Regional Variations in Peucedanum japonicum Antioxidants and Phytochemicals
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Polyphenol and Total Flavonoid Content (TPC and TFC) Assays
2.2. DPPH and ABTS Radical-Scavenging Assays
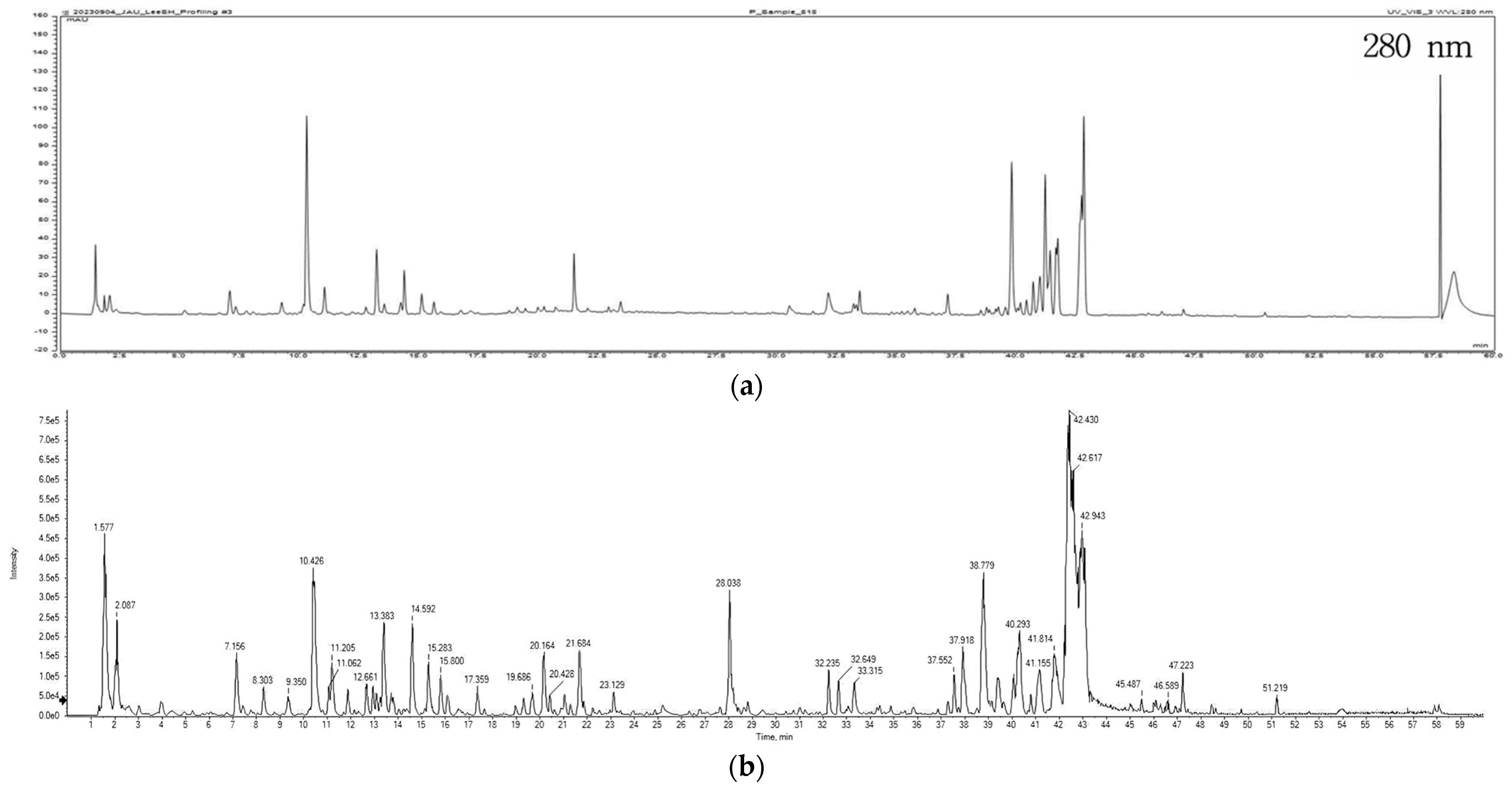
2.3. LC-MS/MS Analysis
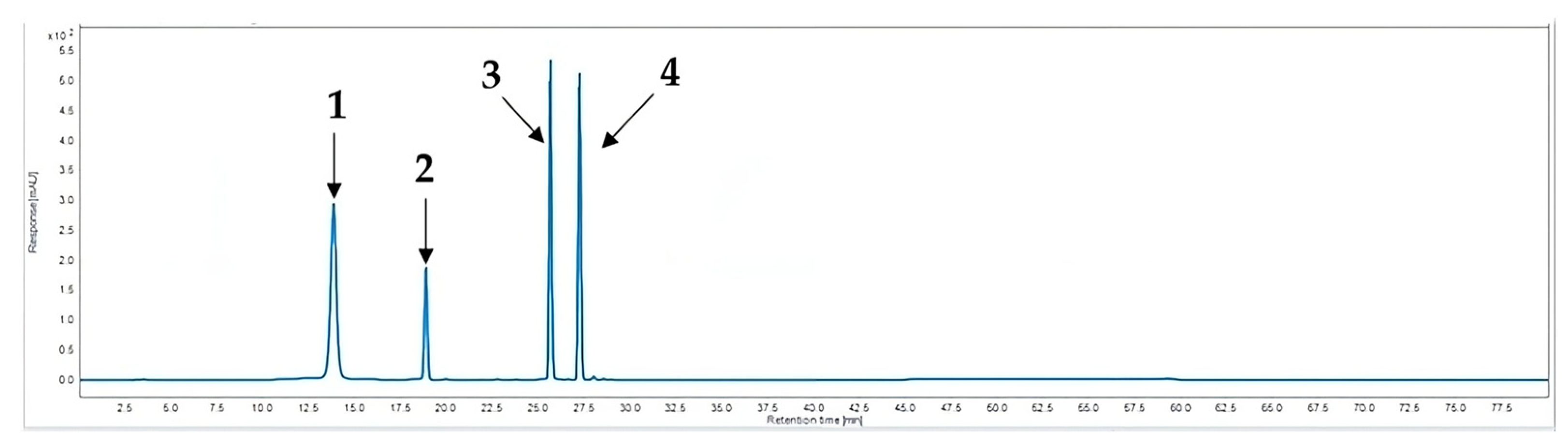
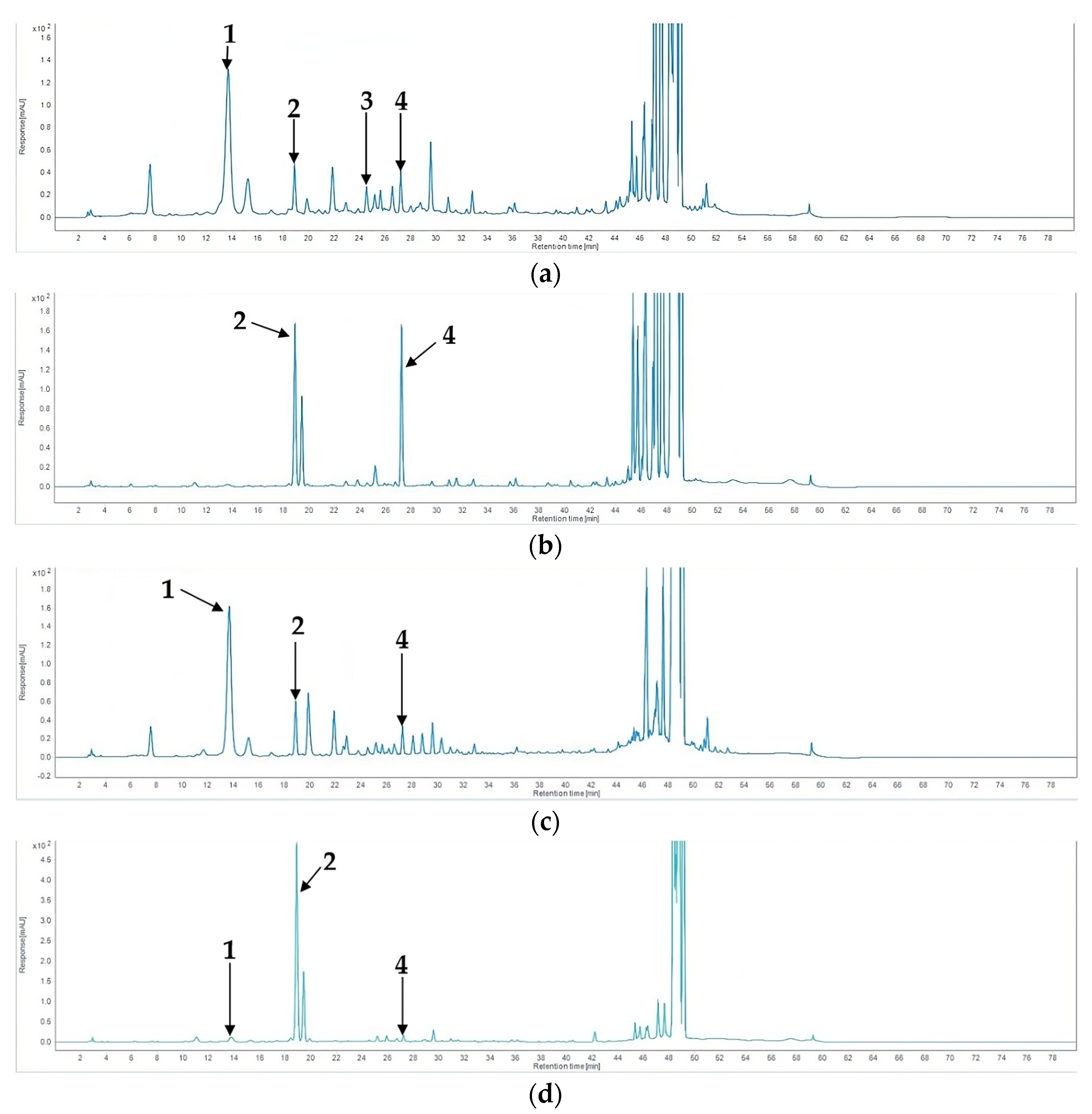
2.4. HPLC Analysis
3. Materials and Methods
3.1. Plant Materials and Extracts
3.2. Chemicals and Apparatus
3.3. Preparation of Samples and Standard Solutions for HPLC Analysis
3.4. TPC Assay
3.5. TFC Assay
3.6. DPPH Radical-Scavenging Assay
3.7. ABTS Radical-Scavenging Assay
3.8. LC-MS/MS Conditions
3.9. HPLC Conditions
3.10. Calibration Curves
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The role of oxidative stress in physiopathology and pharmacological treatment with pro- and antioxidant properties in chronic diseases. Oxid. Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef]
- Markitantova, Y.V.; Simirskii, B.H. Endogenous and exogenous regulation of redox homeostasis in retinal pigment epithelium cells: An updated antioxidant perspective. Int. J. Mol. Sci. 2023, 24, 10776. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants, and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Li, C. Dietary regulation of oxidative stress in chronic metabolic diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef]
- Aune, D. Plant foods, antioxidant biomarkers, and the risk of cardiovascular disease, cancer, and mortality: A review of the evidence. Adv. Nutr. 2019, 10, S404–S421. [Google Scholar] [CrossRef]
- Christensen, L.P. Bioactive C17 and C18 acetylenic oxylipins from terrestrial plants as potential lead compounds for anticancer drug development. Molecules 2020, 25, 2568. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, J.H.; Shin, J.Y.; Kang, E.S.; Cho, B.O. Anti-inflammatory effects of Peucedanum japonicum Thunberg leaves extract in lipopolysaccharide-stimulated RAW264.7 cells. J. Ethnopharmacol. 2023, 309, 116362. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Ryu, H.W.; Park, J.W.; Kim, J.; Kim, D.-Y.; Oh, J.; Kwon, O.; Han, S.; Ahn, K.H. Effects of 3′-isovaleryl-4′-senecioylkhellactone from Peucedanum japonicum Thunberg on PMA-stimulated inflammatory response in A549 human lung epithelial cells. J. Microbiol. Biotechnol. 2022, 32, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.M.; Lee, A.Y.; Kim, J.S.; Choi, G.; Kim, S.-H. Protective effects of Peucedanum japonicum extract against osteoarthritis in an animal model using a combined systems approach for compound-target prediction. Nutrients 2018, 10, 754. [Google Scholar] [CrossRef]
- Abifarin, T.O.; Otunola, G.A.; Afolayan, A.J. Assessment of the phytochemical, antioxidant and antibacterial activities of Heteromorpha arborescens (Spreng.) Cham & Schltdl. leaf extracts. F1000 Res. 2020, 9, 1079. [Google Scholar]
- Kim, J.; Kang, H.M.; Jang, D.C.; Na, J.K.; Choi, K. Effect of light intensity and temperature on the growth and functional compounds in the baby leaf vegetable plant Peucedanum japonicum Thunb. Korean J. Hortic. Sci. Technol. 2020, 38, 822–829. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Makuch-Pietraś, I.; Grabek-Lejko, D.; Górka, A.; Kasprzyk, I. Antioxidant activities in relation to the transport of heavy metals from the soil to different parts of Betula pendula (Roth.). J. Biol. Eng. 2023, 17, 9. [Google Scholar] [CrossRef]
- Petruk, G.; Del Giudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from plants protect against skin photoaging. Oxid. Med. Cell. Longev. 2018, 2018, 1454936. [Google Scholar] [CrossRef]
- Maina, S.; Ryu, D.H.; Bakari, G.G.; Misinzo, G.; Nho, C.W.; Kim, H.-Y. Variation in phenolic compounds and antioxidant activity of various organs of African cabbage (Cleome gynandra L.) accessions at different growth stages. Antioxidants 2021, 10, 1952. [Google Scholar] [CrossRef] [PubMed]
- Goszcz, K.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Bioactive polyphenols and cardiovascular disease: Chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br. J. Pharmacol. 2017, 174, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-G.; Hu, Q.-P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lim, J.H.; Cho, S.K. Effect of antioxidant and anti-inflammatory on bioactive components of carrot (Daucus carota L.) leaves from Jeju Island. Appl. Biol. Chem. 2023, 66, 34. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2016, 20, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Ding, Y.; Li, L.; Ge, M.; Ban, G.-G.; Yang, H.; Dai, J.; Zhang, L. Effects and mechanism of chlorogenic acid on weight loss. Curr. Pharm. Biotechnol. 2020, 21, 1099–1106. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Choi, J.-W.; Lee, H.-D.; Cho, H.; Lee, C.-D.; Tran, G.H.; Kim, H.; Moon, S.; Lee, S. Antioxidative phenolic compounds from the aerial parts of Cyperus exaltatus var. iwasakii and their HPLC analysis. Appl. Biol. Chem. 2023, 66, 61. [Google Scholar] [CrossRef]
- Satari, A.; Ghasemi, S.; Habtemariam, S.; Asgharian, S.; Lorigooini, Z. Rutin: A flavonoid as an effective sensitizer for anticancer therapy; Insights into multifaceted mechanisms and applicability for combination therapy. Evid. Based Complement. Altern. Med. 2021, 2021, 9913179. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Kim, S.-H. Rutin suppresses atopic dermatitis and allergic contact dermatitis. Exp. Biol. Med. 2013, 238, 410–417. [Google Scholar] [CrossRef]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gándara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem: X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Hisamoto, M.; Kikuzaki, H.; Ohigashi, H.; Nakatani, N. Antioxidant compounds from the leaves of Peucedanum japonicum Thunb. J. Agric. Food Chem. 2003, 51, 5255–5261. [Google Scholar] [CrossRef]
- Lee, S.O.; Choi, S.Z.; Lee, J.H.; Chung, S.H.; Park, S.H.; Kang, H.Y.; Yang, E.Y.; Cho, H.J.; Lee, K.R. Antidiabetic coumarin and cyclitol compounds from Peucedanum japonicum. Arch. Pharm. Res. 2004, 27, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-S.; Chang, C.; Sheen, W.-S.; Chen, T.; Tsai, I.; Duh, C.; Ko, F. Coumarins and antiplatelet aggregation constituents from formosan Peucedanum japonicum. Phytochemistry 1996, 41, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Altemimi, A.B.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Srivastava, R.P.; Kumar, S.; Singh, L.; Madhukar, M.; Singh, N.; Saxena, G.; Pandey, S.; Singh, A.; Devkota, H.P.; Verma, P.C.; et al. Major phenolic compounds, antioxidant, antimicrobial, and cytotoxic activities of Selinum carvifolia (L.) collected from different altitudes in India. Front. Nutr. 2023, 10, 1180225. [Google Scholar] [CrossRef]
- Lim, H.; Kim, I.; Jeong, Y. Antioxidant activities of Peucedanum japonicum Thunberg root extracts. J. Korean Soc. Food Sci. Nutr. 2019, 48, 32–39. [Google Scholar] [CrossRef]
- Upadhyay, R.; Chaurasia, J.K.; Tiwari, K.N.; Singh, K. Antioxidant property of aerial parts and root of Phyllanthus fraternus Webster, an important medicinal plant. Sci. World J. 2014, 2014, 692392. [Google Scholar] [CrossRef] [PubMed]
- Thiviya, P.; Gamage, A.; Piumali, D.; Merah, O.; Madhujith, T. Apiaceae as an important source of antioxidants and their applications. Cosmetics 2021, 8, 111. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.M.; Ritmejerytė, E.; Wangchuk, P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Guo, W.; Xing, Y.; Luo, X.; Li, F.; Ren, M.; Liang, Y. Reactive oxygen species: A crosslink between plant and human eukaryotic cell systems. Int. J. Mol. Sci. 2023, 24, 13052. [Google Scholar] [CrossRef]
- Yang, D.-D.; Chen, Y.; Guo, F.; Huang, B.; Okyere, S.A.; Wang, H. Comparative analysis of the chemical composition, antioxidant and antimicrobial activities of leaves, leaf tea and root from Codonopsis pilosula. Ind. Crops Prod. 2019, 142, 111844. [Google Scholar] [CrossRef]
- Liigand, P.; Kaupmees, K.; Haav, K.; Liigand, J.; Leito, I.; Girod, M.; Antoine, R.; Kruve, A. Think negative: Finding the best electrospray ionization/MS mode for your analyte. Anal. Chem. 2017, 89, 5665–5668. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, T.; Juhler, R.K.; Svensmark, B.; Cech, N.B. The relative influences of acidity and polarity on the responsiveness of small organic molecules to analysis with negative ion electrospray ionization mass spectrometry (ESI-MS). JASMS 2005, 16, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Bowen, B.P.; Northen, T. Mass spectrometry—Based metabolomics, analysis of metabolite-protein interactions, and imaging. Biotechniques 2010, 49, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Agrawal, D.C.; Lee, M.; Lee, R.; Kuo, C.; Wu, C.; Tsay, H.; Chang, H. Influence of LED light spectra on in vitro somatic embryogenesis and LC–MS analysis of chlorogenic acid and rutin in Peucedanum japonicum Thunb.: A medicinal herb. Bot. Stud. 2016, 57, 9. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.Y.; Kim, E.; Choi, H.-S.; Lee, K.H.; Kim, K.J.; Lim, D.; Choi, S.H.; Kim, Y.; Son, S.Y.; Kim, J.S.; et al. Therapeutic potential of Peucedanum japonicum Thunb. and its active components in a delayed corneal wound healing model following blue light irradiation-induced oxidative stress. Antioxidants 2023, 12, 1171. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tsao, R.; Deng, Z. Factors affecting the antioxidant potential and health benefits of plant foods. Can. J. Plant Sci. 2012, 92, 1101–1111. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- De Queiroz, A.R.; Hines, C.; Brown, J.; Sahay, S.; Vijayan, J.; Stone, J.M.; Bickford, N.; Wuellner, M.R.; Głowacka, K.; Buan, N.R.; et al. The effects of exogenously applied antioxidants on plant growth and resilience. Phytochem. Rev. 2023, 22, 407–447. [Google Scholar] [CrossRef]
- Molole, G.J.; Gure, A.; Abdissa, N. Determination of total phenolic content and antioxidant activity of Commiphora mollis (Oliv.) Engl. Resin. BMC Chem. 2022, 16, 48. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Kitanov, G. Radical scavenging and antioxidant activities of methanolic extracts from Hypericum species growing in Bulgaria. Pharmacogn. Mag. 2010, 6, 74–78. [Google Scholar] [CrossRef]
- Wen, J.; Kang, L.; Liu, H.; Xiao, Y.; Zhang, X.; Chen, Y. A validated UV-HPLC method for determination of chlorogenic acid in Lepidogrammitis drymoglossoides (Baker) Ching, Polypodiaceae. Pharmacogn. Res. 2012, 4, 148. [Google Scholar]
- Kwun, H.J.; Park, J.; Kim, H.S.; Kim, J.-H.; Park, H.-S. Checklist of the tidal pool fishes of Jeju Island, Korea. Zookeys 2017, 709, 135–154. [Google Scholar] [CrossRef]




| Sample | TPC (mg TAE/g Extract) | TFC (mg QE/g Extract) |
|---|---|---|
| PAU | 18.89 ± 6.2 a | 6.88 ± 4.9 a |
| PRU | 8.29 ± 3.8 d | 4.64 ± 1.2 c |
| PAJ | 15.58 ± 4.3 b | 5.83 ± 2.5 b |
| PRJ | 10.34 ± 5.4 c | 6.37 ± 4.6 a |
| Sample | Concentration (mg/mL) | DPPH | ABTS | ||
|---|---|---|---|---|---|
| Scavenging Activity (%) | IC50 (mg/mL) | Scavenging Activity (%) | IC50 (mg/mL) | ||
| PAU | 10 | 68.72 ± 0.11 | 5.59 ± 0.13 a | 83.90 ± 0.26 | 2.71 ± 0.08 a |
| 5 | 46.94 ± 0.78 | 66.03 ± 0.66 | |||
| 2.5 | 36.03 ± 2.93 | 48.85 ± 0.75 | |||
| 1.25 | 18.91 ± 1.25 | 30.50 ± 2.55 | |||
| 0.625 | 7.70 ± 0.76 | 18.71 ± 2.36 | |||
| PRU | 10 | 13.73 ± 0.23 | - | 50.97 ± 0.20 | 9.57 ± 0.14 b |
| 5 | 7.11 ± 1.61 | 38.96 ± 1.41 | |||
| 2.5 | 5.02 ± 0.17 | 31.88 ± 1.04 | |||
| 1.25 | 4.61 ± 0.80 | 25.05 ± 0.88 | |||
| 0.625 | 2.86 ± 0.96 | 21.54 ± 0.61 | |||
| PAJ | 10 | 58.07± 1.23 | 8.92 ± 0.07 a | 83.41 ± 0.33 | 3.15 ± 0.35 a |
| 5 | 24.27 ± 2.45 | 67.31 ± 3.49 | |||
| 2.5 | 13.44 ± 2.68 | 43.44 ± 3.75 | |||
| 1.25 | 9.34 ± 5.05 | 29.00 ± 1.83 | |||
| 0.625 | 4.65 ± 2.46 | 24.11 ± 0.95 | |||
| PRJ | 10 | 18.76 ± 3.35 | - | 50.86 ± 0.52 | 9.60 ± 0.26 b |
| 5 | 12.39 ± 0.79 | 38.48 ± 1.09 | |||
| 2.5 | 7.96 ± 3.70 | 30.22 ± 0.12 | |||
| 1.25 | 7.18 ± 5.04 | 26.16 ± 0.86 | |||
| 0.625 | 3.50 ± 4.31 | 22.76 ± 0.36 | |||
| aAA | 0.2 | 68.83 ± 0.84 | 0.09 ± 0.00 b | 86.12 ± 1.46 | 0.10 ± 0.00 c |
| 0.16 | 49.62 ± 3.95 | 63.67 ± 0.52 | |||
| 0.12 | 38.01 ± 0.84 | 49.41 ± 0.18 | |||
| 0.08 | 19.17 ± 0.34 | 27.96 ± 0.31 | |||
| 0.04 | 3.35 ± 0.93 | 15.47 ± 0.10 | |||
| Retention Time (min) | Molecular Weight | Tentative Identification |
|---|---|---|
| 7.16 | 354.1 | Neochlorogenic acid |
| 7.44 | 138.0 | Protocatechuic aldehyde |
| 9.34 | 338.1 | 3-p-Coumaroylquinic acid |
| 10.42 | 354.1 | Chlorogenic acid |
| 11.19 | 354.1 | Cryptochlorogenic acid |
| 12.93 | 354.1 | 1-Caffeoylquinic acid |
| 13.38 | 338.1 | Coumaroylquinic acid |
| 14.39 | 164.0 | p-Coumaric acid |
| 15.28 | 368.1 | 3-O-Feruloylquinic acid |
| 15.80 | 338.1 | 3-p-Coumaroylquinic acid |
| 19.29 | 464.1 | Isoquercetin |
| 19.31 | 610.2 | Rutin |
| 19.63 | 464.1 | Hyperoside |
| 20.43 | 434.1 | Guaiaverin |
| 21.67 | 610.2 | Hesperidin |
| Compound | tR | Calibration Equation a | Correlation Factor, r2 b |
|---|---|---|---|
| 1 | 13.80 | Y = 11.617x − 34.047 | 0.9989 |
| 2 | 18.83 | Y = 26.264x + 14.37 | 0.9999 |
| 3 | 25.62 | Y = 18.475x + 5.1917 | 1.0000 |
| 4 | 27.20 | Y = 13.764x + 27.381 | 0.9999 |
| Sample | Content (mg/g ext.) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Total | |
| PAU | 5.03 ± 0.14 a | 1.31 ± 0.07 a | 0.79 ± 0.04 | 0.26 ± 0.01 b | 6.96 ± 0.23 |
| PRU | tr | 4.89 ± 0.00 b | ND | 1.76 ± 0.02 d | 6.65 ± 0.02 |
| PAJ | 5.83 ± 0.12 b | 1.69 ± 0.05 a | ND | 0.29 ± 0.00 c | 7.81 ± 0.18 |
| PRJ | 0.34 ± 0.05 c | 14.33 ± 0.33 c | ND | 0.13 ± 0.00 a | 14.8 ± 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uy, N.P.; Kim, H.; Ku, J.; Lee, S. Regional Variations in Peucedanum japonicum Antioxidants and Phytochemicals. Plants 2024, 13, 377. https://doi.org/10.3390/plants13030377
Uy NP, Kim H, Ku J, Lee S. Regional Variations in Peucedanum japonicum Antioxidants and Phytochemicals. Plants. 2024; 13(3):377. https://doi.org/10.3390/plants13030377
Chicago/Turabian StyleUy, Neil Patrick, Hoon Kim, Jajung Ku, and Sanghyun Lee. 2024. "Regional Variations in Peucedanum japonicum Antioxidants and Phytochemicals" Plants 13, no. 3: 377. https://doi.org/10.3390/plants13030377






