Synergistic Influence of Melatonin-Hydrocolloid Coating on Decay and Senescence of Nectarine (Prunus persica var. nucipersica) during Supermarket Storage Conditions
Abstract
:1. Introduction
2. Results
2.1. Weight Loss and Percent Decay Incidence
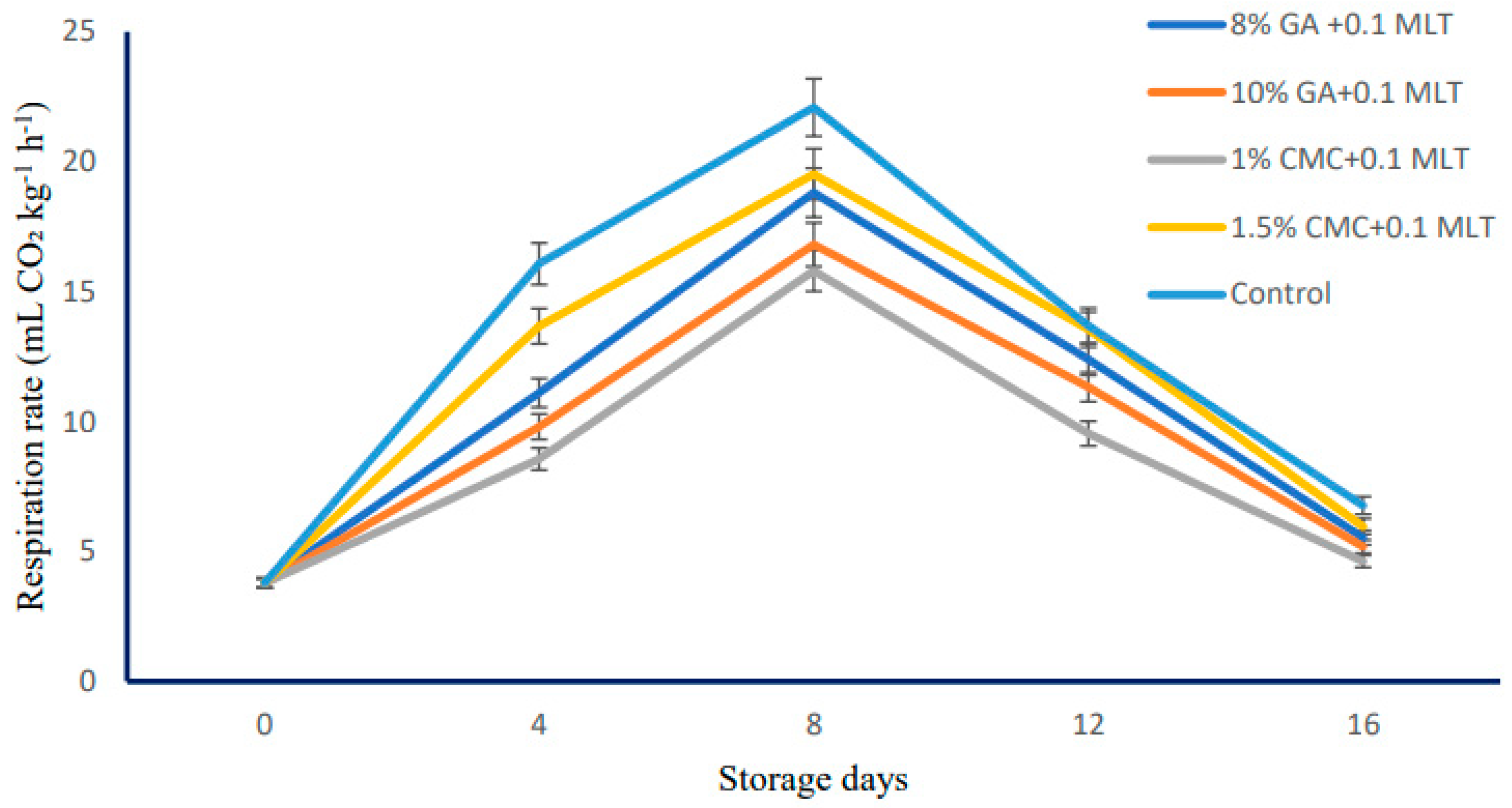
2.2. Firmness and Respiration Rate
2.3. Total Phenolic Content (TPC) and Antioxidant Activity
2.4. Lipoxygenase and Pectin Methylesterase Activity
2.5. Overall Acceptability
3. Experimental Materials and Protocol
3.1. Procurement of Raw Materials
3.2. Weight Loss
3.3. Firmness
3.4. Respiration Rate
3.5. Decay Incidence
3.6. Total Phenol Content
3.7. Total Antioxidant (AOX) Activity
3.8. Lipoxygenase (LOX) Activity
3.9. Pectin Methylesterase (PME) Activity
3.10. Overall Acceptability
3.11. Statistical Design and Analysis of Data
4. Discussion
4.1. Weight Loss and Percent Decay Incidence
4.2. Firmness and Respiration Rate
4.3. Total Phenolic Content (TPC) and Antioxidant Activity
4.4. Lipoxygenase and Pectin Methylesterase Activity
4.5. Overall Acceptability
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petruccelli, R.; Bonetti, A.; Ciaccheri, L.; Ieri, F.; Ganino, T.; Faraloni, C. Evaluation of the Fruit Quality and Phytochemical Compounds in Peach and Nectarine Cultivars. Plants 2023, 12, 1618. [Google Scholar] [CrossRef]
- Jayarajan, S.; Sharma, R.R.; Sethi, S.; Saha, S.; Sharma, V.K.; Singh, S. Chemical and nutritional evaluation of major genotypes of nectarine (Prunus persica var nectarina) grown in North-Western Himalayas. J. Food Sci. Technol. 2019, 56, 4266–4273. [Google Scholar] [CrossRef]
- Mihaylova, D.; Popova, A.; Goranova, Z.; Petkova, D.; Doykina, P.; Lante, A. The perspective of nectarine 372 fruit as a sugar substituent in puddings prepared with corn and rice starch. Foods 2021, 10, 2563. [Google Scholar] [CrossRef] [PubMed]
- Jayarajan, S.; Sharma, R.R. Impact of ethylene absorbents on fruit firmness and quality of nectarine (Prunus persica var. nectarina) fruits during storage at super market conditions. Madridge J. Food Technol. 2018, 3, 150–153. [Google Scholar] [CrossRef]
- Puig, B.A.; García-Melón, M.; López-Cortés, I.; Ortolá, M.D. Combining sensory panels with Analytic Hierarchy Process (AHP) to assess nectarine and peach quality. Cogent Food Agric. 2023, 9, 2161184. [Google Scholar] [CrossRef]
- Jayarajan, S.; Sharma, R.R. Impact of nitric oxide on shelf life and quality of nectarine (Prunus persica var. nucipersica). Acta Physiol. Plant 2018, 40, 207–212. [Google Scholar] [CrossRef]
- Jayarajan, S.; Sharma, R.R. Postharvest life and quality of ‘Snow Queen’ nectarine (Prunus persica var. nucipersica) as influenced by edible coatings during cold storage. Acta Physiol. Plant 2020, 42, 123. [Google Scholar] [CrossRef]
- Mahajan, B.V.C.; Dhillon, W.S.; Kumar, M.; Singh, B. Effect of different packaging films on shelf life and quality of peach under super and ordinary market conditions. J. Food Sci. Technol. 2015, 52, 3756–3762. [Google Scholar] [CrossRef]
- Thakur, K.S.; Reddy, V.C.M.; Lal-Kaushal, B.B. Use of polyethylene box liners and ethylene absorbents for retention of quality of Starking Delicious apples during marketing. Acta Hortic. 2005, 696, 463–465. [Google Scholar] [CrossRef]
- Totad, M.G.; Sharma, R.R.; Sethi, S.; Verma, M.K. Effect of edible coatings on ‘Misty’ blueberry (Vaccinium corymbosum) fruits stored at low temperature. Acta Physiol. Plant. 2019, 41, 183–190. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in flowering, fruit set and fruit ripening. Plant Reprod. 2020, 33, 77–87. [Google Scholar] [CrossRef]
- Jayarajan, S.; Sharma, R.R. Melatonin: A blooming biomolecule for postharvest management of perishable fruits and vegetables. Trends Food Sci. Technol. 2021, 116, 318–328. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020, 71, 4677–4689. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Naga, K.C.; Kumar, R.; Chourasia, K.N.; Subhash, S.; Kumar, D.; Sharma, S. Emerging roles of melatonin in mitigating abiotic and biotic stresses of horticultural crops. Sci. Hortic. 2020, 272, 109592. [Google Scholar] [CrossRef]
- Wu, C.; Hao, W.; Yan, L.; Zhang, H.; Zhang, J.; Liu, C.; Zheng, L. Postharvest melatonin treatment enhanced antioxidant activity and promoted GABA biosynthesis in yellow-flesh peach. Food Chem. 2023, 419, 136088. [Google Scholar] [CrossRef]
- Chacon, S.R.; Esquivel JC, C.; Montanez, J.; Carbo, A.F.A.; Vega, M.L.R.; Rodriguez, R.D.P.; Brambila, G.S. Guar gum as an edible coating for enhancing shelf life and improving postharvest quality of Roma tomato. J. Food Qual. 2017, 2017, 8608304. [Google Scholar] [CrossRef]
- Prasad, K.; Sharma, R.R.; Asrey, R.; Sethi, S.; Srivastav, M.; Singh, D.; Arora, A. Hydrocolloid edible coatings extend shelf life, reduce postharvest decay, and maintain keeping quality of mango fruits (Mangifera indica L.) under ambient storage. J. Food Biochem. 2022, 46, e14481. [Google Scholar] [CrossRef] [PubMed]
- Batool, M.; Bashir, O.; Amin, T.; Wani, S.M.; Masoodi, F.A.; Jan, N.; Bhat, S.A.; Gul, A. Investigating the effect of oxalic acid and salicylic acid treatments on the postharvest life of temperate grown apricot varieties (Prunus armeniaca) during controlled atmosphere storage. Food Sci. Technol. Int. 2022, 28, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Lu, Z.; Yang, Y.; Wang, D.; Yang, T.; Cao, M.; Cao, W. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism. Food Chem. 2018, 245, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Bashir, O.; Hussain, S.Z.; Gani, G.; Jan, N.; Rather, A.H.; Reshi, M.; Amin, T. Evaluating the physicochemical and antioxidant characteristics of apricot juice prepared through pectinase enzyme-assisted extraction from Halman variety. J. Food Meas. Charact. 2021, 15, 2645–2658. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E. Novel total antioxidants capacity index for dietary polyphenol and vitamins C and E using their cupric ion reducing capability in the presence of neocuprine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Rabiei, V.; Kakavand, F.; Zaare-Nahandi, F.; Razavi, F.; Aghdam, M.S. Nitric oxide and γ-aminobutyric acid treatments delay senescence of cornelian cherry fruits during postharvest cold storage by enhancing antioxidant system activity. Sci. Hortic. 2019, 243, 268–273. [Google Scholar] [CrossRef]
- García-Betanzos, C.I.; Hernández-Sánchez, H.; Bernal-Couoh, T.F.; Quintanar-Guerrero, D.; de la Luz Zambrano-Zaragoza, M. Physicochemical, total phenols and pectin methylesterase changes on quality maintenance on guava fruit (Psidium guajava L.) coated with candeuba wax solid lipid nanoparticles-xanthan gum. Food Res. Int. 2017, 101, 218–227. [Google Scholar] [CrossRef]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Singh, S.; Varghese, E. Improving the shelf life of fresh-cut ‘Royal Delicious’ apple with edible coatings and antibrowning agents. J. Food Sci. Technol. 2018, 55, 3767–3778. [Google Scholar] [CrossRef] [PubMed]
- Panse, V.G.; Sukhatme, P.V. Statistical Methods for Agricultural Workers, 3rd ed.; Indian Council of Agricultural Research: New Delhi, India, 1984. [Google Scholar]
- Aghdam, M.S.; Luo, Z.; Li, L.; Jannatizadeh, A.; Fard, J.R.; Pirzad, F. Melatonin treatment maintains nutraceutical properties of pomegranate fruits during cold storage. Food Chem. 2020, 303, 125385. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Shi, X.C.; Wang, R.; Wang, H.L.; Liu, F.; Laborda, P. Melatonin in fruit production and postharvest preservation: A review. Food Chem. 2020, 320, 126642. [Google Scholar] [CrossRef] [PubMed]
- Bal, E. Physicochemical changes in ‘Santa Rosa’plum fruit treated with melatonin during cold storage. J. Food Meas. Charact. 2019, 13, 1713–1720. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Zhao, R.; Li, R.; Zhang, S.; Yu, W.; Shen, L. Melatonin induces disease resistance to Botrytis cinerea in tomato fruit by activating jasmonic acid signaling pathway. J. Agric. Food Chem. 2019, 67, 6116–6124. [Google Scholar] [CrossRef]
- Aloui, H.; Khwaldia, K.; Licciardello, F.; Mazzaglia, A.; Muratore, G.; Hamdi, M.; Restuccia, C. Efficacy of the combined application of chitosan and locust bean gum with different citrus essential oils to control postharvest spoilage caused by Aspergillus flavus in dates. Int. J. Food Microbiol. 2014, 170, 21–28. [Google Scholar] [CrossRef]
- Jhalegar, M.J.; Sharma, R.R.; Singh, D. In vitro and in vivo activity of essential oils against major postharvest pathogens of Kinnow (Citrus nobilis × C. deliciosa) mandarin. J. Food Sci. Technol. 2015, 52, 2229–2237. [Google Scholar] [CrossRef]
- Saleem, M.S.; Ejaz, S.; Anjum, M.A.; Ali, S.; Hussain, S.; Ercisli, S.; Ilhan, G.; Marc, R.A.; Skrovankova, S.; Mlcek, J. Improvement of Postharvest Quality and Bioactive Compounds Content of Persimmon Fruits after Hydrocolloid-Based Edible Coating Application. Horticulturae 2022, 8, 1045. [Google Scholar] [CrossRef]
- Gol, N.B.; Chaudhari, M.L.; Rao, T.V.R. Effect of edible coatings on quality and shelf life of carambola (Averrhoa carambola L.) fruit during storage. J. Food Sci. Technol. 2015, 52, 78–91. [Google Scholar] [CrossRef]
- Liu, S.; Huang, H.; Donald, I.; Huber, J.; Pan, Y.; Shi, X.; Zhang, Z. Delay of ripening and softening in ‘Guifei’ mango fruit by postharvest application of melatonin. Postharvest Biol. Technol. 2020, 163, 111136. [Google Scholar] [CrossRef]
- Fan, S.; Xiong, T.; Lei, Q.; Tan, Q.; Cai, J.; Song, Z.; Yang, M.; Chen, W.; Li, X.; Zhu, X. Melatonin Treatment Improves Postharvest Preservation and Resistance of Guava Fruit (Psidium guajava L.). Foods 2022, 11, 262. [Google Scholar] [CrossRef]


| Treatment | Storage Period (Days) | Mean | |||
|---|---|---|---|---|---|
| 4 | 8 | 12 | 16 | ||
| 8% G.A. + 0.1 mM MLT | 3.40 ± 0.2 bD | 6.80 ± 0.10 bC | 9.50 ± 0.10 bB | 12.84 ± 0.20 bA | 8.13 ± 3.62 |
| 10% GA + 0.1 mM MLT | 2.74 ± 0.03 cD | 5.10 ± 0.10 dC | 7.80 ± 0.20 dB | 10.06 ± 0.02 dA | 6.41 ± 2.86 |
| 1% CMC + 0.1 mM MLT | 2.81 ± 0.02 cD | 4.23 ± 1.33 eC | 7.19 ± 0.01 eB | 9.71 ± 0.05 eA | 5.98 ± 2.84 |
| 1.5% CMC + 0.1 mM MLT | 3.44 ± 0.01 bD | 6.00 ± 0.20 cC | 9.03 ± 0.06 cB | 12.42 ± 0.05 cA | 7.72 ± 3.50 |
| Control | 4.56 ± 0.35 aD | 8.10 ± 0.20 aC | 12.92 ± 0.10 aB | 15.98 ± 0.11 aA | 10.39 ± 4.58 |
| Mean | 3.39 ± 0.69 | 6.04 ± 1.47 | 9.28 ± 2.06 | 12.19 ± 2.34 | |
| Treatment | Storage Period (Days) | Mean | ||||
|---|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | 16 | ||
| 8% G.A. + 0.1 mM MLT | 10.76 ± 0.34 aA | 8.48 ± 0.34 bB | 6.91 ± 0.26 bC | 5.22 ± 0.20 cC | 3.63 ± 0.30 dC | 7.00 ± 2.58 |
| 10% GA + 0.1 mM MLT | 10.61 ± 0.34 aA | 9.75 ± 0.36 aA | 7.67 ± 0.18 bB | 6.48 ± 0.18 bB | 4.50 ± 0.29 cB | 7.80 ± 1.98 |
| 1% CMC + 0.1 mM MLT | 10.86 ± 0.34 aA | 9.45 ± 0.39 aA | 8.41 ± 0.20 bA | 7.29 ± 0.18 bA | 5.17 ± 0.11 cA | 8.23 ± 2.33 |
| 1.5% CMC + 0.1 mM MLT | 10.16 ± 0.34 aA | 9.49 ± 0.30 aA | 6.97 ± 0.09 bC | 5.78 ± 0.30 bC | 3.83 ± 0.07 cC | 7.24 ± 2.59 |
| Control | 10.46 ± 0.34 aA | 9.02 ± 0.13 aB | 6.76 ± 0.10 bC | 5.03 ± 0.06 cD | 3.17 ± 0.18 dD | 6.88 ± 2.81 |
| Mean | 10.57 ± 0.29 | 9.23 ± 0.53 | 7.34 ± 0.65 | 5.96 ± 0.88 | 4.06 ± 0.74 | |
| Treatment | Storage Period (Days) | Mean | ||||
|---|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | 16 | ||
| 8% G.A. + 0.1 mM MLT | 13.00 ± 0.03 cB | 14.59 ± 0.02 cA | 12.19 ± 0.08 bC | 9.52 ± 0.15 cD | 7.75 ± 0.07 cE | 11.41 ± 2.54 |
| 10% GA + 0.1 mM MLT | 13.47 ± 0.03 aB | 14.80 ± 0.05 bA | 11.80 ± 0.04 cC | 9.15 ± 0.34 bD | 7.88 ± 0.04 bE | 11.42 ± 2.60 |
| 1% CMC + 0.1 mM MLT | 13.32 ± 0.03 bC | 15.51 ± 0.54 aA | 14.15 ± 0.11 aB | 10.71 ± 0.0 aD | 8.78 ± 0.31 aE | 12.51 ± 2.49 |
| 1.5% CMC + 0.1 mM MLT | 13.43 ± 0.03 aB | 13.79 ± 0.10 dA | 10.16 ± 0.09 dC | 8.20 ± 0.14 dE | 5.16 ± 0.13 dF | 10.15 ± 3.04 |
| Control | 13.41 ± 0.03 aA | 11.04 ± 0.03 eB | 9.92 ± 0.08 eC | 6.50 ± 0.28 eD | 4.16 ± 0.13 eE | 9.01 ± 3.29 |
| Mean | 13.32 ± 0.02 | 13.95 ± 1.69 | 11.64 ± 1.58 | 8.83 ± 1.49 | 6.74 ± 1.83 | |
| Treatment | Storage Period (Days) | Mean | ||||
|---|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | 16 | ||
| 8% GA + 0.1 mM MLT | 20.12 ± 0.09 aC | 23.04 ± 0.18 bA | 20.99 ± 0.06 bB | 17.41 ± 0.54 bD | 12.60 ± 0.04 bE | 18.83 ± 3.73 |
| 10% GA + 0.1 mM MLT | 20.12 ± 0.09 aC | 22.77 ± 0.09 cA | 20.78 ± 0.10 cB | 17.47 ± 0.05 bD | 12.41 ± 0.06 eE | 18.71 ± 3.70 |
| 1% CMC + 0.1 mM MLT | 20.12 ± 0.09 aC | 24.92 ± 0.95 aA | 23.22 ± 0.11 aB | 19.15 ± 0.10 aD | 14.76 ± 0.12 aE | 20.43 ± 3.65 |
| 1.5% CMC + 0.1 mM MLT | 20.12 ± 0.09 aB | 21.18 ± 0.10 dA | 18.27 ± 0.04 dC | 14.51 ± 0.20 cD | 10.40 ± 0.10 dE | 16.89 ± 4.10 |
| Control | 20.12 ± 0.09 aA | 19.15 ± 0.05 eB | 17.29 ± 0.06 eC | 13.54 ± 0.12 dD | 8.67 ± 0.07 eE | 15.75 ± 4.34 |
| Mean | 20.12 ± 0.07 | 22.21 ± 2.03 | 20.11 ± 2.18 | 16.42 ± 2.15 | 11.77 ± 2.16 | |
| Treatment | Storage Period (Days) | Mean | ||||
|---|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | 16 | ||
| 8% G.A. + 0.1 mM MLT | 1.33 ± 0.02 aE | 1.77 ± 0.01 cD | 2.42 ± 0.25 cC | 3.08 ± 0.11 cA | 2.78 ± 0.12 cB | 2.28 ± 0.67 |
| 10% GA + 0.1 mM MLT | 1.31 ± 0.01 aE | 1.72 ± 0.02 dD | 2.36 ± 0.01 dC | 2.72 ± 0.03 dA | 2.42 ± 0.02 dB | 2.11 ± 0.52 |
| 1% CMC + 0.1 mM MLT | 1.32 ± 0.02 aE | 1.64 ± 0.06 eD | 2.06 ± 0.06 eB | 2.24 ± 0.03 eA | 1.94 ± 0.05 eC | 1.84 ± 0.33 |
| 1.5% CMC + 0.1 mM MLT | 1.34 ± 0.02 aE | 1.93 ± 0.03 bD | 2.76 ± 0.04 bC | 3.28 ± 0.05 bA | 2.98 ± 0.05 bB | 2.45 ± 0.74 |
| Control | 1.33 ± 0.02 aE | 2.42 ± 0.11 aD | 3.06 ± 0.10 aC | 3.79 ± 0.02 aA | 3.49 ± 0.02 Ab | 2.81 ± 0.90 |
| Mean | 1.32 ± 0.02 | 1.89 ± 0.2 | 2.53 ± 0.37 | 3.02 ± 0.54 | 2.72 ± 0.54 | |
| Treatment | Storage Period (Days) | Mean | ||||
|---|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | 16 | ||
| 8% G.A. + 0.1 mM MLT | 5.54 ± 0.01 aC | 5.74 ± 0.02 Eb | 6.25 ± 0.01 eA | 5.40 ± 0.20 dD | 5.30 ± 0.10 eD | 5.64 ± 0.35 |
| 10% GA + 0.1 mM MLT | 5.53 ± 0.01 aD | 6.50 ± 0.10 bB | 8.00 ± 0.20 bA | 6.10 ± 0.10 bC | 6.11 ± 0.01 bC | 6.45 ± 0.86 |
| 1% CMC + 0.1 mM MLT | 5.56 ± 0.03 aD | 6.77 ± 0.15 aB | 8.13 ± 0.11 aA | 6.30 ± 0.10 aC | 6.30 ± 0.20 aC | 6.61 ± 0.88 |
| 1.5% CMC + 0.1 mM MLT | 5.54 ± 0.01 aD | 6.63 ± 0.15 cB | 7.70 ± 0.20 cA | 5.83 ± 0.05 cC | 5.83 ± 0.15 cC | 6.30 ± 0.81 |
| Control | 5.47 ± 0.01 aE | 6.40 ± 0.10 dB | 7.10 ± 0.20 dA | 5.21 ± 0.20 eC | 4.36 ± 0.05 eD | 5.50 ± 0.84 |
| Mean | 5.55 ± 0.01 | 6.40 ± 0.38 | 7.59 ± 0.72 | 5.92 ± 0.34 | 5.75 ± 0.36 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayarajan, S.; Sethi, S.; Awasthi, O.P.; Sharma, A.; Bukvički, D. Synergistic Influence of Melatonin-Hydrocolloid Coating on Decay and Senescence of Nectarine (Prunus persica var. nucipersica) during Supermarket Storage Conditions. Plants 2024, 13, 822. https://doi.org/10.3390/plants13060822
Jayarajan S, Sethi S, Awasthi OP, Sharma A, Bukvički D. Synergistic Influence of Melatonin-Hydrocolloid Coating on Decay and Senescence of Nectarine (Prunus persica var. nucipersica) during Supermarket Storage Conditions. Plants. 2024; 13(6):822. https://doi.org/10.3390/plants13060822
Chicago/Turabian StyleJayarajan, Smruthi, Shruti Sethi, Om Prakash Awasthi, Abhishek Sharma, and Danka Bukvički. 2024. "Synergistic Influence of Melatonin-Hydrocolloid Coating on Decay and Senescence of Nectarine (Prunus persica var. nucipersica) during Supermarket Storage Conditions" Plants 13, no. 6: 822. https://doi.org/10.3390/plants13060822






