Resistance to Frankliniella occidentalis during Different Plant Life Stages and under Different Environmental Conditions in the Ornamental Gladiolus
Abstract
:1. Introduction
- Do Robinetta and Charming Beauty show consistent differences in WFT resistance over all development stages?
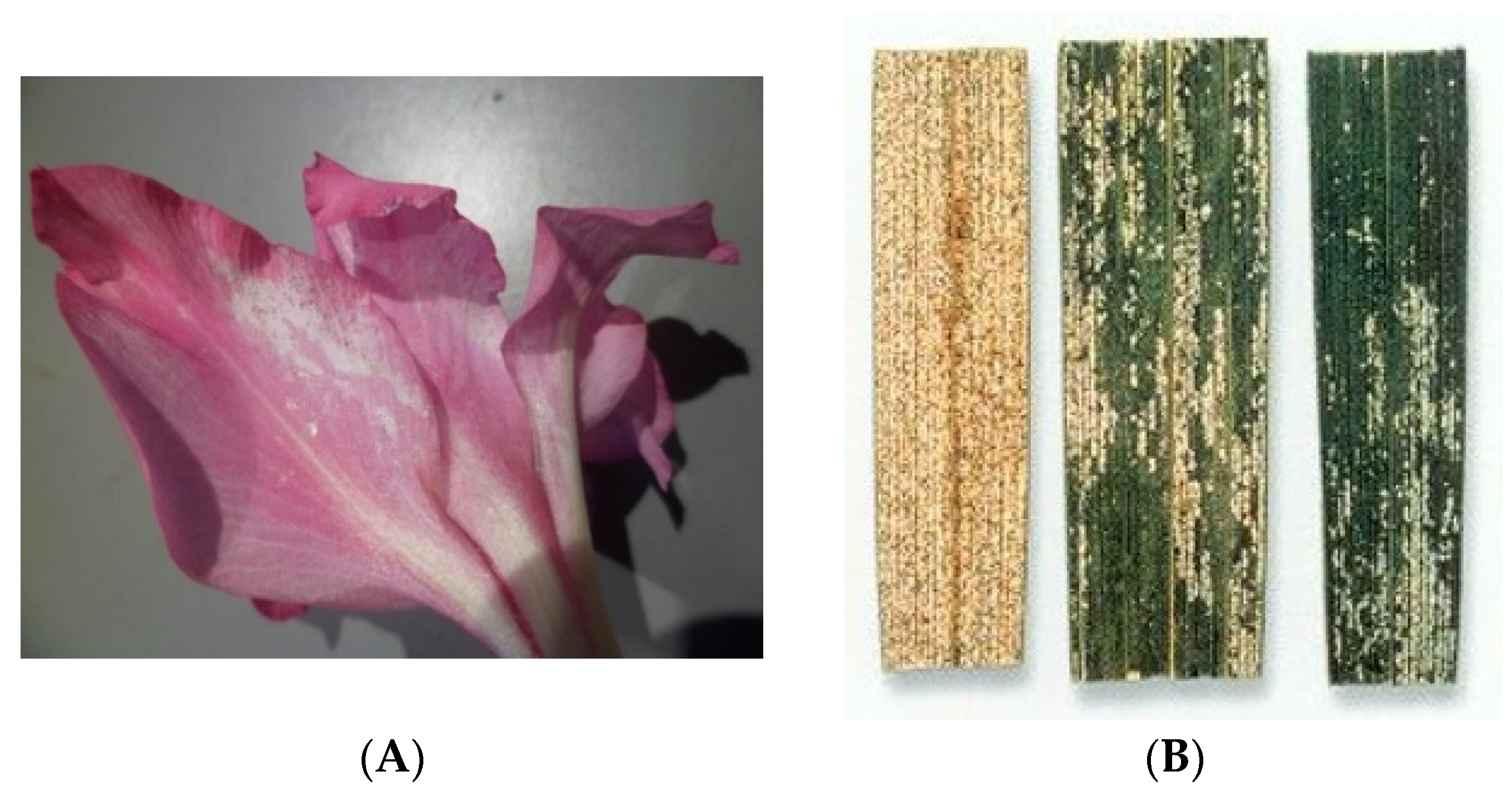
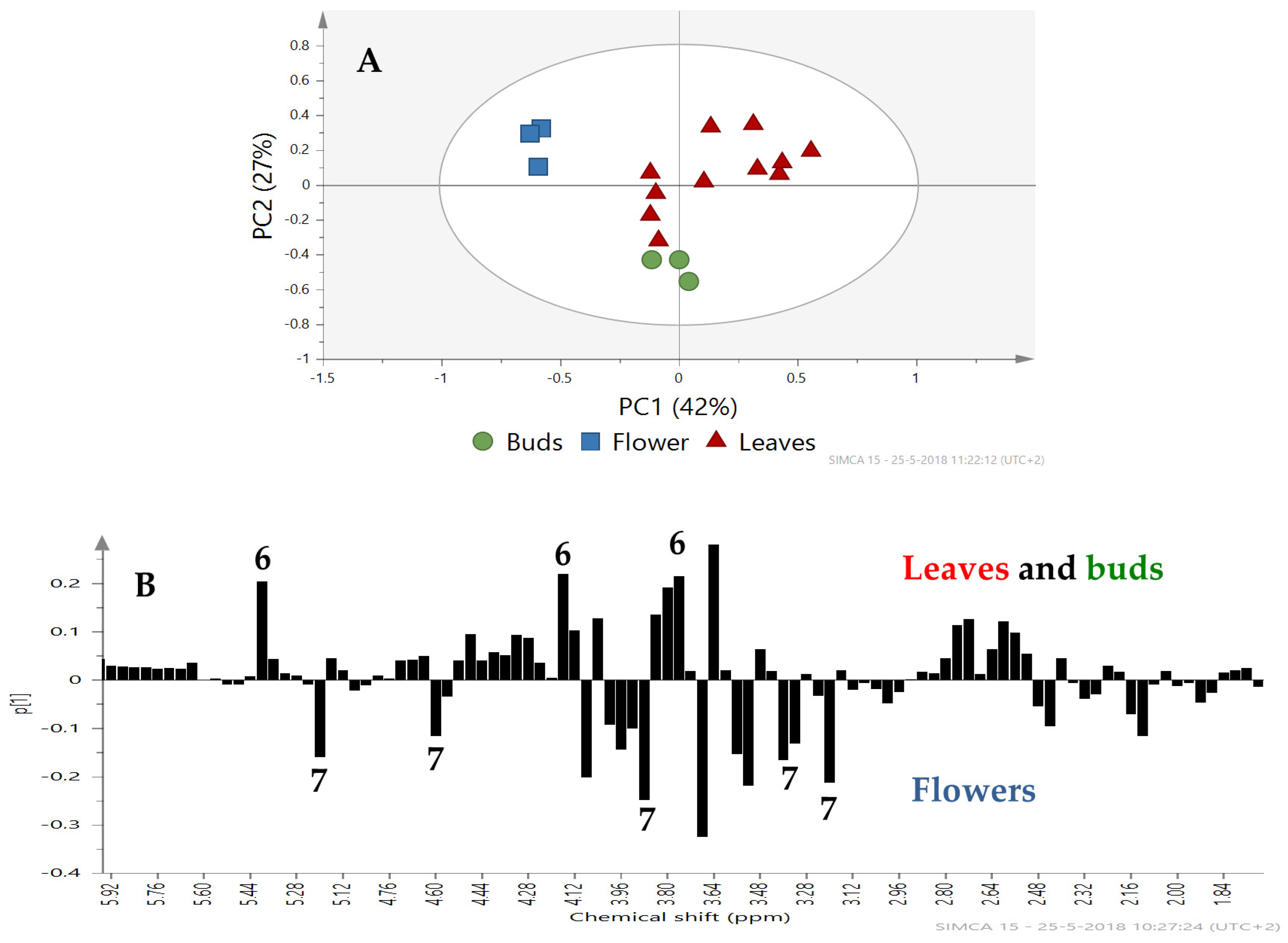
- Does WFT damage differ between plant organs?
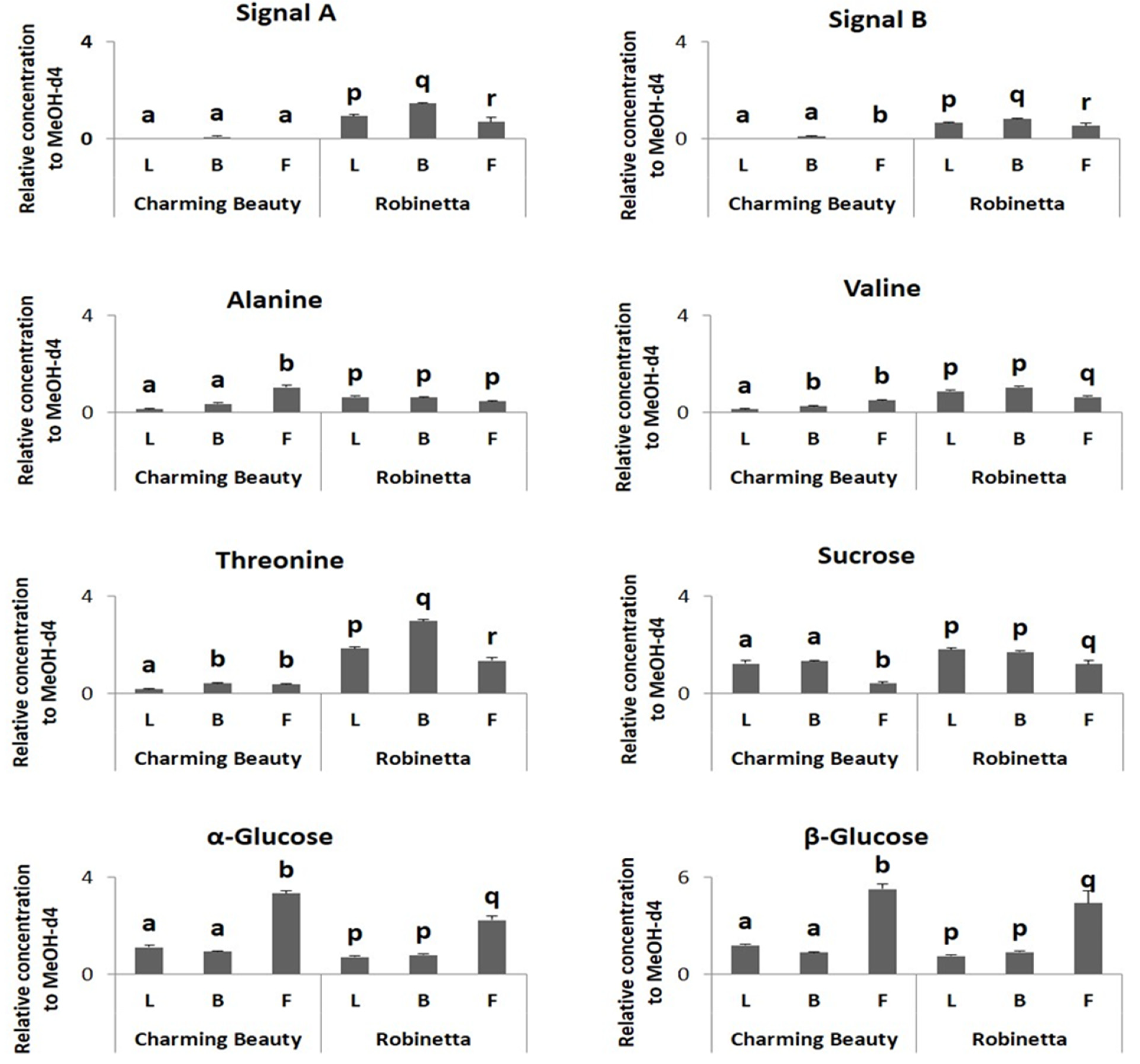
- Does WFT damage to leaves differ among plant development stages?
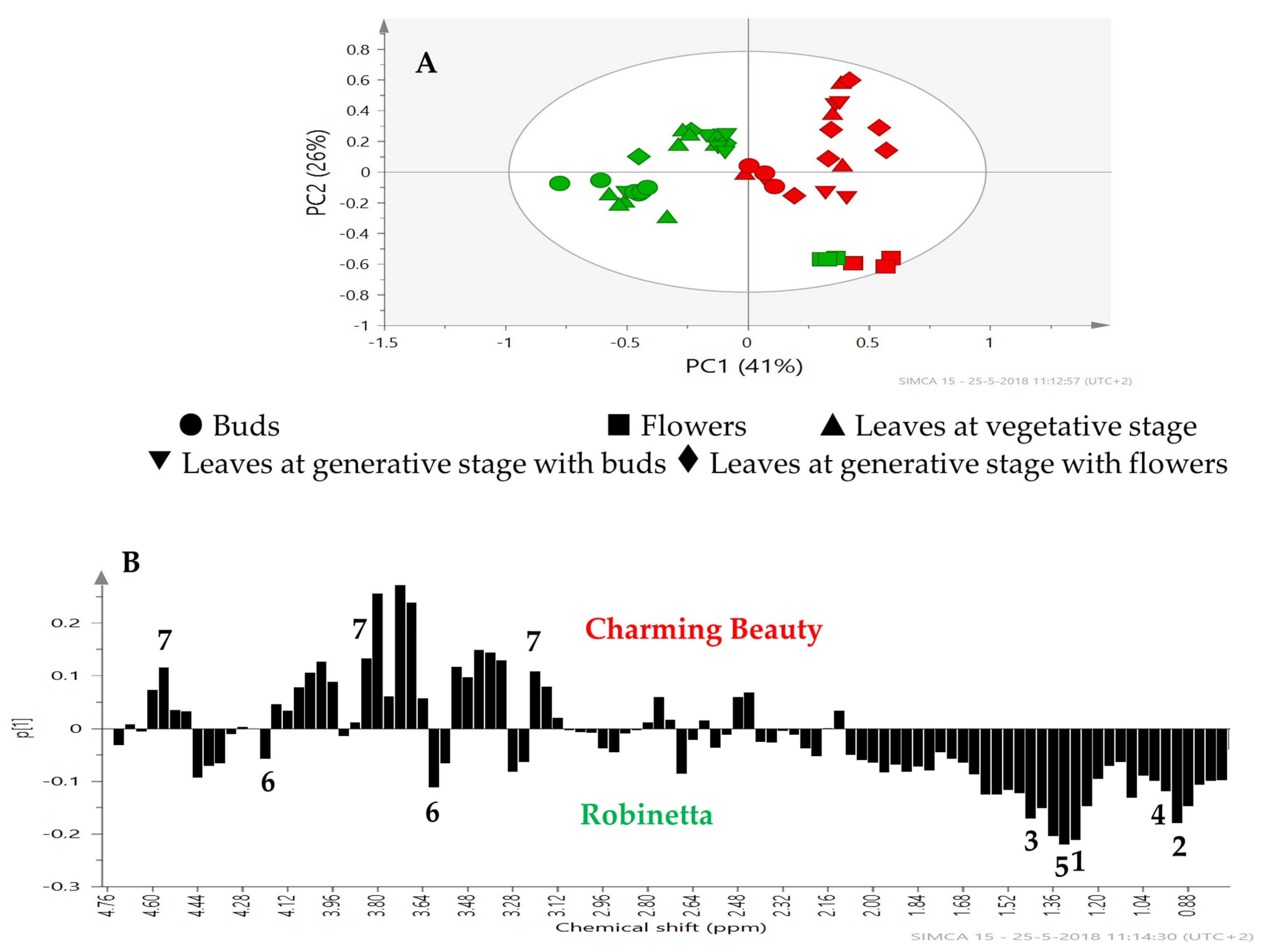
- Are differences between the metabolomic profiles of Robinetta and Charming Beauty consistent across developmental stages?
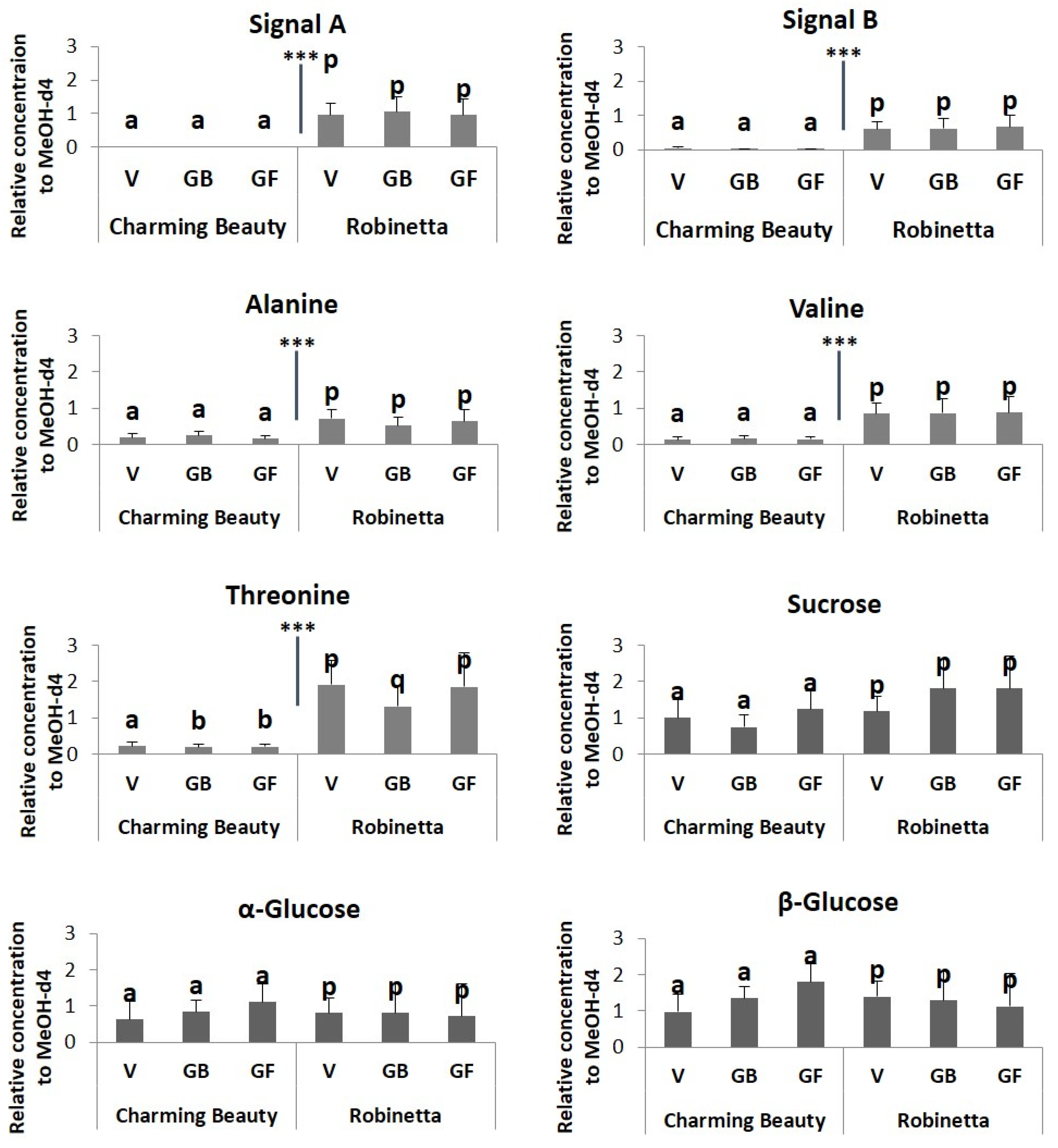
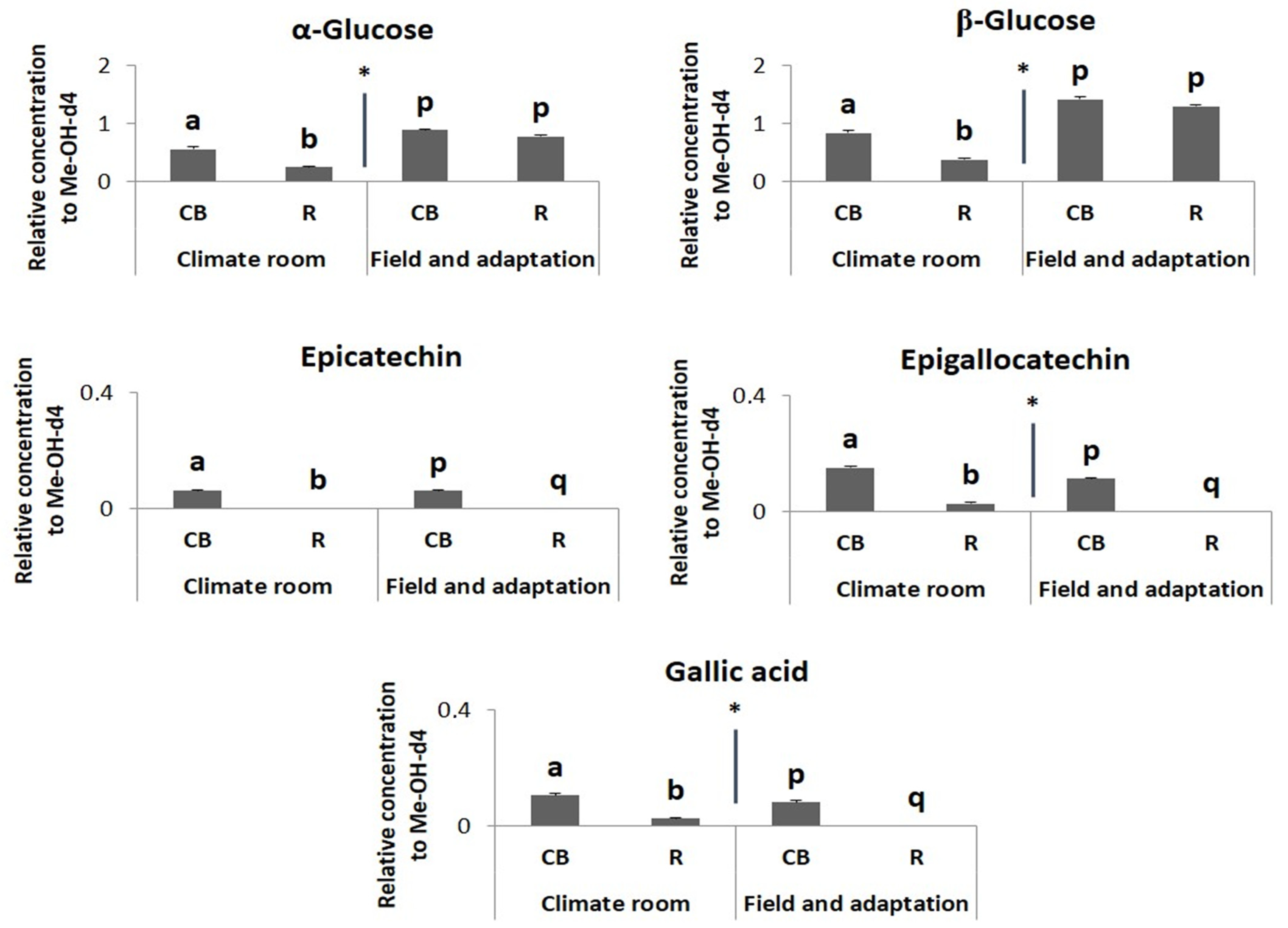
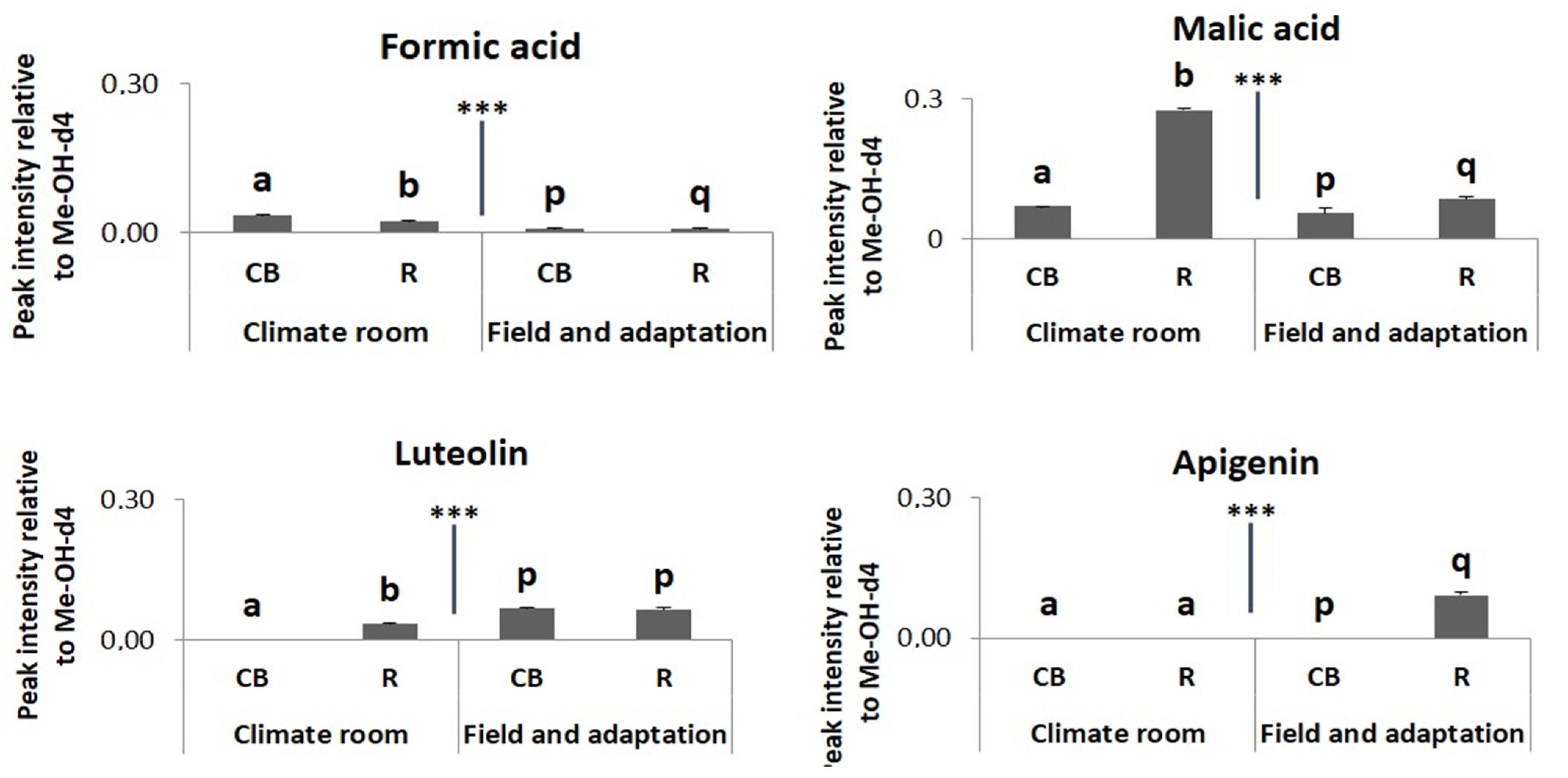
- Do the concentrations of defence compounds related to WFT resistance differ among plant organs?
- Do the concentrations of compounds that were related to WFT resistance alter with the development stages of the plant?
- Are the metabolic profiles of the plants dependent on the environmental conditions?
- And if so: Is there a change in the concentration of compounds related to thrips resistance?
2. Results
2.1. Thrips Whole Plant Bioassay
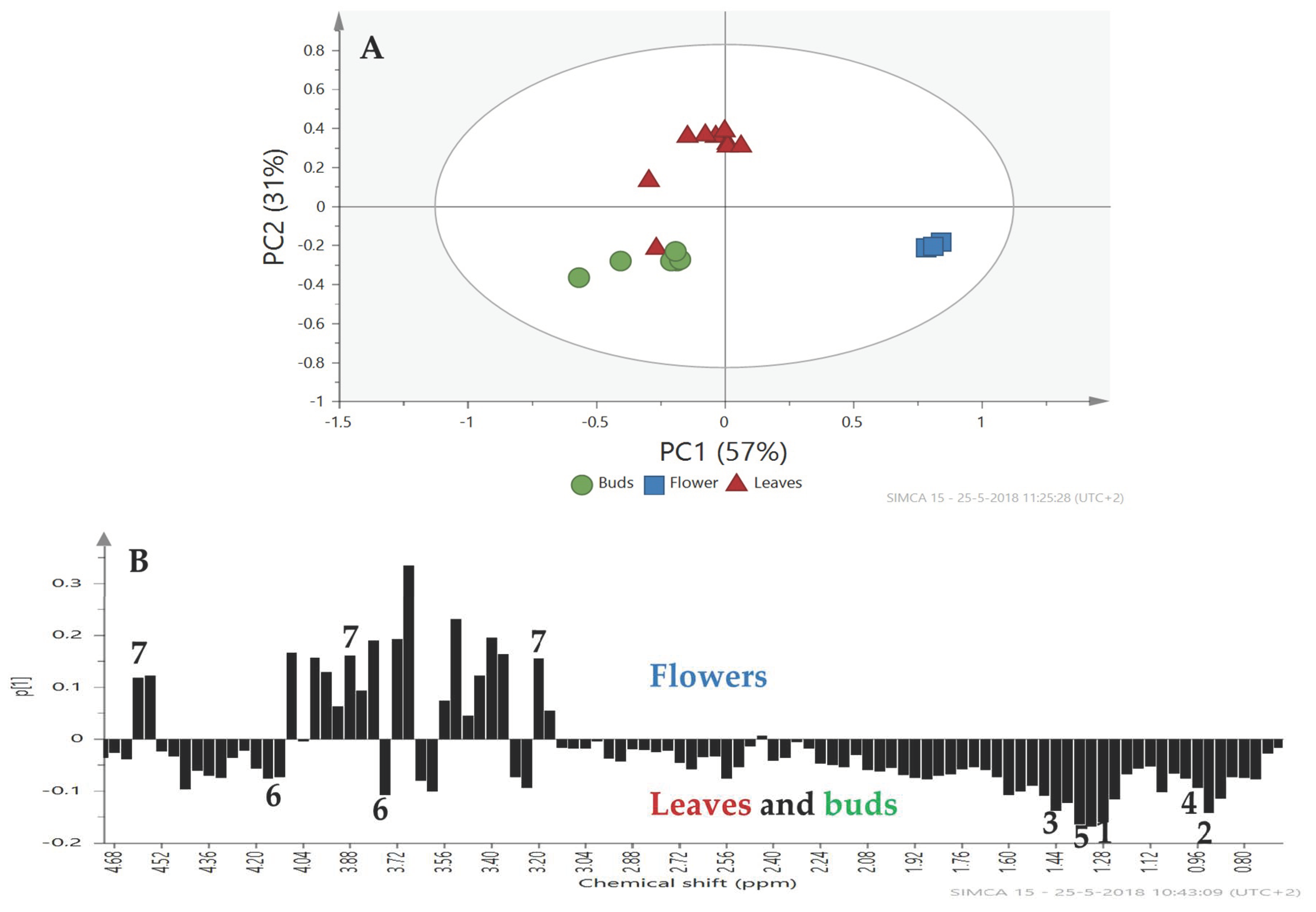
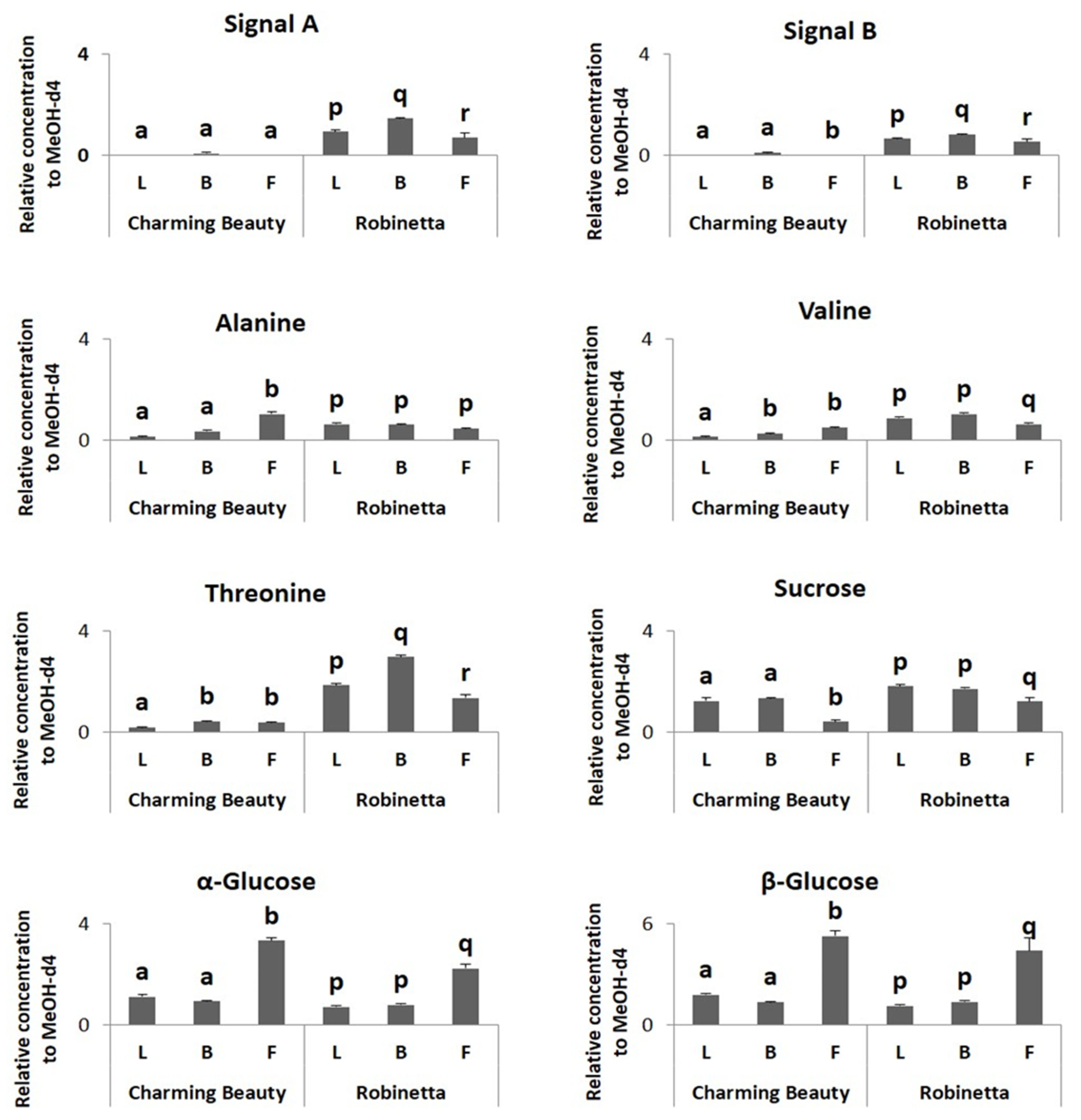
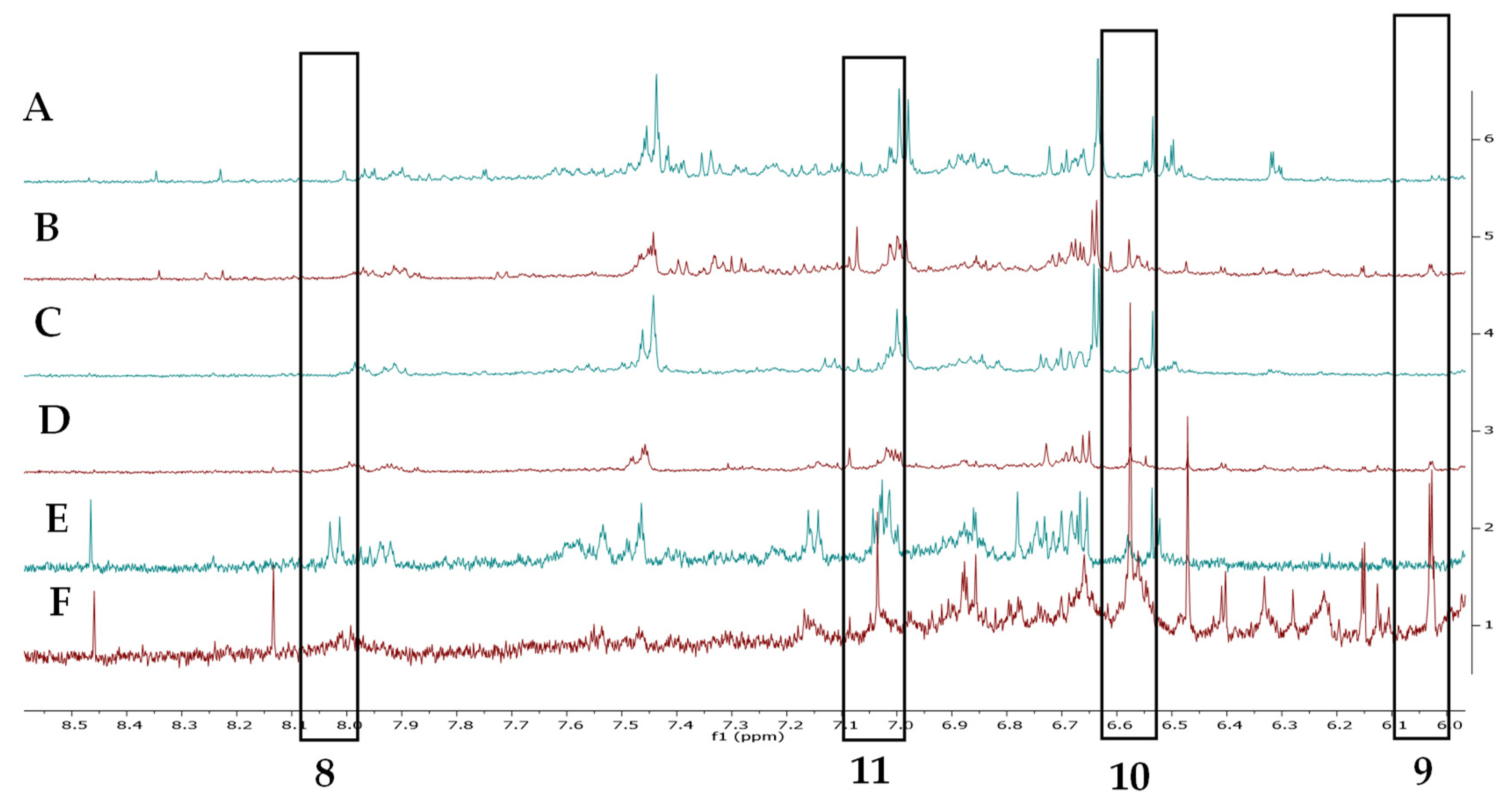
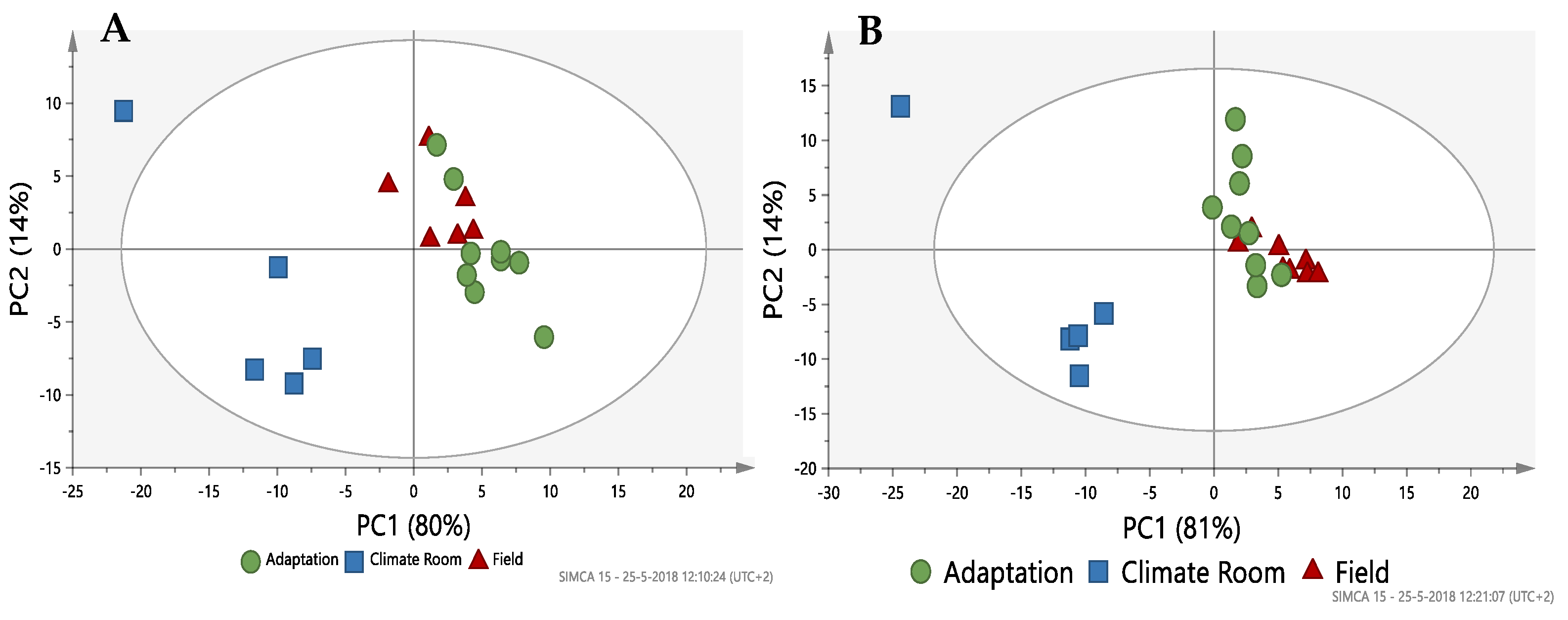
2.2. Metabolomic Profiling
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Plant Development Stages
4.3. Different Environmental Conditions
4.4. Thrips Whole Plant Bioassay
4.5. Metabolomic Profiling
4.5.1. Extraction of Plant Materials for NMR Metabolomics
4.5.2. NMR Analysis
4.5.3. Data Processing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szakiel, A.; Pączkowski, C.; Henry, M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem. Rev. 2011, 10, 471–491. [Google Scholar] [CrossRef]
- Jänkänpää, H.J.; Mishra, Y.; Schröder, W.P.; Jansson, S. Metabolic profiling reveals metabolic shifts in Arabidopsis plants grown under different light conditions. Plant Cell Environ. 2012, 35, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.U.; Prasannalaxmi, K. Water stress induced physiological and biochemical changes in Piper betle L. and Ricinus communis L. plants and their effects on Spodoptera litura. Allelopath. J. 2014, 33, 25–41. [Google Scholar]
- Boege, K.; Marquis, R.J. Facing herbivory as you grow up: The ontogeny of resistance in plants. Trends Ecol. Evol. 2005, 20, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.E.; Koricheva, J. The Ontogeny of Plant Defense and Herbivory: Characterizing General Patterns Using Meta-Analysis. Am. Nat. 2010, 175, 481–493. [Google Scholar] [CrossRef] [PubMed]
- De Jong, T.J.; Van Der Meijden, E. On the correlation between allocation to defence and regrowth in plants. Oikos 2000, 88, 503–508. [Google Scholar] [CrossRef]
- Sun, H.H.; Wang, L.; Zhang, B.Q.; Ma, J.H.; Hettenhausen, C.; Cao, G.Y.; Sun, G.; Wu, J.; Wu, J. Scopoletin is a phytoalexin against Alternaria alternata in wild tobacco dependent on jasmonate signalling. J. Exp. Bot. 2014, 65, 4305–4315. [Google Scholar] [CrossRef]
- Van Dam, N.M.; Horn, M.; Mareš, M.; Baldwin, I.T. Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. J. Chem. Ecol. 2001, 27, 547–568. [Google Scholar] [CrossRef]
- Damle, M.S.; Giri, A.P.; Sainani, M.N.; Gupta, V.S. Higher accumulation of proteinase inhibitors in flowers than leaves and fruits as a possible basis for differential feeding preference of Helicoverpa armigera on tomato (Lycopersicon esculentum Mill, Cv. Dhanashree). Phytochemistry 2005, 66, 2659–2667. [Google Scholar] [CrossRef]
- Ugine, T.A.; Sanderson, J.P.; Wraight, S.P. Within-plant and temporal distribution of nymphal and adult western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae), on flowers and foliage of greenhouse impatiens, Impatiens wallerana, and implications for pest population sampling. Environ. Entomol. 2006, 35, 507–515. [Google Scholar] [CrossRef]
- Kirk, W.D.J. Aggregation and mating of thrips in flowers of Calystegia sepium. Ecol. Entomol. 1985, 10, 433–440. [Google Scholar] [CrossRef]
- Mirnezhad, M.; Romero-González, R.R.; Leiss, K.A.; Choi, Y.H.; Verpoorte, R.; Klinkhamer, P.G.L. Metabolomic analysis of host plant resistance to thrips in wild and cultivated tomatoes. Phytochem. Anal. 2010, 21, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Leiss, K.A.; Choi, Y.H.; Abdel-Farid, I.B.; Verpoorte, R.; Klinkhamer, P.G.L. NMR metabolomics of thrips (Frankliniella occidentalis) resistance in Senecio hybrids. J. Chem. Ecol. 2009, 35, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Kirk, W.D.J.; Terry, L.I. The spread of the western flower thrips Frankliniella occidentalis (Pergande). Agric. For. Entomol. 2003, 5, 301–310. [Google Scholar] [CrossRef]
- Jensen, S.E. Insecticide resistance in the western flower thrips, Frankliniella occidentalis. Integr. Pest Manag. Rev. 2000, 5, 131–146. [Google Scholar] [CrossRef]
- Buitenhuis, R.; Shipp, J.L. Influence of plant species and plant growth stage on Frankliniella occidentalis pupation behaviour in greenhouse ornamentals. J. Appl. Entomol. 2008, 132, 86–88. [Google Scholar] [CrossRef]
- Ullman, D.E.; Sherwood, J.L.; German, T.L. Thrips as vectors of plant pathogens. In Thrips as Crop Pests; Cabi Publishing: Oxfordshire, UK, 1997; pp. 539–565. [Google Scholar]
- Wahyuni, D.S.C.; Young, H.C.; Leiss, K.; Klinkhamer, P.G.L. Morphological and Chemical Factors Related to Western Flower Thrips Resistance in the Ornamental Gladiolus. Plants 2021, 10, 3390. [Google Scholar] [CrossRef]
- Deckmyn, G.; Impens, I. The ratio UV-B/photosynthetically active radiation (PAR) determines the sensitivity of rye to increased UV-B radiation. Environ. Exp. Bot. 1997, 37, 3–12. [Google Scholar] [CrossRef]
- Muller, V.; Lankes, C.; Albert, A.; Winkler, J.B.; Zimmermann, B.F.; Noga, G.; Hunsche, M. Concentration of hinokinin, phenolic acids and flavonols in leaves and stems of Hydrocotyle leucocephala is differently influenced by PAR and ecologically relevant UV-B level. J. Plant Physiol. 2015, 173, 105–115. [Google Scholar] [CrossRef]
- Markham, K.R.; Ryan, K.G.; Bloor, S.J.; Mitchell, K.A. An increase in the luteolin: Apigenin ratio in Marchantia polymorpha on UV-B enhancement. Phytochemistry 1998, 48, 791–794. [Google Scholar] [CrossRef]
- Gershenzon, J. Metabolic costs of terpenoid accumulation in higher plants. J. Chem. Ecol. 1994, 20, 1281–1328. [Google Scholar] [CrossRef]
- Kim, H.K.; Wilson, E.G.; Choi, Y.H.; Verpoorte, R. Metabolomics: A tool for anticancer lead-finding from natural products. Plant. Med. 2010, 76, 1094–1102. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahyuni, D.S.C.; Klinkhamer, P.G.L.; Choi, Y.H.; Leiss, K.A. Resistance to Frankliniella occidentalis during Different Plant Life Stages and under Different Environmental Conditions in the Ornamental Gladiolus. Plants 2024, 13, 687. https://doi.org/10.3390/plants13050687
Wahyuni DSC, Klinkhamer PGL, Choi YH, Leiss KA. Resistance to Frankliniella occidentalis during Different Plant Life Stages and under Different Environmental Conditions in the Ornamental Gladiolus. Plants. 2024; 13(5):687. https://doi.org/10.3390/plants13050687
Chicago/Turabian StyleWahyuni, Dinar S. C., Peter G. L. Klinkhamer, Young Hae Choi, and Kirsten A. Leiss. 2024. "Resistance to Frankliniella occidentalis during Different Plant Life Stages and under Different Environmental Conditions in the Ornamental Gladiolus" Plants 13, no. 5: 687. https://doi.org/10.3390/plants13050687




