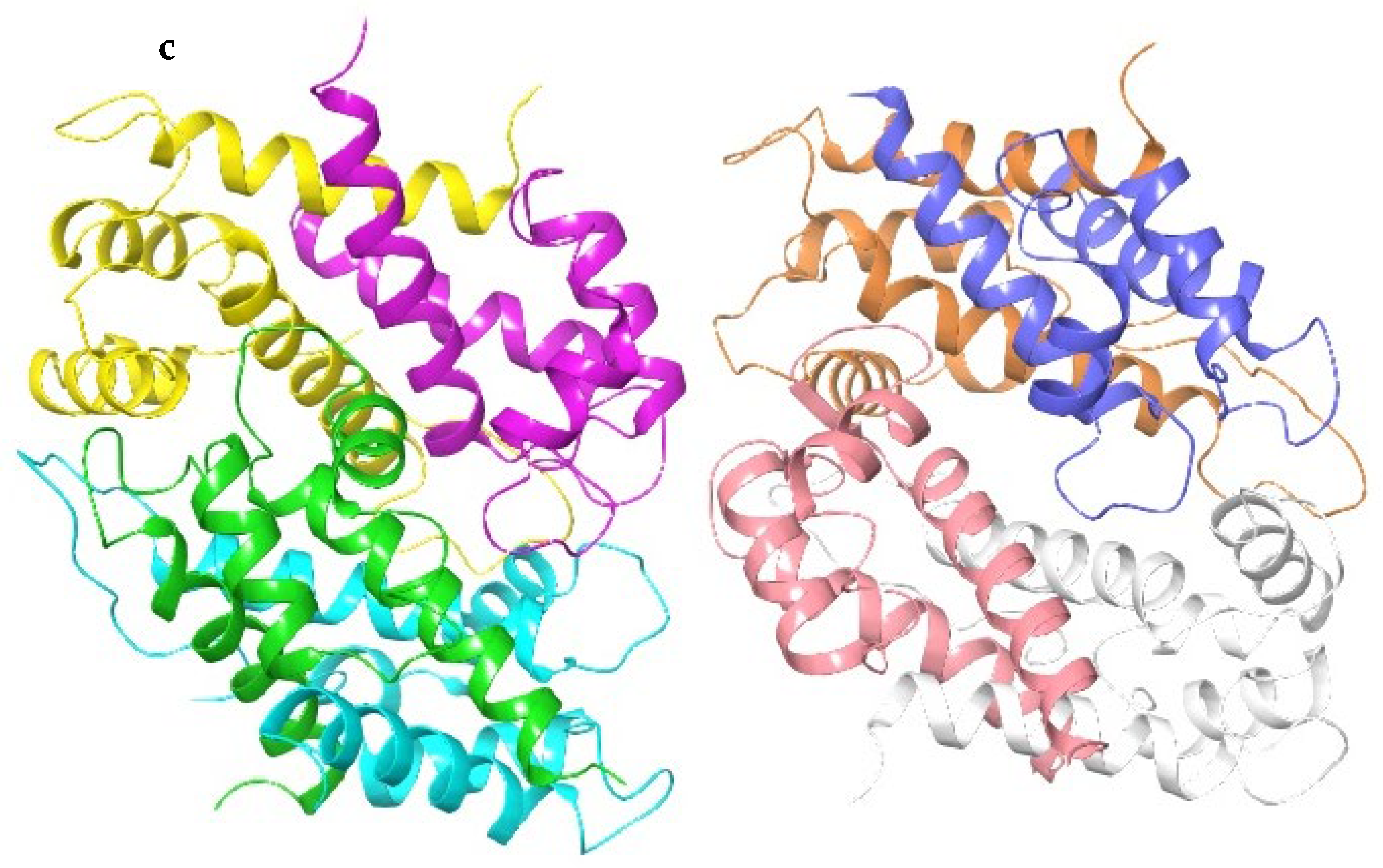
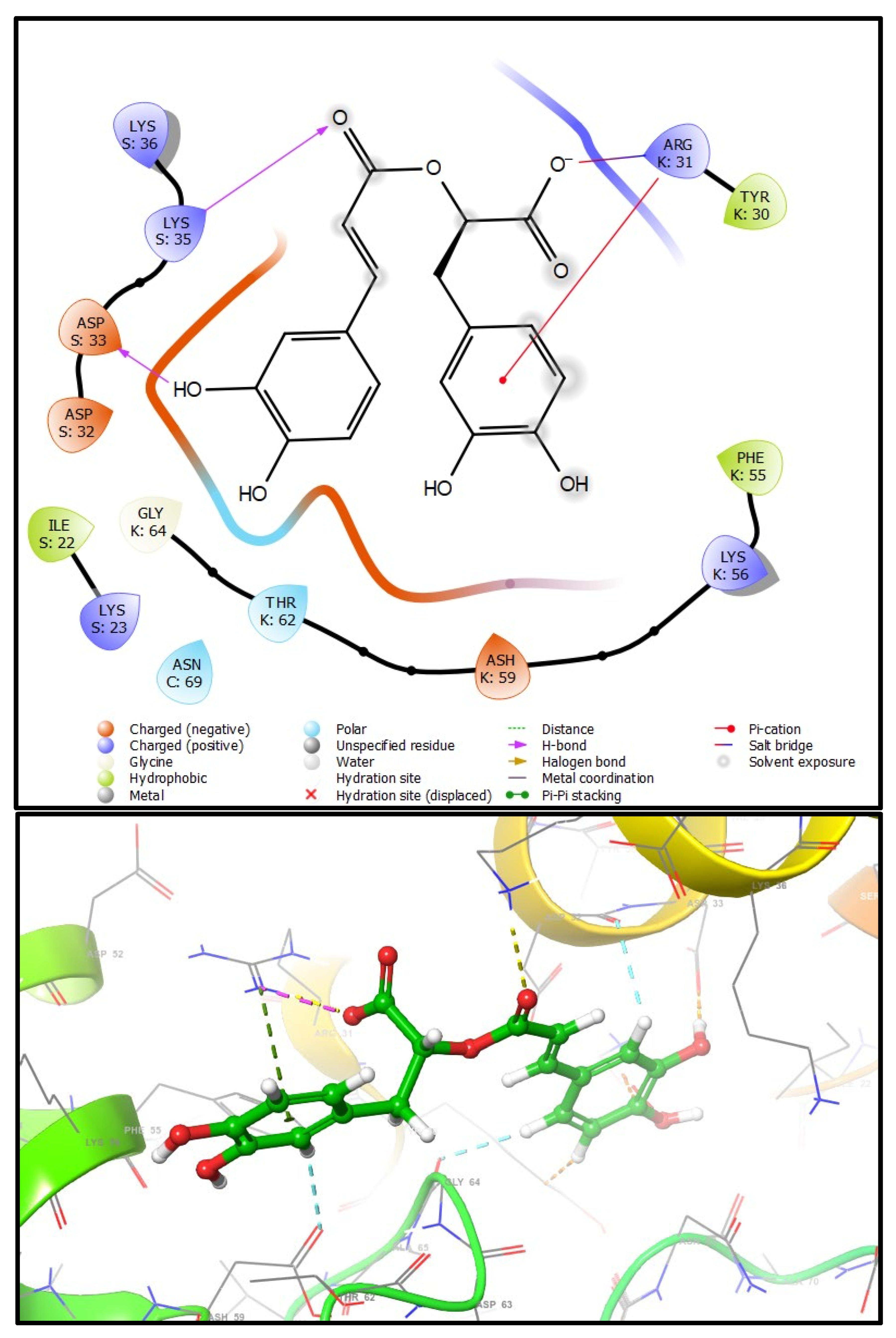
4.3. Docking Studies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, K.; Kumar, V.; Sharma, A.K.; Gupta, G.K. Isoxazoline Containing Natural Products as Anticancer Agents: A Review. Eur. J. Med. Chem. 2014, 77, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, M.V.N.L.; Ramanunny, A.K.; Babu, M.R.; Gulati, M.; Vishwas, S.; Singh, T.G.; Chellappan, D.K.; Adams, J.; Dua, K.; Singh, S.K. Journey of Rosmarinic Acid as Biomedicine to Nano-Biomedicine for Treating Cancer: Current Strategies and Future Perspectives. Pharmaceutics 2022, 14, 2401. [Google Scholar] [CrossRef]
- Ziani, I.; Bouakline, H.; Yahyaoui, M.I.; Belbachir, Y.; Fauconnier, M.L.; Asehraou, A.; Tahani, A.; Talhaoui, A.; El Bachiri, A. The Effect of Ethanol/Water Concentration on Phenolic Composition, Antioxidant, and Antimicrobial Activities of Rosmarinus Tournefortii de Noé Hydrodistillation Solid Residues. J. Food Meas. Charact. 2022, 17, 1602–1615. [Google Scholar] [CrossRef]
- Santana-Méridas, O.; Polissiou, M.; Izquierdo-Melero, M.E.; Astraka, K.; Tarantilis, P.A.; Herraiz-Peñalver, D.; Sánchez-Vioque, R. Polyphenol Composition, Antioxidant and Bioplaguicide Activities of the Solid Residue from Hydrodistillation of Rosmarinus officinalis L. Ind. Crops Prod. 2014, 59, 125–134. [Google Scholar] [CrossRef]
- Lesellier, E.; Lefebvre, T.; Destandau, E. Recent developments for analysis and the extraction of bioactive compounds from Rosmarinus officinalis and medicinal plants of the Lamiaceae family. Trends Anal. Chem. 2021, 135, 11658. [Google Scholar] [CrossRef]
- Chen, H.; Gu, Z.; Yang, L.; Yang, R.; Ji, Y.; Zeng, Q.; Xiao, F.; Huang, P. Optimization Extraction of Rosemary Essential Oils Using Hydrodistillation with Extraction Kinetics Analysis. Food Sci. Nutr. 2021, 9, 6069–6077. [Google Scholar] [CrossRef] [PubMed]
- Lešnik, S.; Furlan, V.; Bren, U. Rosemary (Rosmarinus officinalis L.): Extraction Techniques, Analytical Methods and Health-Promoting Biological Effects. Phytochem. Rev. 2021, 20, 1273–1328. [Google Scholar] [CrossRef]
- Bouakline, H.; Elkabous, M.; Ziani, I.; Karzazi, Y.; Tahani, A.; El Bachiri, A. Antioxidative Activity of Pistacia Lentiscus Leaf Extract Main Components: Experimental and Theoretical Study. Mater. Today Proc. 2023, 72, 3275–3279. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Vella, F.M.; Laratta, B. Rosmary Essential Oil Extraction and Residue Valorization by Means of Plyphenol Recovery. Biol. Life Sci. Forum 2023, 26, 8. [Google Scholar] [CrossRef]
- Macheix, J.J.; Fleuriet, A.; Jay-Allemand, C. Phenolic Compounds Plants: An Example of Secondary Metabolites of Economic Importance; Polytechnic and University Romandes Presses: Lausanne, Italie, 2005; p. 192. [Google Scholar]
- Anwar, S.; Shahwan, M.; Hasan, G.M.; Islam, A.; Hassan, M.I. Microtubule-Affinity Regulating Kinase 4: A Potential Drug Target for Cancer Therapy. Cell. Signal. 2022, 99, 110434. [Google Scholar] [CrossRef]
- Yar Khan, H.; Zubair, H.; Fahad Ullah, M.; Ahmad, A.; Mumtaz Hadi, S. A Prooxidant Mechanism for the Anticancer and Chemopreventive Properties of Plant Polyphenols. Curr. Drug Targets 2012, 13, 1738–1749. [Google Scholar] [CrossRef]
- Scuto, M.; Ontario, M.L.; Salinaro, A.T.; Caligiuri, I.; Rampulla, F.; Zimbone, V.; Modafferi, S.; Rizzolio, F.; Canzonieri, V.; Calabrese, E.J.; et al. Redox Modulation by Plant Polyphenols Targeting Vitagenes for Chemoprevention and Therapy: Relevance to Novel Anti-Cancer Interventions and Mini-Brain Organoid Technology. Free Radic. Biol. Med. 2022, 179, 59–75. [Google Scholar] [CrossRef]
- Yeddes, W.; Majdi, H.; Gadhoumi, H.; Affes, T.G.; Mohamed, S.N.; Aidi Wannes, W.; Saidani-Tounsi, M. Optimizing Ethanol Extraction of Rosemary Leaves and Their Biological Evaluations. J. Explor. Res. Pharmacol. 2022, 7, 85–94. [Google Scholar] [CrossRef]
- Richheimer, S.L.; Bernart, M.W.; King, G.A.; Kent, M.C.; Beiley, D.T. Antioxidant Activity of Lipid-Soluble Phenolic Diterpenes from Rosemary. J. Am. Oil Chem. Soc. 1996, 73, 507–514. [Google Scholar] [CrossRef]
- Birtić, S.; Dussort, P.; Pierre, F.-X.; Bily, A.C.; Roller, M. Carnosic Acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, L.; Zu, Y.; Chen, X.; Wang, F.; Liu, F. Oxidative Stability of Sunflower Oil Supplemented with Carnosic Acid Compared with Synthetic Antioxidants during Accelerated Storage. Food Chem. 2010, 118, 656–662. [Google Scholar] [CrossRef]
- Huang, S.-W.; Hopia, A.; Schwarz, K.; Frankel, E.N.; German, J.B. Antioxidant Activity of α-Tocopherol and Trolox in Different Lipid Substrates: Bulk Oils vs. Oil-in-Water Emulsions. J. Agric. Food Chem. 1996, 44, 444–452. [Google Scholar] [CrossRef]
- Fadili, K.; Amalich, S.; N’dedianhoua, S.K.; Bouachrine, M.; Mahjoubi, M.; El Hilali, F.; Zair, T. Polyphenols Content and Antioxidant Activity of Two Species from Moroccan High Atlas: Rosmarinus officinalis and Thymus satureioides. Int. J. Innov. Sci. Res. 2015, 4, 24–33. [Google Scholar]
- Borras Linares, I.; Arraez-Rman, D.; Herrero, M.; Ibanez, E.; Segura-Carretero, A.; Fernandez-Gutiérrez, A. Comparison of different extraction procedures for the comprehensive characterization of bioactve phenolic compounds in Rosmarinus officinalis by reversed-phase high-performance liquid chromatography with diode array detection coupled to elecrospray time-of-flight mass spectrometry. J. Chromatogr. A 2011, 1218, 7682–7690. [Google Scholar] [CrossRef] [PubMed]
- Almela, L.; Sanchez-Munoz, B.; Fernndez-Lopez, J.A.; Roca, M.J.; Rabe, V. Liquid chromatograpic-mass spectrometric analysis of phenolics and free radical scavenging activity of rosemary extract from defferent raw material. J. Chromatogh. A 2006, 1120, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Huang, J.; Zhao, D.; Du, B.; Wang, M. Protective Effect of Rosmarinic Acid and Carnosic Acid against Streptozotocin-Induced Oxidation, Glycation, Inflammation and Microbiota Imbalance in Diabetic Rats. Food Funct. 2018, 9, 851–860. [Google Scholar] [CrossRef]
- Ben Jemia, M.; Tundis, R.; Maggio, A.; Rosselli, S.; Senatore, F.; Menichini, F.; Bruno, M.; Kchouk, M.E.; Loizzo, M.R. NMR-Based Quantification of Rosmarinic and Carnosic Acids, GC–MS Profile and Bioactivity Relevant to Neurodegenerative Disorders of Rosmarinus officinalis L. Extracts. J. Funct. Foods 2013, 5, 1873–1882. [Google Scholar] [CrossRef]
- Lim, S.H.; Nam, K.H.; Kim, K.; Yi, S.A.; Lee, J.; Han, J.-W. Rosmarinic Acid Methyl Ester Regulates Ovarian Cancer Cell Migration and Reverses Cisplatin Resistance by Inhibiting the Expression of Forkhead Box M1. Pharmaceuticals 2020, 13, 302. [Google Scholar] [CrossRef]
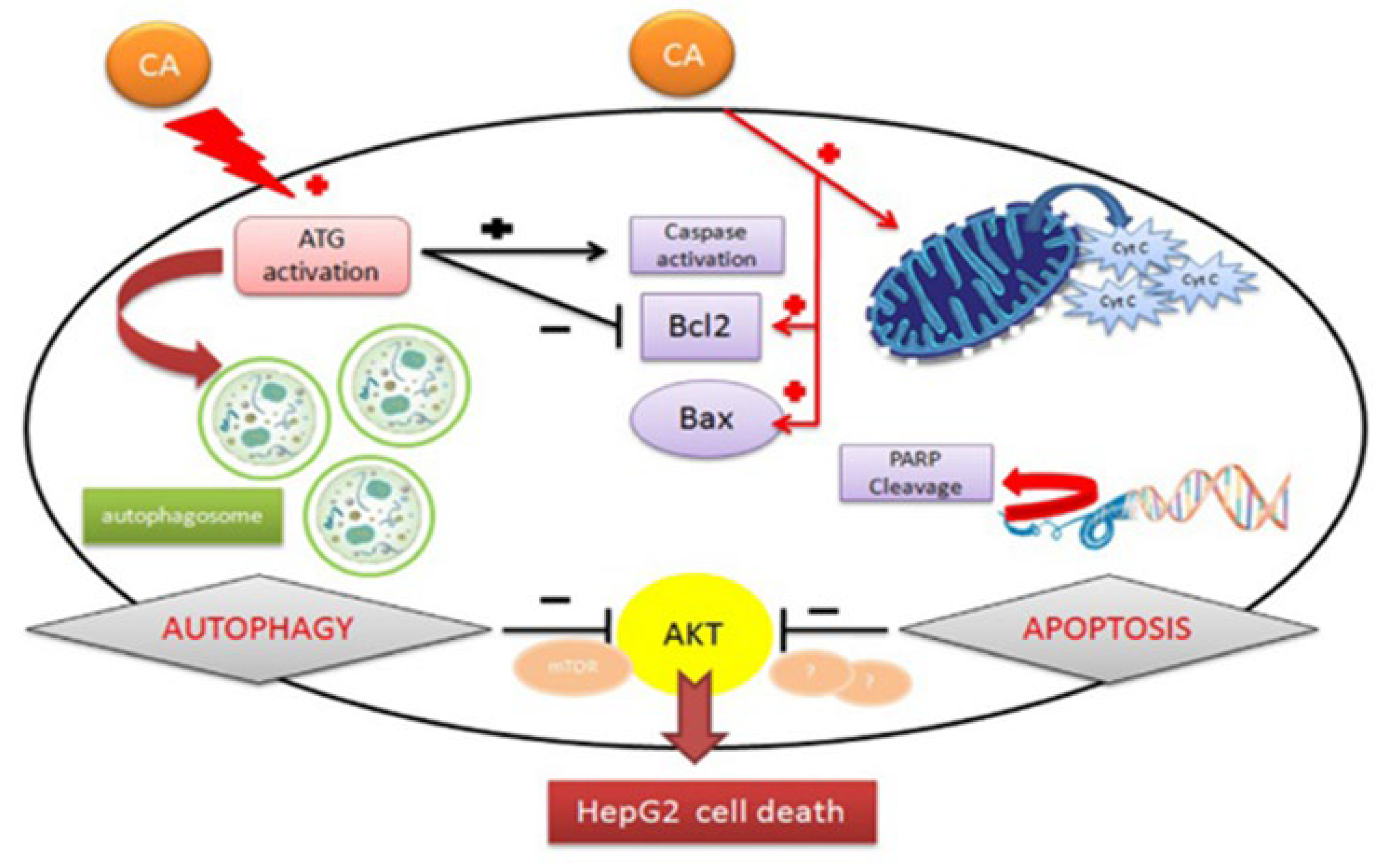
- Min, F.; Liu, X.; Li, Y.; Dong, M.; Qu, Y.; Liu, W. Carnosic Acid Suppresses the Development of Oral Squamous Cell Carcinoma via Mitochondrial-Mediated Apoptosis. Front. Oncol. 2021, 11, 760861. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, S.; Iqbal, J.; Abbasi, B.A.; Ullah, Z.; Yaseen, T.; Kanwal, S.; Mahmood, T.; Sydykbayeva, S.; Ydyrys, A.; Almarhoon, Z.M.; et al. Rosmarinic Acid and Its Derivatives: Current Insights on Anticancer Potential and Other Biomedical Applications. Biomed. Pharmacother. 2023, 162, 114687. [Google Scholar] [CrossRef]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef]
- Chen, X.; Su, H.Z.Z. Detailed Studies on the Anticancer Action of Rosmarinic Acid in Human Hep-G2 Liver Carcinoma Cells: Evaluating Its Effects on Cellular Apoptosis, Caspase Activation and Suppression of Cell Migration and Invasion. J. BUON. 2020, 25, 2011–2016. [Google Scholar]
- Tai, J.; Cheung, S.; Wu, M.; Hasman, D. Antiproliferation Effect of Rosemary (Rosmarinus officinalis) on Human Ovarian Cancer Cells in Vitro. Phytomedicine 2012, 19, 436–443. [Google Scholar] [CrossRef]
- González-Vallinas, M.; Reglero, G.; Ramirez de Molina, A. Rosemary (Rosmarinus officinalis L.) Extract as a Potential Complementary Agent in Anticancer Therapy. Nutr. Cancer 2015, 67, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Khella, K.F.; El Maksoud, A.I.A.; Hassan, A.; Abdel-Ghany, S.E.; Elsanhoty, R.M.; Aladhadh, M.A.; Abdel-Hakeem, M.A. Carnosic Acid Encapsulated in Albumin Nanoparticles Induces Apoptosis in Breast and Colorectal Cancer Cells. Molecules 2022, 27, 4102. [Google Scholar] [CrossRef]
- Manimaran, A.; Manoharan, S.; Karthikeyan, S.; Nirmal, M. Anti-Cell Proliferative, Anti-Inflammatory and Anti-Angiogenic Potential of Lupeol in 7, 12-Dimethylbenz (a) Anthracene Induced Hamster Buccal Pouch Carcinogenesis. Br. J. Med. Med. Res. 2015, 6, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Bahri, S.; Jameleddine, S.; Shlyonsky, V. Relevance of Carnosic Acid to the Treatment of Several Health Disorders: Molecular Targets and Mechanisms. Biomed. Pharmacother. 2016, 84, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Petiwala, S.M.; Puthenveetil, A.G.; Johnson, J.J. Polyphenols from the Mediterranean Herb Rosemary (Rosmarinus officinalis) for Prostate Cancer. Front. Pharmacol. 2013, 4, 00029. [Google Scholar] [CrossRef]
- Chou, S.T.; Ho, B.Y.; Tai, Y.T.; Huang, C.J.; Chao, W.W. Bidirect Effects from Cisplatin Combine with Rosmarinic Acid (RA) or Hot Water Extracts of Glechoma Hederacea (HWG) on Renal Cancer Cells. Chin. Med. 2020, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. Anticancer Activity of Rosmarinus officinalis L.: Mechanisms of Action and Therapeutic Potentials. Nutrients 2020, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Agulló-Chazarra, L.; Herranz-López, M.; Valdés, A.; Cifuentes, A.; Micol, V. Rosemary (Rosmarinus officinalis) Extract Causes ROS-Induced Necrotic Cell Death and Inhibits Tumor Growth in Vivo. Sci. Rep. 2019, 9, 808. [Google Scholar] [CrossRef] [PubMed]
- Faustino, C.; Neto, Í.; Fonte, P.; Macedo, A. Cytotoxicity and Chemotherapeutic Potential of Natural Rosin Abietane Diterpenoids and Their Synthetic Derivatives. Curr. Pharm. Des. 2018, 24, 4362–4375. [Google Scholar] [CrossRef]
- Han, S.; Yang, S.; Cai, Z.; Pan, D.; Li, Z.; Huang, Z.; Zhang, P.; Zhu, H.; Lei, L.; Wang, W. Anti-Warburg Effect of Rosmarinic Acid via MiR-155 in Gastric Cancer Cells. Drug Des. Devel. Ther. 2015, 9, 2695–2703. [Google Scholar] [CrossRef]
- Jaglanian, A.; Termini, D.; Tsiani, E. Rosemary (Rosmarinus officinalis L.) Extract Inhibits Prostate Cancer Cell Proliferation and Survival by Targeting Akt and MTOR. Biomed. Pharmacother. 2020, 131, 110717. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Shamsi, A.; Shahbaaz, M.; Queen, A.; Khan, P.; Hasan, G.M.; Islam, A.; Alajmi, M.F.; Hussain, A.; Ahmad, F. Rosmarinic Acid Exhibits Anticancer Effects via MARK4 Inhibition. Sci. Rep. 2020, 10, 10300. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Jung, K.-J.; Kwon, T.K. Carnosic Acid Induces Apoptosis through Reactive Oxygen Species-Mediated Endoplasmic Reticulum Stress Induction in Human Renal Carcinoma Caki Cells. J. Cancer Prev. 2014, 19, 170. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, D.; Eriksson, P.; Krawczyk, K.; Nilsson, H.; Hansson, J.; Veerla, S.; Sjölund, J.; Höglund, M.; Johansson, M.E.; Axelson, H. Cell-Type-Specific Gene Programs of the Normal Human Nephron Define Kidney Cancer Subtypes. Cell Rep. 2017, 20, 1476–1489. [Google Scholar] [CrossRef]
- Jung, K.-J.; Min, K.; Bae, J.H.; Kwon, T.K. Carnosic Acid Sensitized TRAIL-Mediated Apoptosis through down-Regulation of c-FLIP and Bcl-2 Expression at the Post Translational Levels and CHOP-Dependent up-Regulation of DR5, Bim, and PUMA Expression in Human Carcinoma Caki Cells. Oncotarget 2015, 6, 1556. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, N.; El-Serag, H.B.; Abrams, H.R.; Thrift, A.P. Burden of Pancreatic Cancer: From Epidemiology to Practice. Clin. Gastroenterol. Hepatol. 2021, 19, 876–884. [Google Scholar] [CrossRef]
- Gonzalez-Vallinas, M.; Molina, S.; Vicente, G.; Zarza, V.; Martin-Hernandez, R.; Garcia-Risco, M.R.; Fornari, T.; Reglero, G.; De Molina, A.R. Expression of MicroRNA-15b and the Glycosyltransferase GCNT3 Correlates with Antitumor Efficacy of Rosemary Diterpenes in Colon and Pancreatic Cancer. PLoS ONE 2014, 9, 0098556. [Google Scholar] [CrossRef]
- Reid, P.E.; Brown, N.J.; Holen, I. Breast cancer cells stimulate osteoprotegerin (OPG) production by endothelial cells through direct cell contact. Mol. Cancer 2009, 8, 49. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Ummul Maqsummiya, Z.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Plant-Derived Bioactive Compounds in Colon Cancer Treatment: An Updated Review. Biomed. Pharmacother. 2022, 153, 113384. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Ding, H.; Wang, J.; Chan, P.S.F.; Huang, J. Prevalence and Risk Factors of Colorectal Cancer in Asia. Intest. Res. 2019, 17, 317–329. [Google Scholar] [CrossRef]
- Jang, Y.-G.; Hwang, K.-A.; Choi, K.-C. Rosmarinic Acid, a Component of Rosemary Tea, Induced the Cell Cycle Arrest and Apoptosis through Modulation of HDAC2 Expression in Prostate Cancer Cell Lines. Nutrients 2018, 10, 1784. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, D.; Williams, J.; Wu, Y.; Damm, W.; Shelley, J.; Sherman, W. Prediction of Absolute Solvation Free Energies Using Molecular Dynamics Free Energy Perturbation and the OPLS Force Field. J. Chem. Theory Comput. 2010, 6, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.; Henrick, K.; Nakamura, H.; Markley, J.L. The Worldwide Protein Data Bank (WwPDB): Ensuring a Single, Uniform Archive of PDB Data. Nucleic Acids Res. 2007, 35, D301–D303. [Google Scholar] [CrossRef]
- Bourhou, C.; Benouda, H.; Bellaouchi, R.; Merzouki, M.; Fraj, E.; Harit, T.; Challioui, A.; Asehraou, A.; Touzani, R.; Ozdemir, I. Synthesis of Novel Tetrazolic Derivatives and Evaluation of Their Antimicrobial Activity. J. Mol. Struct. 2023, 1278, 134913. [Google Scholar] [CrossRef]
- Merzouki, M.; Challioui, A.; Bourassi, L.; Abidi, R.; Bouammli, B.; El Farh, L. In Silico Evaluation of Antiviral Activity of Flavone Derivatives and Commercial Drugs against SARS-CoV-2 Main Protease (3CLpro). Moroc. J. Chem. 2023, 11, 11. [Google Scholar] [CrossRef]
- Diass, K.; Merzouki, M.; Elfazazi, K.; Azzouzi, H.; Challioui, A.; Azzaoui, K.; Hammouti, B.; Touzani, R.; Depeint, F.; Ayerdi Gotor, A. Essential Oil of Lavandula officinalis: Chemical Composition and Antibacterial Activities. Plants 2023, 12, 1571. [Google Scholar] [CrossRef]
| Tested Compounds in IC50 (μΜ) | References | |||
|---|---|---|---|---|
| Rosemary Extract | Carnosic Acid | Rosmarinic Acid | ||
| Radical-scavenging activity DPPH | 54.0 ± 1.4 | 33.1 ± 1.7 | 72.3 ± 3.3 | [32] |
| Oral cancer | 27.4–68.2 μM | 7.5–20 μM | 50–104.2 μM | [33,34,35] |
| Kidney cancer | - | 20 μΜ | 240.2 µM | [36,37] |
| Pancreatic Cancer | 20–120 µM | 6.02–54.15 µM | 100 μΜ | [36,38] |
| Colon cancer | 20–40 µg | 8.29–27.43 mg/mL | 240.2 μM | [39,40,41] |
| Prostate cancer | 19.72 μg/mL | 41.1 μM | 14 μM | [42,43,44] |
| Free Binding Energy | (kcal/mol) | |
|---|---|---|
| Rosmarinic Acid | Carnosic Acid | |
| S100A8 | −4.688 | |
| S100A9 | −5.446 | −4.850 |
| S100A8–S100A9 | −6.720 | −3.506 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouammali, H.; Zraibi, L.; Ziani, I.; Merzouki, M.; Bourassi, L.; Fraj, E.; Challioui, A.; Azzaoui, K.; Sabbahi, R.; Hammouti, B.; et al. Rosemary as a Potential Source of Natural Antioxidants and Anticancer Agents: A Molecular Docking Study. Plants 2024, 13, 89. https://doi.org/10.3390/plants13010089
Bouammali H, Zraibi L, Ziani I, Merzouki M, Bourassi L, Fraj E, Challioui A, Azzaoui K, Sabbahi R, Hammouti B, et al. Rosemary as a Potential Source of Natural Antioxidants and Anticancer Agents: A Molecular Docking Study. Plants. 2024; 13(1):89. https://doi.org/10.3390/plants13010089
Chicago/Turabian StyleBouammali, Haytham, Linda Zraibi, Imane Ziani, Mohammed Merzouki, Lamiae Bourassi, Elmehdi Fraj, Allal Challioui, Khalil Azzaoui, Rachid Sabbahi, Belkheir Hammouti, and et al. 2024. "Rosemary as a Potential Source of Natural Antioxidants and Anticancer Agents: A Molecular Docking Study" Plants 13, no. 1: 89. https://doi.org/10.3390/plants13010089







