Functional Characterization of the MsFKF1 Gene Reveals Its Dual Role in Regulating the Flowering Time and Plant Height in Medicago sativa L.
Abstract
:1. Introduction
2. Results
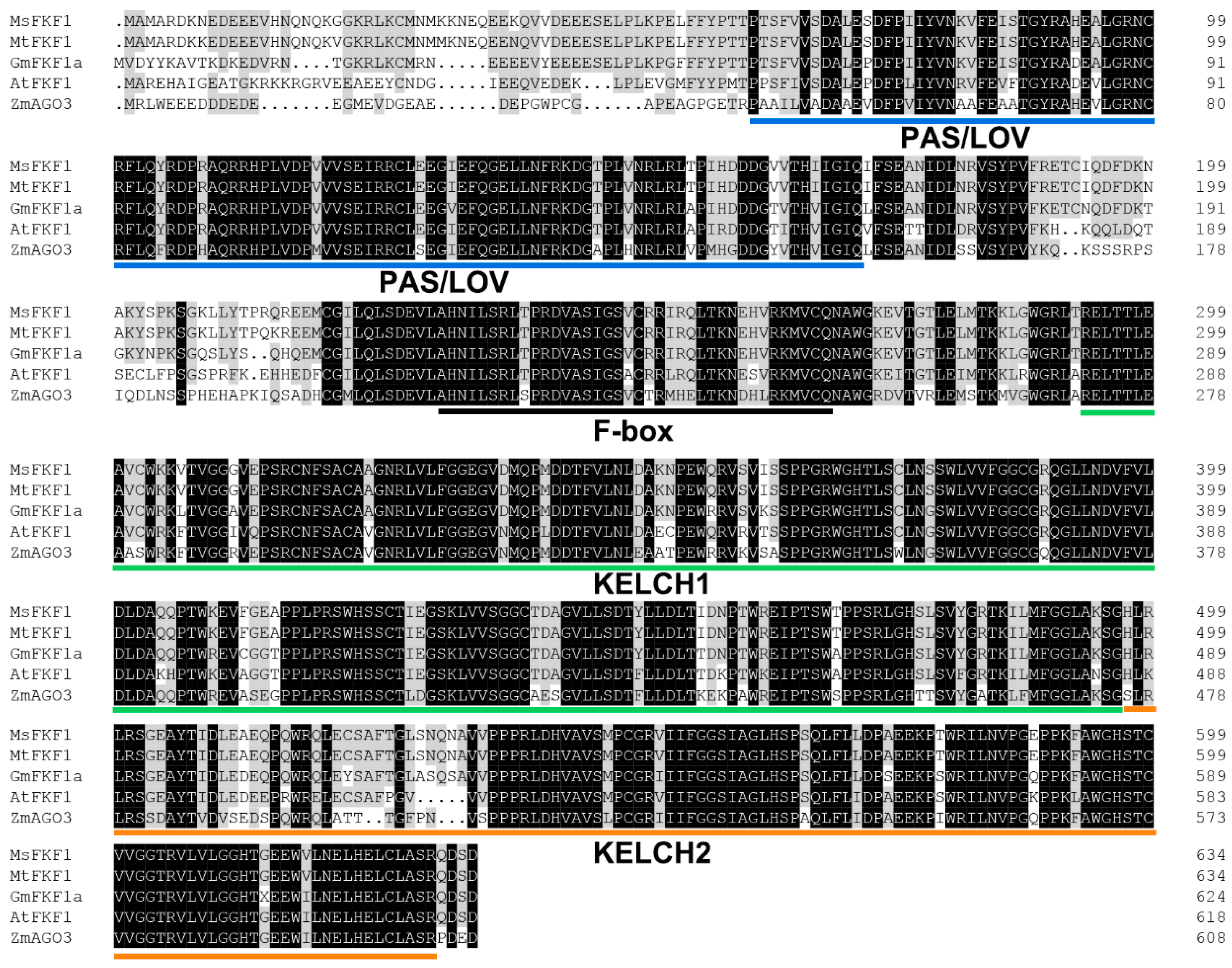
2.1. MsFKF1 Encodes a Putative Photoreceptor Protein That Shares High Sequence Similarity with FKF1 Orthologs
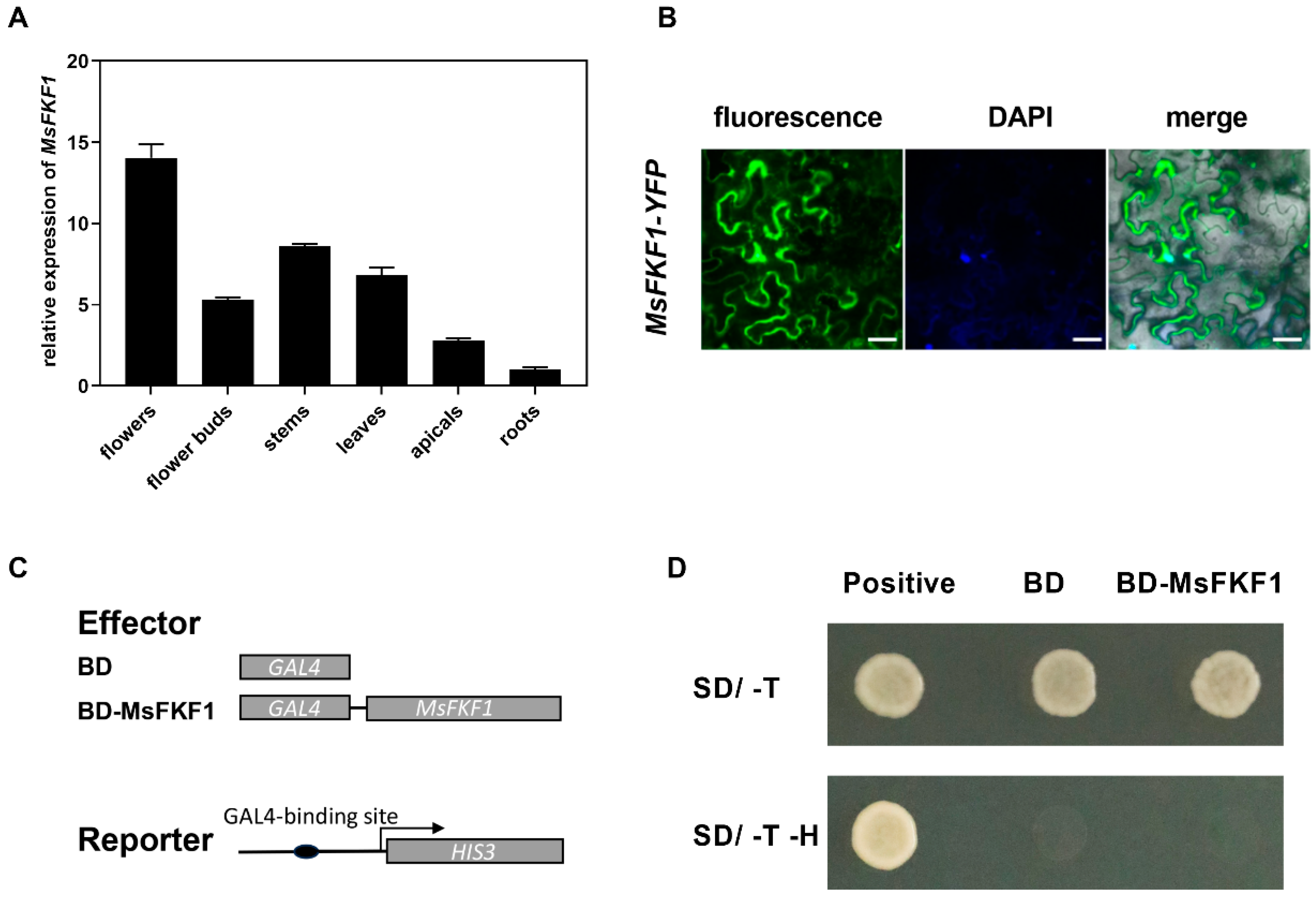
2.2. Expression Analysis of MsFKF1 in Alfalfa Tissues and Mainly Located in the Nucleus
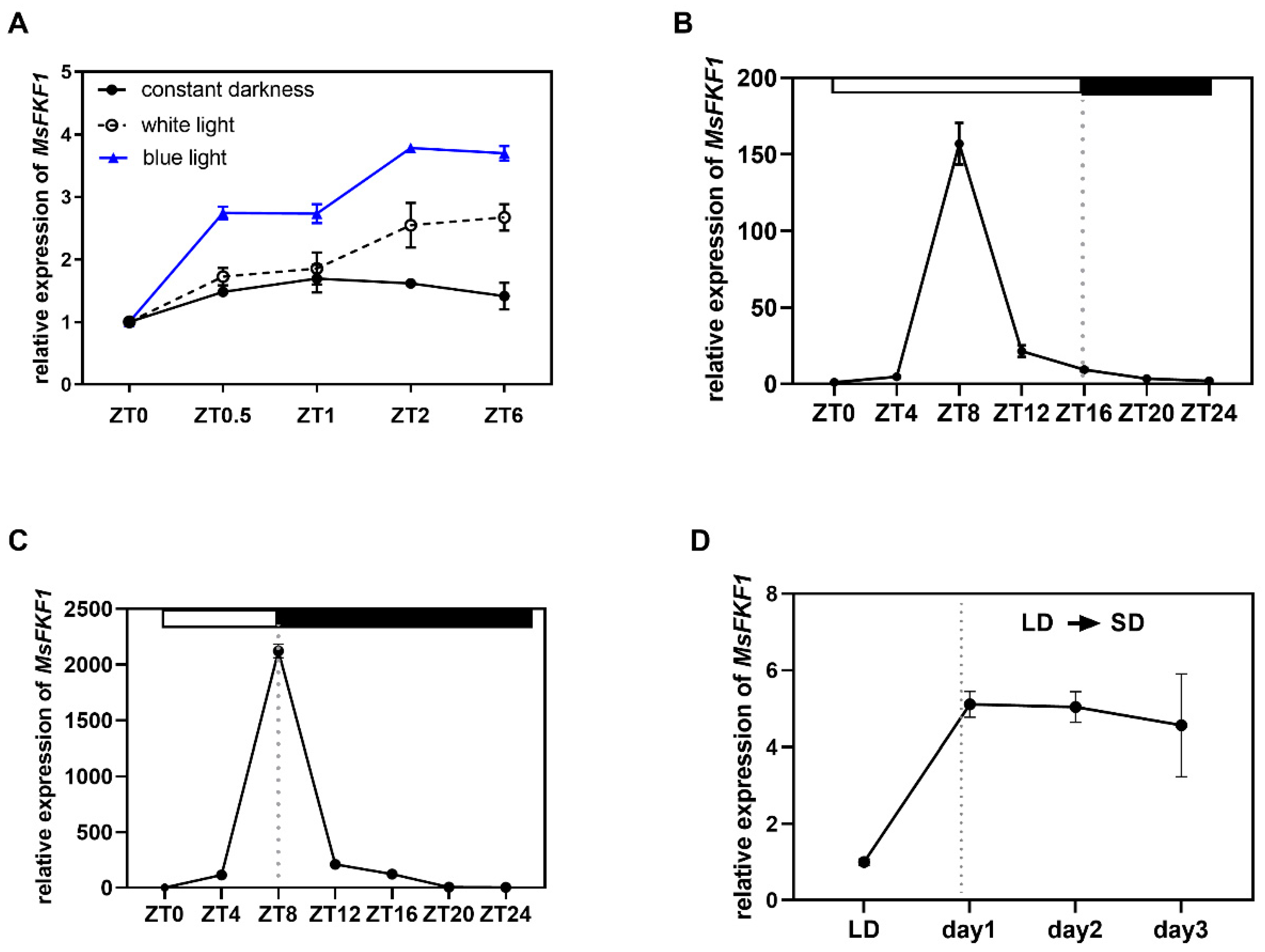
2.3. MsFKF1 Response to Light and Showed a Circadian Rhythm Expression Pattern
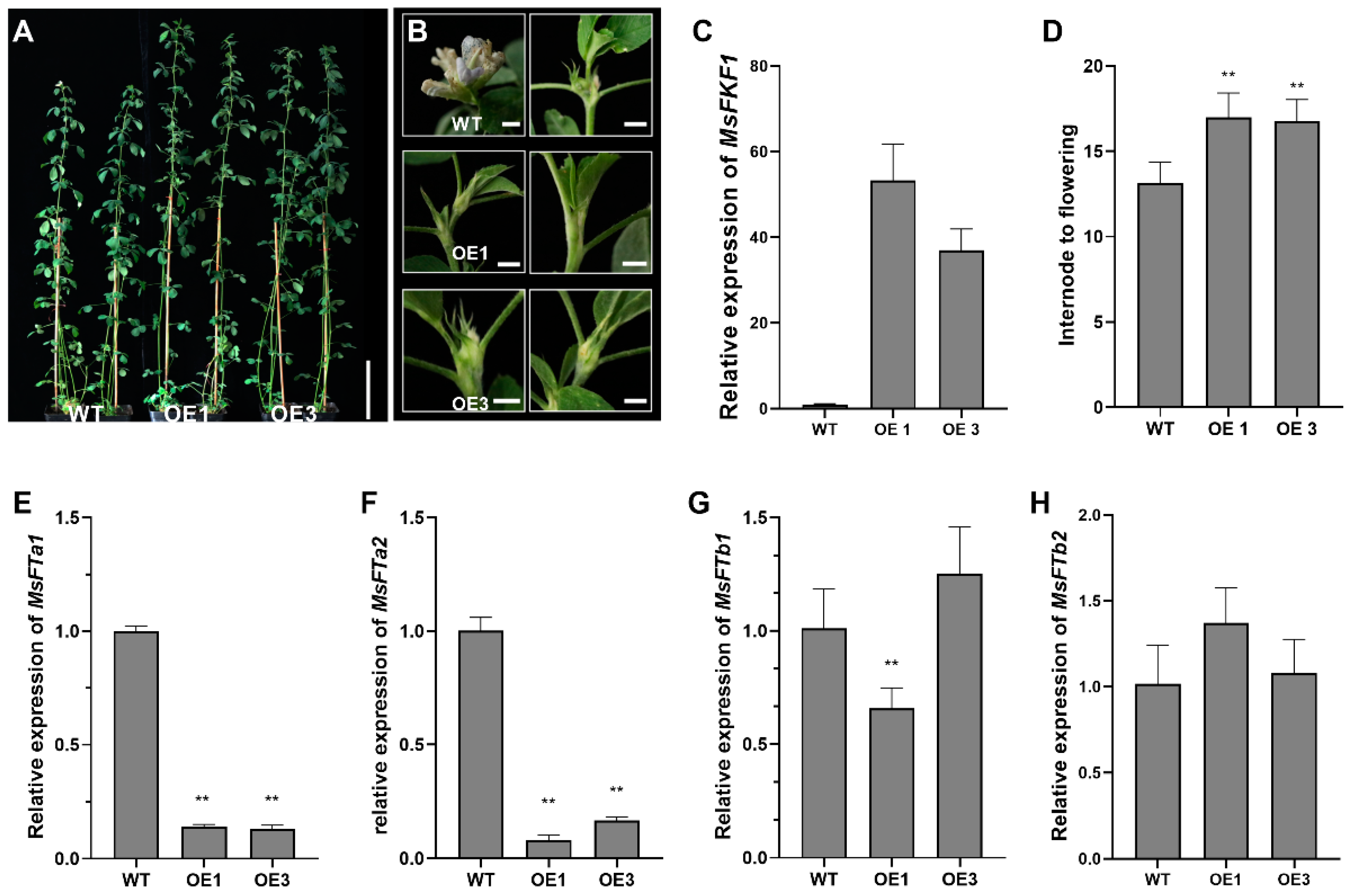
2.4. Overexpression of MsFKF1 Results in Delayed Flowering in M. sativa
2.5. MsFKF1 Mediated the Expression of Photoperiod Pathway Genes
2.6. MsFKF1 Increases Plant Height and Affects Forage Digestibility
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. RT-qPCR
4.3. Isolation and Phylogenetic Analysis of Alfalfa MsFKF1 Homologs
4.4. Plasmid Construction and Transgenic Alfalfa Generation
4.5. Plant and Flowering Time Assays with Alfalfa
4.6. Subcellular Localization and Transactive Assay
4.7. Determination of the Forage Quality Assays
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wolabu, T.W.; Mahmood, K.; Jerez, I.T.; Cong, L.; Yun, J.; Udvardi, M.; Tadege, M.; Wang, Z.; Wen, J. Multiplex CRISPR/Cas9-mediated mutagenesis of alfalfa FLOWERING LOCUS Ta1 (MsFTa1) leads to delayed flowering time with improved forage biomass yield and quality. Plant Biotechnol. J. 2023, 21, 1383–1392. [Google Scholar] [CrossRef]
- Bao, Q.; Wolabu, T.W.; Zhang, Q.; Zhang, T.; Liu, Z.; Sun, J.; Wang, Z.-Y. Application of CRISPR/Cas9 technology in forages. Grassland Res. 2022, 1, 244–251. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, Y.; Yang, Y.; Huang, L.; Tang, B.; Zhang, H.; Hao, F.; Liu, W.; Li, Y.; Liu, Y.; et al. Allele-aware chromosome-level genome assembly and efficient transgene-free genome editing for the autotetraploid cultivated alfalfa. Nat. Commun. 2020, 11, 2494. [Google Scholar] [CrossRef]
- Lorenzo, C.D.; García-Gagliardi, P.; Antonietti, M.S.; Sánchez-Lamas, M.; Mancini, E.; Dezar, C.A.; Vazquez, M.; Watson, G.; Yanovsky, M.J.; Cerdán, P.D. Improvement of alfalfa forage quality and management through the down-regulation of MsFTa1. Plant Biotechnol. J. 2020, 18, 944–954. [Google Scholar] [CrossRef]
- Zhou, C.; Han, L.; Pislariu, C.; Nakashima, J.; Fu, C.; Jiang, Q.; Quan, L.; Blancaflor, E.B.; Tang, Y.; Bouton, J.H.; et al. From model to crop: Functional analysis of a STAY-GREEN gene in the model legume Medicago truncatula and effective use of the gene for alfalfa improvement. Plant Physiol. 2011, 157, 1483–1496. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, T.; Guo, T.; Ding, W.; Long, R.; Yang, Q.; Wang, Z. Isolation and Functional Characterization of MsFTa, a Flowering Locus T Homolog from Alfalfa (Medicago sativa). Int. J. Mol. Sci. 2019, 20, 1968. [Google Scholar] [CrossRef]
- Wang, Y.; Chantreau, M.; Sibout, R.; Hawkins, S. Plant cell wall lignification and monolignol metabolism. Front. Plant Sci. 2013, 4, 220. [Google Scholar] [CrossRef] [PubMed]
- Kalu, B.A.; Fick, G.W. Morphological Stage of Development as a Predictor of Alfalfa Herbage Quality. Crop Sci. 1983, 23, 1167–1172. [Google Scholar] [CrossRef]
- Gao, R.; Feyissa, B.A.; Croft, M.; Hannoufa, A. Gene editing by CRISPR/Cas9 in the obligatory outcrossing Medicago sativa. Planta 2018, 247, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Freytes, S.N.; Canelo, M.; Cerdán, P.D. Regulation of Flowering Time: When and Where? Curr. Opin. Plant Biol. 2021, 63, 102049. [Google Scholar] [CrossRef] [PubMed]
- Devlin, P.F.; Kay, S.A. Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity. Plant Cell 2000, 12, 2499–2510. [Google Scholar] [CrossRef]
- Lin, C. Blue light receptors and signal transduction. Plant Cell 2002, 14, S207–S225. [Google Scholar] [CrossRef]
- Nagy, F.; Schäfer, E. Phytochromes control photomorphogenesis by differentially regulated, interacting signaling pathways in higher plants. Annu. Rev. Plant Biol. 2002, 53, 329–355. [Google Scholar] [CrossRef]
- Nelson, D.C.; Lasswell, J.; Rogg, L.E.; Cohen, M.A.; Bartel, B. FKF1, a clock-controlled gene that regulates the transition to flowering in Arabidopsis. Cell 2000, 101, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Zoltowski, B.D.; Imaizumi, T. Structure and Function of the ZTL/FKF1/LKP2 Group Proteins in Arabidopsis. Enzymes 2014, 35, 213–239. [Google Scholar] [PubMed]
- Suetsugu, N.; Wada, M. Evolution of Three LOV Blue Light Receptor Families in Green Plants and Photosynthetic Stramenopiles: Phototropin, ZTL/FKF1/LKP2 and Aureochrome. Plant Cell Physiol. 2013, 54, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, Y.; Zikihara, K.; Tokutomi, S.; Terazima, M. Kinetics of Conformational Changes of the FKF1-LOV Domain upon Photoexcitation. Biophys. J. 2010, 99, 3831–3839. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Song, Y.H.; Imaizumi, T. LOV Domain-Containing F-Box Proteins: Light-Dependent Protein Degradation Modules in Arabidopsis. Mol. Plant 2012, 5, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; González-Guzmán, M.; Serrano, R.; Rodríguez, P.L. A combination of the F-box motif and kelch repeats defines a large Arabidopsis family of F-box proteins. Plant Mol. Biol. 2001, 46, 603–614. [Google Scholar] [CrossRef]
- Imaizumi, T.; Tran, H.G.; Swartz, T.E.; Briggs, W.R.; Kay, S.A. FKF1 is essential for photoperiodic-specific light signalling in Arabidopsis. Nature 2003, 426, 302–306. [Google Scholar] [CrossRef]
- Han, S.H.; Yoo, S.C.; Lee, B.D.; An, G.; Paek, N.C. Rice Flavin-Binding, Kelch Repeat, F-BOX 1 (OsFKF1) promotes flowering independent of photoperiod. Plant Cell Environ. 2015, 38, 2527–2540. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Du, H.P.; He, M.L.; Wang, J.H.; Wang, F.; Yuan, W.J.; Huang, Z.R.; Cheng, Q.; Gou, C.J.; Chen, Z.; et al. Natural variation of FKF1 controls flowering and adaptation during soybean domestication and improvement. New Phytol. 2023, 238, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, X.; Hu, R.; Wu, F.; Ma, J.; Meng, Y.; Fu, Y. Identification and molecular characterization of FKF1 and GI homologous genes in soybean. PLoS ONE 2013, 8, e79036. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, T.; Nishiyama, M.; Kato, K.; Kanayama, Y. Characterization of the FLAVIN-BINDING, KELCH REPEAT, F-BOX 1 Homolog SlFKF1 in Tomato as a Model for Plants with Fleshy Fruit. Int. J. Mol. Sci. 2021, 22, 1735. [Google Scholar] [CrossRef]
- Huang, F.N.; Fu, Z.Y.; Zeng, L.H.; Morley-Bunker, M. Isolation and characterization of GI and FKF1 homologous genes in the subtropical fruit tree Dimocarpus longan. Mol. Breed. 2017, 37, 90. [Google Scholar] [CrossRef]
- Ding, J.; Böhlenius, H.; Rühl, M.G.; Chen, P.; Sane, S.; Zambrano, J.A.; Zheng, B.; Eriksson, M.E.; Nilsson, O. GIGANTEA-like genes control seasonal growth cessation in Populus. New Phytol. 2018, 218, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Andrés, F.; Coupland, G. The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 2012, 13, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Lv, X.; Liu, R.; He, H.; Liang, S.; Chen, L.; Zhang, F.; Chen, L.; He, Y.; Du, J. Molecular basis of CONSTANS oligomerization in FLOWERING LOCUS T activation. J. Integr. Plant Biol. 2022, 64, 731–740. [Google Scholar] [CrossRef]
- Fornara, F.; Panigrahi, K.C.; Gissot, L.; Sauerbrunn, N.; Rühl, M.; Jarillo, J.A.; Coupland, G. Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. Dev Cell. 2009, 17, 75–86. [Google Scholar] [CrossRef]
- Song, Y.H.; Smith, R.W.; To, B.J.; Millar, A.J.; Imaizumi, T. FKF1 conveys timing information for CONSTANS stabilization in photoperiodic flowering. Science 2012, 336, 1045–1049. [Google Scholar] [CrossRef]
- Imaizumi, T.; Schultz, T.F.; Harmon, F.G.; Ho, L.A.; Kay, S.A. FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science 2005, 309, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Sawa, M.; Nusinow, D.A.; Kay, S.A.; Imaizumi, T. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 2007, 318, 261–265. [Google Scholar] [CrossRef]
- Lee, B.D.; Kim, M.R.; Kang, M.Y.; Cha, J.Y.; Han, S.H.; Nawkar, G.M.; Sakuraba, Y.; Lee, S.Y.; Imaizumi, T.; McClung, C.R.; et al. The F-box protein FKF1 inhibits dimerization of COP1 in the control of photoperiodic flowering. Nat. Commun. 2017, 8, 2259. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.D.; Cha, J.Y.; Kim, M.R.; Shin, G.I.; Paek, N.C.; Kim, W.Y. Light-dependent suppression of COP1 multimeric complex formation is determined by the blue-light receptor FKF1 in Arabidopsis. Biochem. Biophys. Res. Commun. 2019, 508, 191–197. [Google Scholar] [CrossRef]
- Hecht, V.; Laurie, R.E.; Vander Schoor, J.K.; Ridge, S.; Knowles, C.L.; Liew, L.C.; Sussmilch, F.C.; Murfet, I.C.; Macknight, R.C.; Weller, J.L. The pea GIGAS gene is a FLOWERING LOCUS T homolog necessary for graft-transmissible specification of flowering but not for responsiveness to photoperiod. Plant Cell 2011, 23, 147–161. [Google Scholar] [CrossRef]
- Laurie, R.E.; Diwadkar, P.; Jaudal, M.; Zhang, L.; Hecht, V.; Wen, J.; Tadege, M.; Mysore, K.S.; Putterill, J.; Weller, J.L.; et al. The Medicago FLOWERING LOCUS T homolog, MtFTa1, is a key regulator of flowering time. Plant Physiol. 2011, 156, 2207–2224. [Google Scholar] [CrossRef]
- Wong, A.C.; Hecht, V.F.; Picard, K.; Diwadkar, P.; Laurie, R.E.; Wen, J.; Mysore, K.; Macknight, R.C.; Weller, J.L. Isolation and functional analysis of CONSTANS-LIKE genes suggests that a central role for CONSTANS in flowering time control is not evolutionarily conserved in Medicago truncatula. Front Plant Sci. 2014, 5, 486. [Google Scholar] [CrossRef]
- Zhang, X.; Zhai, H.; Wang, Y.; Tian, X.; Zhang, Y.; Wu, H.; Lü, S.; Yang, G.; Li, Y.; Wang, L.; et al. Functional conservation and diversification of the soybean maturity gene E1 and its homologs in legumes. Sci. Rep. 2016, 6, 29548. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Lu, S.; Wang, K.; Dong, L.; Li, S.; Xie, Q.; Xu, X.; Cheng, Q.; Chen, L.; Fang, C.; et al. A critical role of the soybean evening complex in the control of photoperiod sensitivity and adaptation. Proc. Natl. Acad. Sci. USA 2021, 118, e2010241118. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.D.; Li, X.M.; Zeng, B.J.; Zhong, M.; Yang, J.X.; Yang, P.; Li, X.; He, C.S.; Lin, J.Z.; Liu, X.M.; et al. FKF1 F-box protein promotes flowering in part by negatively regulating DELLA protein stability under long-day photoperiod inArabidopsis. J. Integr. Plant Biol. 2020, 62, 1717–1740. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Coupland, G. ZEITLUPE and FKF1: Novel connections between flowering time and circadian clock control. Trends Plant Sci. 2000, 5, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Baudry, A.; Ito, S.; Song, Y.H.; Strait, A.A.; Kiba, T.; Lu, S.; Henriques, R.; Pruneda-Paz, J.L.; Chua, N.H.; Tobin, E.M.; et al. F-Box Proteins FKF1 and LKP2 Act in Concert with ZEITLUPE to Control Arabidopsis Clock Progression. Plant Cell 2010, 22, 606–622. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Han, L.; Liu, X.; Wang, H.; Wen, L.; Yu, X.; Xu, X.; Kong, F.; Fu, C.; Mysore, K.S.; et al. The nodulation and nyctinastic leaf movement is orchestrated by clock gene LHY in Medicago truncatula. J. Integr. Plant Biol. 2020, 62, 1880–1895. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Fang, C.; Cheng, Q.; Su, T.; Kou, K.; Kong, L.; Zhang, C.; Li, H.; Hou, Z.; Zhang, Y.; et al. Genetic basis and adaptation trajectory of soybean from its temperate origin to tropics. Nat. Commun. 2021, 12, 5445. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, N.; Jiang, B.; Li, M.; Wang, H.; Jiang, Z.; Pan, H.; Xia, Q.; Ma, Q.; Han, T.; et al. A Single Nucleotide Deletion in J Encoding GmELF3 Confers Long Juvenility and Is Associated with Adaption of Tropic Soybean. Mol. Plant 2017, 10, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Dong, L.; Fang, C.; Liu, S.; Kong, L.; Cheng, Q.; Chen, L.; Su, T.; Nan, H.; Zhang, D.; et al. Stepwise selection on homeologous PRR genes controlling flowering and maturity during soybean domestication. Nat. Genet. 2020, 52, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, A.; Thomson, G.; Kerr-Phillips, M.; Phan, C.; Krueger, T.; Jaudal, M.; Wen, J.; Mysore, K.S.; Putterill, J. Overexpression of Medicago MtCDFd1_1 Causes Delayed Flowering in Medicago via Repression of MtFTa1 but Not MtCO-Like Genes. Front. Plant Sci. 2019, 10, 1148. [Google Scholar] [CrossRef]
- Li, W.; Ma, Q.; Yin, P.; Wen, J.; Pei, Y.; Niu, L.; Lin, H. The GA 20-Oxidase Encoding Gene MSD1 Controls the Main Stem Elongation in Medicago truncatula. Front. Plant Sci. 2021, 12, 709625. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Ban, L.; Wu, Y.; Wu, X.; Wang, Y.; Wen, H.; Chapurin, V.; Dzyubenko, N.; Li, Z.; et al. Functional characterization of a gibberellin receptor and its application in alfalfa biomass improvement. Sci. Rep. 2017, 7, 41296. [Google Scholar] [CrossRef]
- Sun, T.P.; Gubler, F. Molecular mechanism of gibberellin signaling in plants. Annu. Rev. Plant Biol. 2004, 55, 197–223. [Google Scholar] [CrossRef]
- Long, R.; Zhang, F.; Zhang, Z.; Li, M.; Chen, L.; Wang, X.; Liu, W.; Zhang, T.; Yu, L.X.; He, F.; et al. Genome Assembly of Alfalfa Cultivar Zhongmu-4 and Identification of SNPs Associated with Agronomic Traits. Genom. Proteom. Bioinform. 2022, 20, 14–28. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Weigel, D.; Glazebrook, J. Transformation of agrobacterium using the freeze-thaw method. CSH Protoc. 2006, 2006, pdb.prot4666. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Fu, C.; Wang, Z.Y. A Unified Agrobacterium-Mediated Transformation Protocol for Alfalfa (Medicago sativa L.) and Medicago truncatula. Methods Mol. Biol. 2019, 1864, 153–163. [Google Scholar] [PubMed]
- Jiang, X.; Cui, H.; Wang, Z.; Kang, J.; Yang, Q.; Guo, C. Genome-Wide Analysis of the LATERAL ORGAN BOUNDARIES Domain (LBD) Members in Alfalfa and the Involvement of MsLBD48 in Nitrogen Assimilation. Int. J. Mol. Sci. 2023, 24, 4644. [Google Scholar] [CrossRef] [PubMed]


| Genotype | Plant Height (cm) | Stem NO. | Fresh Weight (g) | % ADF | % NDF | % Lignin | % Digestibility |
|---|---|---|---|---|---|---|---|
| WT | 54.70 ± 9.66 | 21.60 ± 2.19 | 4.74 ± 0.62 | 38.01 ± 0.16 | 51.40 ± 0.18 | 7.94 ± 0.03 | 74.52 ± 0.21 |
| OE1 | 62.31 ± 6.89 ** | 21.29 ± 2.06 | 5.56 ± 1.79 ** | 41.76 ± 0.31 ** | 56.83 ± 0.24 ** | 8.68 ± 0.09 ** | 70.76 ± 0.31 ** |
| OE3 | 61.35 ± 6.03 ** | 21.38 ± 1.51 | 5.92 ± 0.78 ** | 42.96 ± 0.07 ** | 57.23 ± 0.12 ** | 8.69 ± 0.02 ** | 69.81 ± 0.11 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Zhang, L.; Li, Y.; Long, R.; Yang, Q.; Kang, J. Functional Characterization of the MsFKF1 Gene Reveals Its Dual Role in Regulating the Flowering Time and Plant Height in Medicago sativa L. Plants 2024, 13, 655. https://doi.org/10.3390/plants13050655
Jiang X, Zhang L, Li Y, Long R, Yang Q, Kang J. Functional Characterization of the MsFKF1 Gene Reveals Its Dual Role in Regulating the Flowering Time and Plant Height in Medicago sativa L. Plants. 2024; 13(5):655. https://doi.org/10.3390/plants13050655
Chicago/Turabian StyleJiang, Xu, Lili Zhang, Yajing Li, Ruicai Long, Qingchuan Yang, and Junmei Kang. 2024. "Functional Characterization of the MsFKF1 Gene Reveals Its Dual Role in Regulating the Flowering Time and Plant Height in Medicago sativa L." Plants 13, no. 5: 655. https://doi.org/10.3390/plants13050655




