A Catalog of GNI-A1 Genes That Regulate Floret Fertility in a Diverse Bread Wheat Collection
Abstract
:1. Introduction
2. Results
2.1. Allelic Variation of GNI-A1
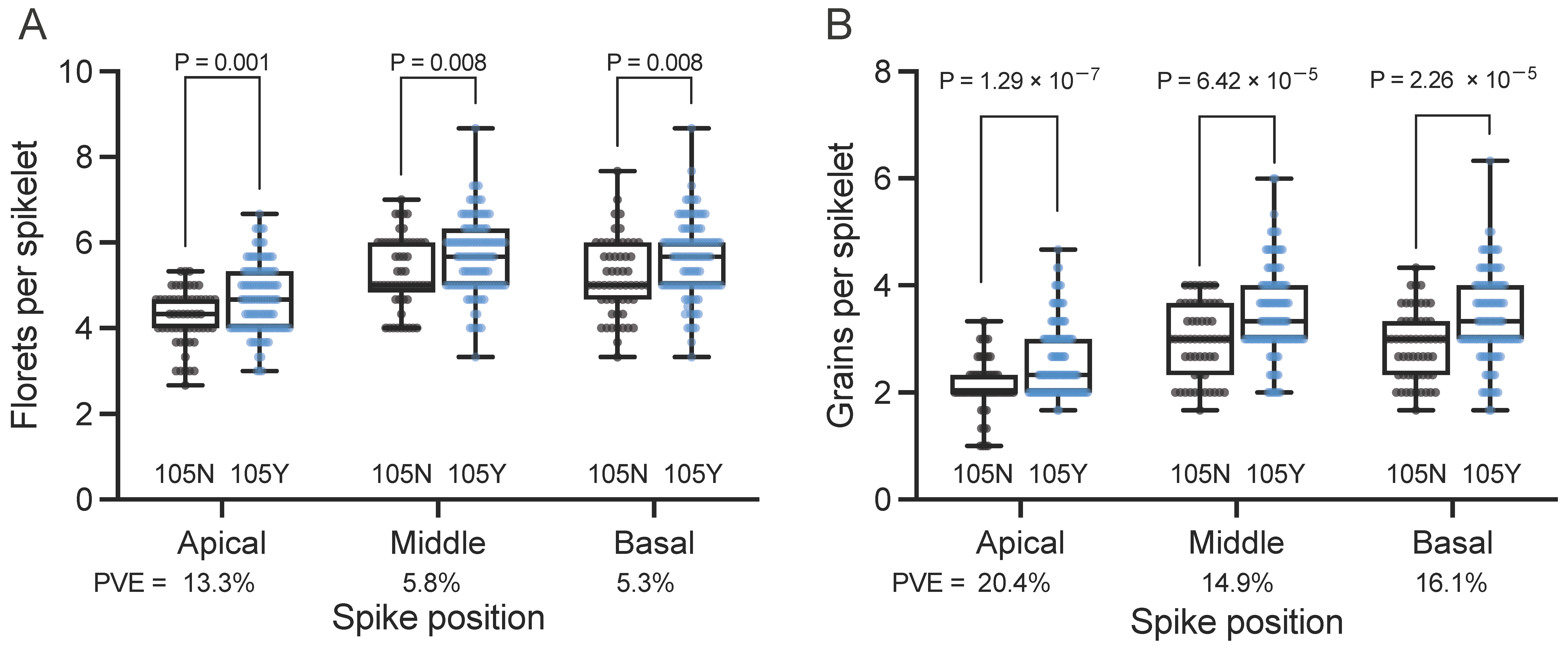
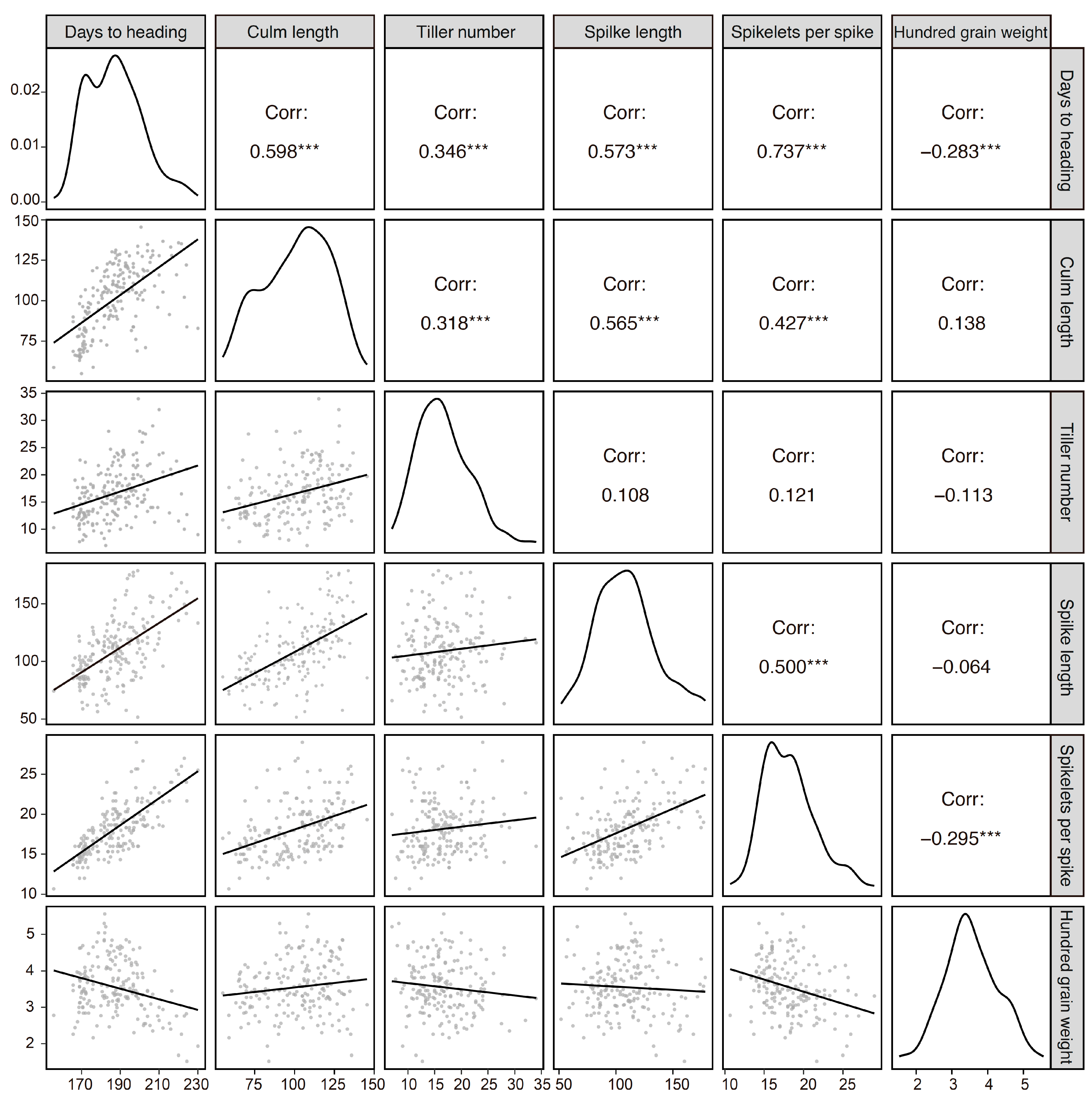
2.2. Association of Agricultural Traits with GNI-A1 Variants
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Resequencing
4.3. Phenotyping
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, J.R.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F.; et al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Zhang, L.; Zhang, Q.; Yang, Y.; Li, D.; Xie, Z.; Cui, G.; Chen, Y.; Wu, L.; Li, Z.; et al. Tiller Number1 encodes an ankyrin repeat protein that controls tillering in bread wheat. Nat. Commun. 2023, 14, 836. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Schnurbusch, T. Of floral fortune: Tinkering with the grain yield potential of cereal crops. New Phytol. 2020, 225, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Voss-Fels, K.P.; Stahl, A.; Wittkop, B.; Lichthardt, C.; Nagler, S.; Rose, T.; Chen, T.-W.; Zetzsche, H.; Seddig, S.; Majid Baig, M.; et al. Breeding improves wheat productivity under contrasting agrochemical input levels. Nat. Plants 2019, 5, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, O.T. Inflorescences of Maize, Wheat, Rye, Barley and Oats: Their Initiation and Development; University of Illinois College of Agriculture, Agricultural Experimental Station: Urbana, IL, USA, 1966; Volume 721, p. 105. [Google Scholar]
- Sakuma, S.; Golan, G.; Guo, Z.; Ogawa, T.; Tagiri, A.; Sugimoto, K.; Bernhardt, N.; Brassac, J.; Mascher, M.; Hensel, G.; et al. Unleashing floret fertility in wheat through the mutation of a homeobox gene. Proc. Natl. Acad. Sci. USA 2019, 116, 5182. [Google Scholar] [CrossRef] [PubMed]
- Komatsuda, T.; Pourkheirandish, M.; He, C.; Azhaguvel, P.; Kanamori, H.; Perovic, D.; Stein, N.; Graner, A.; Wicker, T.; Tagiri, A.; et al. Six-rowed barley originated from a mutation in a homeodomain-leucine zipper I-class homeobox gene. Proc. Natl. Acad. Sci. USA 2007, 104, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Saisho, D.; Pourkheirandish, M.; Kanamori, H.; Matsumoto, T.; Komatsuda, T. Allelic variation of row type gene Vrs1 in barley and implication of the functional divergence. Breed. Sci. 2009, 59, 621–628. [Google Scholar] [CrossRef]
- Golan, G.; Ayalon, I.; Perry, A.; Zimran, G.; Ade-Ajayi, T.; Mosquna, A.; Distelfeld, A.; Peleg, Z. GNI-A1 mediates trade-off between grain number and grain weight in tetraploid wheat. Theor. Appl. Genet. 2019, 132, 2353–2365. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Nitta, M.; Nasuda, S. Population structure and association analyses of the core collection of hexaploid accessions conserved ex situ in the Japanese gene bank NBRP-Wheat. Genes Genet. Syst. 2018, 93, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Kirby, E.J.M.; Appleyard, M. Cereal Development Guide, 1st ed.; Cereal Unit: Kenilworth, UK, 1981; p. 80. [Google Scholar]
- Muqaddasi, Q.H.; Brassac, J.; Koppolu, R.; Plieske, J.; Ganal, M.W.; Roder, M.S. TaAPO-A1, an ortholog of rice ABERRANT PANICLE ORGANIZATION 1, is associated with total spikelet number per spike in elite European hexaploid winter wheat (Triticum aestivum L.) varieties. Sci. Rep. 2019, 9, 13853. [Google Scholar] [CrossRef] [PubMed]
- Kuzay, S.; Xu, Y.; Zhang, J.; Katz, A.; Pearce, S.; Su, Z.; Fraser, M.; Anderson, J.A.; Brown-Guedira, G.; DeWitt, N.; et al. Identification of a candidate gene for a QTL for spikelet number per spike on wheat chromosome arm 7AL by high-resolution genetic mapping. Theor. Appl. Genet. 2019, 132, 2689–2705. [Google Scholar] [CrossRef] [PubMed]
- Wittern, L.M.; Barrero, J.M.; Bovill, W.D.; Verbyla, K.L.; Hughes, T.; Swain, S.M.; Steed, G.; Webb, A.A.R.; Gardner, K.; Greenland, A.; et al. Overexpression of the WAPO-A1 gene increases the number of spikelets per spike in bread wheat. Sci. Rep. 2022, 12, 14229. [Google Scholar] [CrossRef]
- Mizuno, N.; Ishikawa, G.; Kojima, H.; Tougou, M.; Kiribuchi-Otobe, C.; Fujita, M.; Nakamura, K. Genetic mechanisms determining grain number distribution along the spike and their effect on yield components in wheat. Mol. Breed. 2021, 41, 62. [Google Scholar] [CrossRef]
- Boden, S.A.; Cavanagh, C.; Cullis, B.R.; Ramm, K.; Greenwood, J.; Jean Finnegan, E.; Trevaskis, B.; Swain, S.M. Ppd-1 is a key regulator of inflorescence architecture and paired spikelet development in wheat. Nat. Plants 2015, 1, 14016. [Google Scholar] [CrossRef]
- Dixon, L.E.; Greenwood, J.R.; Bencivenga, S.; Zhang, P.; Cockram, J.; Mellers, G.; Ramm, K.; Cavanagh, C.; Swain, S.M.; Boden, S.A. TEOSINTE BRANCHED1 regulates inflorescence architecture and development in bread wheat (Triticum aestivum). Plant Cell 2018, 30, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]

| Genus | Species | 105N | 105Y | 105K | Unknown |
|---|---|---|---|---|---|
| Triticum | aestivum | 41 | 103 | 11 | 4 |
| compactum | 0 | 3 | 1 | 2 | |
| macha | 4 | 0 | 0 | 0 | |
| spelta | 12 | 1 | 0 | 0 | |
| sphaerococcum | 0 | 2 | 0 | 1 | |
| vavilovii | 0 | 2 | 0 | 0 | |
| Synthetic | 0 | 1 | 0 | 0 | |
| Total | 57 | 112 | 12 | 7 |
| Species | Country | 105N | 105Y | 105K | Unknown |
|---|---|---|---|---|---|
| Triticum aestivum | Afghanistan | 1 | 12 | 0 | 1 |
| Armenia | 1 | 1 | 0 | 0 | |
| Australia | 0 | 2 | 0 | 0 | |
| Azerbaijan | 1 | 0 | 0 | 0 | |
| Bhutan | 0 | 4 | 0 | 0 | |
| Canada | 0 | 1 | 0 | 0 | |
| China | 3 | 12 | 0 | 0 | |
| Egypt | 1 | 0 | 0 | 0 | |
| Ethiopia | 0 | 7 | 0 | 1 | |
| Georgia | 3 | 0 | 0 | 0 | |
| Greece | 1 | 1 | 2 | 1 | |
| India | 0 | 1 | 0 | 0 | |
| Iran | 2 | 14 | 1 | 0 | |
| Iraq | 0 | 1 | 1 | 0 | |
| Italy | 1 | 0 | 0 | 0 | |
| Japan | 5 | 24 | 5 | 0 | |
| Jordan | 1 | 0 | 0 | 0 | |
| Lebanon | 1 | 0 | 0 | 0 | |
| Mexico | 1 | 1 | 0 | 0 | |
| Nepal | 1 | 5 | 0 | 0 | |
| Pakistan | 0 | 5 | 0 | 0 | |
| Romania | 2 | 3 | 0 | 0 | |
| Spain | 2 | 2 | 1 | 0 | |
| Syria | 1 | 0 | 0 | 0 | |
| Tanzania | 0 | 1 | 0 | 0 | |
| Turkey | 4 | 4 | 1 | 0 | |
| UK | 6 | 0 | 0 | 0 | |
| USA | 3 | 1 | 0 | 1 | |
| Uzbekistan | 0 | 1 | 0 | 0 | |
| Total | 41 | 103 | 11 | 4 |
| Species | Country | 105N | 105Y | 105K | Unknown |
|---|---|---|---|---|---|
| Triticum aestivum | Australia | 3 | 0 | 0 | 0 |
| Canada | 3 | 0 | 0 | 0 | |
| Germany | 4 | 0 | 0 | 0 | |
| Japan | 4 | 1 | 7 | 0 | |
| Mexico | 1 | 0 | 0 | 0 | |
| Russia | 1 | 0 | 0 | 0 | |
| Switzerland | 1 | 0 | 0 | 0 | |
| Turkey | 0 | 0 | 1 | 0 | |
| UK | 6 | 6 | 0 | 0 | |
| USA | 15 | 6 | 4 | 0 | |
| Yugoslavia | 1 | 0 | 0 | 0 | |
| Total | 39 | 13 | 12 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakuma, S.; Yamashita, Y.; Suzuki, T.; Nasuda, S. A Catalog of GNI-A1 Genes That Regulate Floret Fertility in a Diverse Bread Wheat Collection. Plants 2024, 13, 330. https://doi.org/10.3390/plants13030330
Sakuma S, Yamashita Y, Suzuki T, Nasuda S. A Catalog of GNI-A1 Genes That Regulate Floret Fertility in a Diverse Bread Wheat Collection. Plants. 2024; 13(3):330. https://doi.org/10.3390/plants13030330
Chicago/Turabian StyleSakuma, Shun, Yoko Yamashita, Takako Suzuki, and Shuhei Nasuda. 2024. "A Catalog of GNI-A1 Genes That Regulate Floret Fertility in a Diverse Bread Wheat Collection" Plants 13, no. 3: 330. https://doi.org/10.3390/plants13030330





