Chemical Composition and Bioactivity of Dill Seed (Anethum graveolens L.) Essential Oil from Plants Grown under Shading
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Clevenger Hydrodistillation
2.3. Gas Chromatography–Mass Spectrometry (GC/MS) and Gas Chromatography–Flame Ionization Detection (GC/FID) Analyses
2.4. Antioxidant Activity—DPPH Assay
2.5. Antimicrobial Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Climatic Conditions
3.2. Yield of Fresh Mass, Dry Mass, Seed and Essential Oil
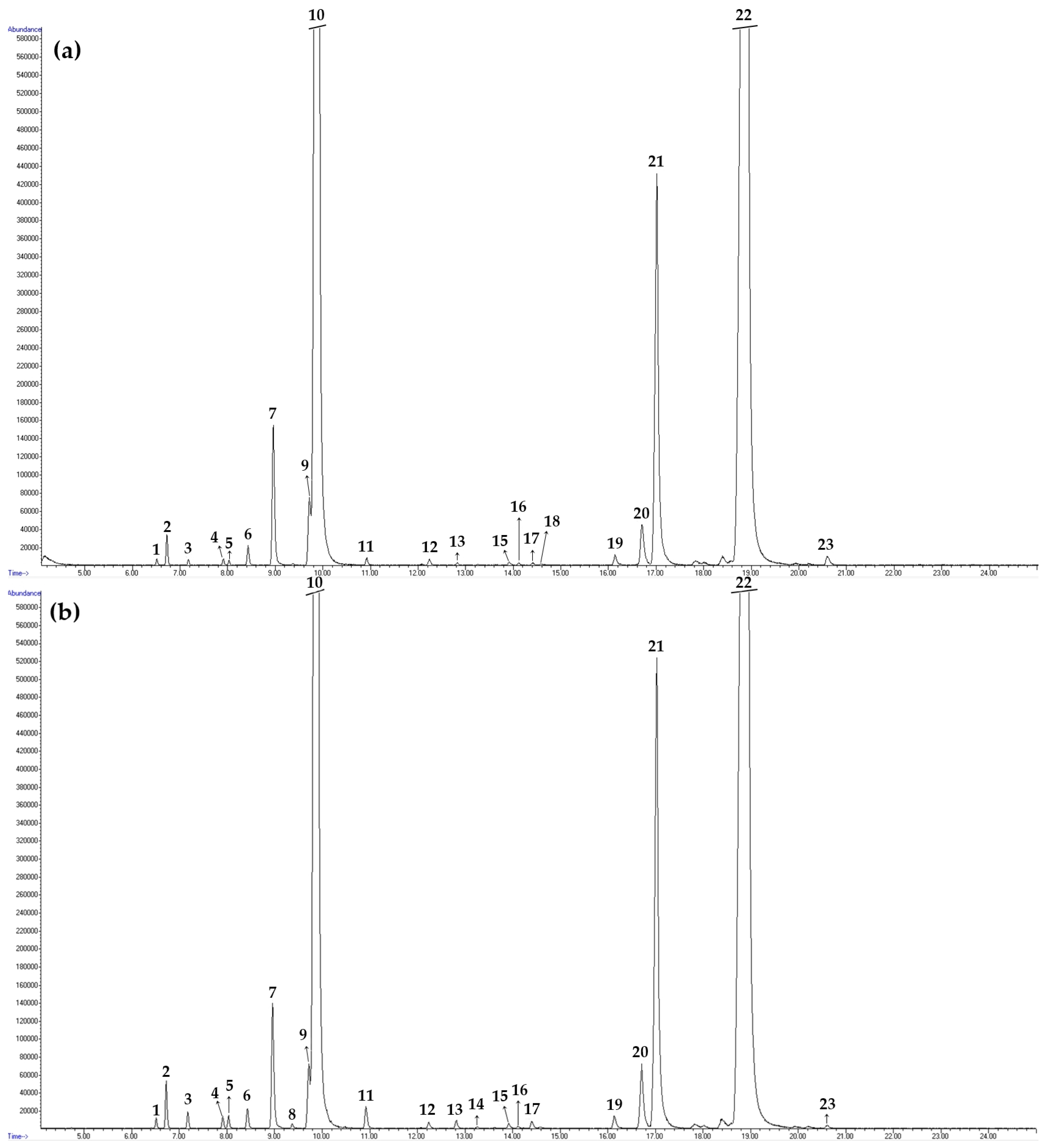
3.3. Dill Seed Essential Oil (DSEO) Compositions
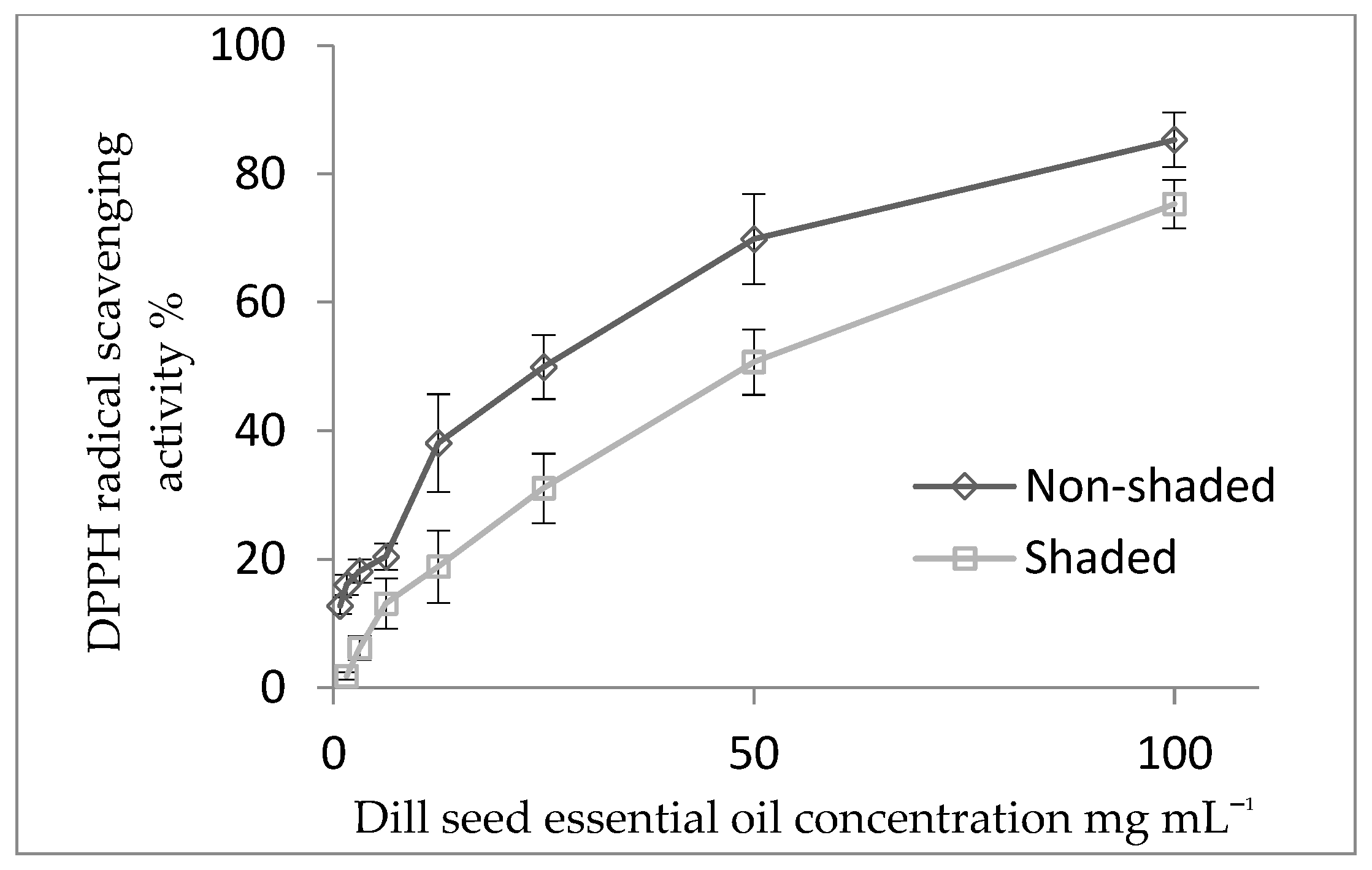
3.4. Antioxidant Activity
3.5. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mohebodini, M.; Farmanpour-Kalalagh, K. Comparative chemical composition of essential oils in dill (Anethum graveolens L.) ecotypes: Focus on Univariate and Factor Analysis. Int. J. Hortic. Sci. Technol. 2021, 8, 81–90. [Google Scholar]
- Olaoluwa, O.; Taiwo, O.; Nahar, L.; Sarker, D.S. Ethnopharmacology, phytochemistry and biological activities of selected African species of the genus Ficus L. Trends Phytochem. Res. 2022, 6, 46–69. [Google Scholar]
- Lazić, B.; Ilić, Z.; Đurovka, M. Organska Proizvodnja Povrća; Centar za Organsku Poljoprivredu iz Selenče i Univerzitet Edukons: Sremska Kamenica, Serbia, 2013. (In Serbian) [Google Scholar]
- Said-Al Ahl, H.A.H.; Sarhan, A.M.; Abou Dahab, A.D.M.; Abou-Zeid, E.-S.N.; Ali, M.S.; Naguib, N.Y.; El-Bendary, M.A. Essential oils of Anethum graveolens L.: Chemical composition and their antimicrobial activities at vegetative, flowering and fruiting stages of development. Int. J. Plant Sci. Ecol. 2015, 1, 98–102. [Google Scholar]
- AguiarCampolina, G.; das Graças Cardoso, M.; Rodrigues-Silva-Caetano, A.; Lee Nelson, D.; Mendes Ramos, E. Essential oil and plant extracts as preservatives and natural antioxidants applied to meat and meat products: A Review. Food Technol. Biotechnol. 2023, 61, 212–225. [Google Scholar]
- Stanojević, L.P.; Radulović, N.S.; Djokić, T.M.; Stanković, B.M.; Ilić, D.P.; Cakić, M.D. The yield, composition and hydrodistillation kinetics of the essential oil of dill seeds (Anethi fructus) obtained by different hydrodistillation techniques. Ind. Crops Prod. 2015, 65, 429–436. [Google Scholar] [CrossRef]
- Ozliman, S.; Yaldiz, G.; Camlica, M.; Ozsoy, N. Chemical components ofessential oils and biological activities of the aqueous extract of Anethum graveolens L. grown under inorganic and organic conditions. Chem. Biol. Technol. Agric. 2021, 8, 20. [Google Scholar] [CrossRef]
- Li, Z.; Xue, Y.; Li, M.; Guo, Q.; Sang, Y.; Wang, C.; Luo, C. The antioxidation of different fractions of dill (Anethum graveolens) and their influences on cytokines in macrophages RAW264.7. J. Oleo Sci. 2018, 67, 1535–1541. [Google Scholar] [CrossRef]
- Ulus, G.; Zeytinoğlu, M.; Kürkçüoğlu, M.; Başer Kemal, H.C.; Koparal, A.T. Dill seed oil as a possible contraceptive agent: Antiangiogenic effects on endothelial cells. Braz. J. Pharm. Sci. 2023, 59, e20060. [Google Scholar]
- Youssef, R.S.A. Medicinal and non-medicinal uses of some plants found in the middle region of Saudi Arabia. J. Med. Plants Res. 2013, 7, 2501–2513. [Google Scholar]
- Aati, H.Y.; Perveen, S.; Aati, S.; Orfali, R.; Alqahtani, J.H.; Al-Taweel, A.M.; Wanner, J.; Aati, A.Y. Headspace solid-phase microextraction method for extracting volatile constituents from the different parts of Saudi Anethum graveolens L. and their antimicrobial activity. Heliyon 2022, 8, e09051. [Google Scholar] [CrossRef]
- Gomaa, S.E.; Gomaa, E.E.D.G. Phenolic content of dill seed extracts as antifungal agent against Aspergillus spp. Middle East. J. Agric. Res. 2021, 10, 1310–1318. [Google Scholar]
- Stanojević, L.; Stanković, M.; Cvetković, D.; Danilović, B.; Stanojević, J. Dill (Anethum graveolens L.) seeds essential oil as a potential natural antioxidant and antimicrobial agent. Biol. Nyssana 2016, 7, 31–39. [Google Scholar]
- Chaubey, M. Insecticidal activities of Anethum graveolens L. and Illicium verum Hook. f. essential oıls against Sitophilus zeamais Motschulsky. Rev. Cienc. Agrícolas 2021, 38, 38–49. [Google Scholar] [CrossRef]
- Karanisa, T.; Akoumianakis, K.; Alexopoulos, A.; Karapanos, I. Effect of postharvest application of carvone on potato tubers grown from true potato seed (TPS). Proced. Environ. Sci. 2015, 29, 166–167. [Google Scholar] [CrossRef]
- Said Al Ahl, H.A.H.; Sarhan, A.M.Z.; Abou Dahab, A.D.M.; Abou Zeid, E.-S.N.; Ali, M.S.; Naguib, N.Y. Volatile oil composition of Anethum graveolens affected by harvest stage. Int. J. Plant Sci. Ecol. 2015, 1, 93–97. [Google Scholar]
- Said-Al Ahl, H.; Omer, E. Impact of cultivar and harvest time on growth, production and essential oil of Anethum graveolens cultivated in Egypt. Int. J. Pharm. Pharm. Sci. 2016, 8, 54–60. [Google Scholar]
- Al-Massarani, S.; Tabanca, N.; Farshori, N.N. Headspace-SPME/GC-MS analysis of the Anethum graveolens L. volatiles from Saudi Arabia with different fibers coatings. Nat. Volatiles Essent. Oils 2018, 5, 29–34. [Google Scholar]
- Jirovetz, L.; Buchbauer, G.; Stoyanova, A.S.; Georgiev, E.V.; Damianova, S.T. Composition, quality control, and antimicrobial activity of the essential oil of long-time stored dill (Anethum graveolens L.) seeds from Bulgaria. J. Agric. Food Chem. 2003, 51, 3854–3857. [Google Scholar] [CrossRef]
- Zawirska-Wojtasiak, R.; Wasowicz, E. Estimation of the main dill seeds odorant carvone by solid-phase micro-extraction and gas chromatography. Nahrung 2002, 46, 357–359. [Google Scholar] [CrossRef]
- Wall, D.A.; Friesen, G.H. The effect of herbicides and weeds on the yields and composition of dill (Anethum graveolens L.) oil. Crop Prot. 1986, 5, 137–142. [Google Scholar] [CrossRef]
- Ilić, S.Z.; Fallik, E. Light quality manipulation improves vegetables quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Lalevic, D.; Ilic, Z.S.; Stanojevic, L.; Milenkovic, L.; Šunic, L.; Kovac, R.; Kovacevic, D.; Danilovic, B.; Milenkovic, A.; Stanojevic, J.; et al. Shade-induced effects on essential oil yield, chemical profiling, and biological activity in some Lamiaceae plants cultivated in Serbia. Horticulturae 2023, 9, 84. [Google Scholar] [CrossRef]
- Ilić, S.Z.; Milenković, L.; Tmušić, N.; Stanojević, L.; Cvetković, D. Essential oils content, composition and antioxidant activity of lemon balm, mint and sweet basil from Serbia. LWT—Food Sci. Technol. 2022, 153, 112210. [Google Scholar] [CrossRef]
- Buthelezi, M.N.D. Effect of Photo-Selective Netting on Postharvest Quality and Bioactive Compounds in Three Selected Summer Herbs (Coriander, Marjoram and Oregano). Master’s Thesis, Department of Crop Sciences, Faculty of Science, Tshwane University of Technology, Pretoria, South Africa, 2015. [Google Scholar]
- Milenković, L.; Ilić, Z.; Šunić, L.; Tmušić, N.; Lalević, D.; Stanojević, L.; Stanojević, J.; Cvetković, D. Modification of light intensity influence essential oils content, composition and antioxidant activity of thyme, marjoram and oregano. Saudi J. Biol. Sci. 2021, 28, 6532–6543. [Google Scholar] [CrossRef] [PubMed]
- Ilić, Z.; Stanojević, L.; Milenković, L.; Šunić, L.; Milenković, A.; Stanojević, J.; Cvetković, D. The yield, chemical composition, and antioxidant activities of essential oils from different plant parts of the wild and cultivated oregano (Origanum vulgare L.). Horticulturae 2022, 8, 1042. [Google Scholar] [CrossRef]
- Stanojević, L.; Stanojević, J.; Milenković, L.; Šunić, L.; Kovač, R.; Cvetković, D.; Babić, M.; Ilić, Z. Aroma profile and antioxidant activity of sweet basil aqueous extracts affect by light modification. J. Essent. Oil Bear. Plants 2022, 25, 1131–1144. [Google Scholar] [CrossRef]
- Ilić, S.Z.; Milenković, L.; Šunić, L.; Tmušić, N.; Mastilović, J.; Kevrešan, Ž.; Stanojević, L.; Danilović, B.; Stanojević, J. Efficiency of basil essential oil antimicrobial agents under different shading treatments and harvest times. Agronomy 2021, 11, 1574. [Google Scholar] [CrossRef]
- Kazemini, M.; Ali Mehrabi, A.; Mahmoudi, R. Chemical composition, biological activities, and nutritional application of Asteraceae family herbs: A systematic review. Trends Phytochem. Res. 2022, 6, 187–213. [Google Scholar]
- Kiehlbauch, J.A.; Hannett, G.E.; Salfinger, M.; Archinal, W.; Monserrat, C.; Carlin, C. Use of the national committee for clinical laboratory standards guidelines for disk diffusion susceptibility testing in New York State Laboratories. J. Clin. Microbiol. 2000, 38, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Stagnari, F.; Di Mattia, C.; Galienia, A.; Santarellia, V.; D’Egidioa, S.; Pagnania, G.; Pisante, M. Light quantity and quality supplies sharply affect growth, morphological, physiological and quality traits of basil. Ind. Crops Prod. 2018, 122, 277–289. [Google Scholar] [CrossRef]
- Ilić, S.Z.; Milenković, L.; Stanojević, L.; Danilović, B.; Šunić, L.J.; Milenković, A.; Kevrešan, Ž.; Stanojević, J.; Cvetković, D. Phytochemical composition and antimicrobial activities of the essential oils from summer savory (Satureja hortensis L.) growing in shading condition. J. Essent. Oil Bear. Plants 2023, 26, 6. [Google Scholar] [CrossRef]
- Popović, V.; Maksimović, L.; Adamović, D.; Sikora, V.; Ugrenović, V.; Filipović, V.; Mačkić, K. Yield of biomass and essential oil of dill (Anethum graveolens L.) grown under irrigation. Ratar. Povrt. 2019, 56, 49–55. [Google Scholar] [CrossRef]
- Bowes, K.M.; Zheljazkov, V.D.; Caldwell, C.D.; Pincock, J.A.; Roberts, J.C. Influence of seeding date and harvest stage on yields and essential oil composition of three cultivars of dill (Anethum graveolens L.) grown in Nova Scotia. Can. J. Plant Sci. 2004, 84, 1155–1160. [Google Scholar] [CrossRef]
- Wander, J.G.N.; Bouwmeester, H.J. Effects of nitrogen fertilization on dill (Anethum graveolens L.) seed and carvone production. Ind. Crops Prod. 1998, 7, 211–216. [Google Scholar] [CrossRef]
- Bailer, J.; Aichinger, T.; Hackl, G.; de Hueber, K.; Dachler, M. Essential oil content and composition in commercially available dill cultivars in comparison to caraway. Ind. Crops Prod. 2001, 14, 229–239. [Google Scholar] [CrossRef]
- Rădulescu, V.; Popescu, M.L.; Ilieş, D.C. Chemical composition of the volatile oil from different plant parts of Anethum graveolens L. (Umbelliferae) cultivated in Romania. Farmacia 2010, 58, 594–600. [Google Scholar]
- Yili, A.; Aisa, H.A.; Maksimov, V.V.; Veshkurova, O.N.; Salikhov, S.I. Chemical composition and antimicrobial activity of essential oil from seeds of Anethum graveolens growing in Uzbekistan. Chem. Nat. Compd. 2009, 45, 280–281. [Google Scholar] [CrossRef]
- Popescu, V.; Ciocarlan, A.; Dragalin, I.; Lungu, L.; Arîcu, A. Chemical composition of essential oil of dill (Anethum graveolens L.) growing in Republic of Moldova. Scientific seminar. In Proceedings of the New Frontiers in Natural Product Chemistry. Book of Abstract, Chișinău, Moldova, 21 May 2021; p. 35. [Google Scholar]
- Khaldi, A.; Meddah, B.; Moussaoui, A.; Sonnet, P.; Akermy, M.M. Chemical composition and antifungal activity of essential oil of Anethum graveolens L. from Southwestern Algeria (Bechar). J. Chem. Pharm. Res. 2015, 7, 615–620. [Google Scholar]
- Salehiarjmand, H.; Ebrahimi, S.N.; Hadian, J.; Ghorbanpour, M. Essential oils main constituents and antibacterial activity of seeds from Iranian local landraces of dill (Anethum graveolens L.). J. Hortic. For. Biotechnol. 2014, 18, 1–9. [Google Scholar]
- Biesiada, A.; Kędra, K.; Godlewska, K.; Szumny, A.; Nawirska-Olszańska, A. Nutritional value of garden dill (Anethum graveolens L.), depending on genotype. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 784–791. [Google Scholar]
- El-Zaeddi, H.; Martínez-Tomé, J.; Calín-Sánchez, A.; Burló, F.; Carbonell-Barrachina, A. Volatile composition of essential oils from different aromatic herbs grown in Mediterranean regions of Spain. Foods 2016, 5, 41. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Sharopov, S.F.; Wink, M.; Gulmurodov, I.S.; Isupov, S.J.; Zhang, H.; Setzer, W.N. Composition and bioactivity of the essential oil of Anethum graveolens L. from Tajikistan. Int. J. Med. Aromat. Plants 2013, 3, 125–130. [Google Scholar]
- Sefidkon, F. Essential oil composition of Anethum graveolens L. Iran. J. Med. Aromat. Plants Res. 2001, 8, 45–62. [Google Scholar]
- Kruger, H.; Hammer, K. A new chemotype of Anethum graveolens L. J. Essent. Oil Res. 1996, 8, 205–206. [Google Scholar] [CrossRef]
- Benlembarek, K.; Lograda, T.; Ramdani, M.; Figueredo, G.; Chalard, P. Chemical composition and biological activities of Anethum graveolens L. essential oil from Algeria. J. Essent. Oil Bear. Plants 2022, 25, 5. [Google Scholar] [CrossRef]
- Chahal, K.K.; Monika, K.D.; Singh, R. Antifungal potential of dill seed essential oil and its constituents. Indian J. Ecol. 2016, 43, 903–906. [Google Scholar]
- Alsahli, A.A. Light effects on growth and essential oil quantity and constituents in some Apiaceae plants. Afr. J. Agric. Res. 2019, 14, 1262–1271. [Google Scholar]
- El-Zaeddi, H.; Martinez-Tome, J.; Calin-Sanchez, A.; Burlo, F.; CarbonellBarrachina, A.A. Irrigation dose and plant density affect the volatile composition and sensory quality of dill (Anethum graveolens L.). J. Sci. Food Agric. 2017, 97, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Jianu, C.; Mişcă, C.; Stoin, D.; Bujancă, G.; Lukinich-Gruia, T.A. Chemical composition and antioxidant properties of dill essential oil. In Proceedings of the International Multidisciplinary Scientific GeoConference: SGEM, Sofia, Bulgaria, 2–8 July 2018; Volume 18, p. 4. [Google Scholar]
- Kazemi, M. Phenolic profile, antioxidant capacity and anti-inflammatory activity of Anethum graveolens L. essential oil. Nat. Prod. Res. 2015, 29, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Nanasombat, S.; Wimuttigosol, P. Antimicrobial and antioxidant activity of spice essential oils. Food Sci. Biotechnol. 2011, 20, 45–53. [Google Scholar] [CrossRef]
- Kaur, V.; Kaur, R.; Bhardaj, U. A review on dill essential oil and its chief compounds as natural biocide. Flavour Fragr. J. 2020, 36, 412–431. [Google Scholar] [CrossRef]
- Dahiya, P.; Purkayastha, S. Phytochemical analysis and antibacterial efficacy of dill seed oil against multi-drug resistant clinical isolates. Asian J. Pharm. Clin. Res. 2012, 5, 62–64. [Google Scholar]
- Kavaz, D.; Idris, M.; Onyebuchi, C. Physiochemical characterization, antioxidative, anticancer cells proliferation and food pathogens antibacterial activity of chitosan nanoparticles loaded with Cyperus articulatus rhizome essential oils. Int. J. Biol. Macromol. 2019, 123, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Delshadi, R.; Bahrami, A.; Tafti, A.G.; Barba, T.J.; Williams, L.L. Micro and nanoencapsulation of vegetable and essential oils to develop functional food products with improved nutritional profiles. Trends Food Sci. Technol. 2020, 104, 72–83. [Google Scholar] [CrossRef]
- Hasheminejad, N.; Khodaiyan, F.; Safari, M. Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem. 2019, 275, 113–122. [Google Scholar] [CrossRef]

| pH in 1 M KCl | pH in H2O | CaCO3 % | Humus % | N % | P2O5 | K2O |
|---|---|---|---|---|---|---|
| mg/100 g | ||||||
| 6.62 | 7.54 | 0.69 | 3.59 | 0.18 | 40 | 40 |
| Time (h) | PAR * (μmol m−2 s−1) | Solar Radiation (W m−2) | Temperature °C | Relative Humidity % | ||||
|---|---|---|---|---|---|---|---|---|
| Non- Shading | Shading Reduction % | Non- Shading | Shading | Non-Shading | Shading Reduction % | Non-Shading | Shading Reduction % | |
| 6:00 | 201.3 | 41.3 | 143.9 | 62.1 | 19.7 | 0.0 | 61.9 | +0.6 |
| 9:00 | 1327.0 | 37.9 | 528.0 | 302.9 | 29.7 | +2.15 | 40.8 | +1.5 |
| 12:00 | 2106.5 | 40.3 | 884.5 | 482.1 | 36.6 | +0.8 | 26.0 | +3.1 |
| 15:00 | 1768.0 | 40.2 | 764.1 | 411.7 | 40.2 | +0.17 | 18.9 | −1.6 |
| 18:00 | 502.0 | 46.9 | 329.7 | 116.4 | 38.8 | +0.51 | 28.2 | 0.0 |
| Method of Production | Yield of Fresh Biomass kg/ha | Yield of Dry Mass kg/ha | Number of Umbels/Plants | Number of Non-Fertile Umbels | Seed Yield kg/ha | Content % EO in Seed | Yield of EO kg/ha |
|---|---|---|---|---|---|---|---|
| Control (non-shaded) | 10.8 a | 2.73 a | 6.47 a | 1.9 b | 1.6 a | 4.6 a | 66.7 a |
| Shaded plants | 13.6 b | 3.40 b | 6.92 a | 1.0 a | 2.1 b | 4.8 b | 80.6 b |
| ANOVA | * | * | NS | ** | * | * | ** |
| Method | Umbel | Umbel Diameter (cm) | Number of Umbelets/Umbel | Ratio of External/Internal Parts of Umbel | Seed Weight/Umbel (g) | Absolute Mass 1000 Seeds (g) |
|---|---|---|---|---|---|---|
| Non-shading | Primary | 18.5 d | 20.7 c | 1.68 b | 1.7 d | 1.6 bc |
| Secondary | 10.8 bc | 13.6 ab | 1.33 a | 0.8 c | 1.2 a | |
| Tertiary | 6.9 a | 10.8 a | - | 0.2 a | 1.2 a | |
| Shading | Primary | 18.2 d | 20.7 c | 1.62 ab | 2.1 e | 1.9 d |
| Secondary | 12.8 c | 16.8 b | 1.44 ab | 0.8 c | 1.7 cd | |
| Tertiary | 9.4 ab | 13.2 ab | - | 0.5 b | 1.4 ab | |
| Shading | NS | NS | NS | ** | ** | |
| Umbel | ** | ** | * | ** | ** | |
| Shading x Umbel | NS | NS | NS | NS | NS |
| No | tret, min | Compound | RIexp | RIlit | Method of Identification | Content % | |
|---|---|---|---|---|---|---|---|
| Non-Shaded | Shaded Plants | ||||||
| 1. | 6.51 | α-Thujene | 927 | 924 | RI, MS | tr | tr |
| 2. | 6.73 | α-Pinene | 934 | 932 | RI, MS, Co-I | 0.2 ± 0.01 | 0.3 ± 0.01 |
| 3. | 7.18 | Camphene | 950 | 946 | RI, MS | tr | 0.1 ± 0.00 |
| 4. | 7.91 | Sabinene | 975 | 969 | RI, MS | tr | tr |
| 5. | 8.03 | β-Pinene | 979 | 974 | RI, MS | tr | tr |
| 6. | 8.43 | Myrcene | 993 | 988 | RI, MS | 0.2 ± 0.01 | 0.2 ± 0.00 |
| 7. | 8.96 | α-Phellandrene | 1008 | 1002 | RI, MS | 1.4 ± 0.02 | 1.2 ± 0.02 |
| 8. | 9.37 | α-Terpinene | 1020 | 1014 | RI, MS | - | tr |
| 9. | 9.71 | p-Cymene | 1028 | 1020 | RI, MS | 0.7 ± 0.01 | 0.7 ± 0.01 |
| 10. | 9.91 | Limonene | 1032 | 1024 | RI, MS, Co-I | 43.8 ± 0.05 | 37.8 ± 0.43 |
| 11. | 10.93 | γ-Terpinene | 1061 | 1054 | RI, MS | tr | 0.2 ± 0.00 |
| 12. | 12.24 | p-Cymenene | 1097 | 1089 | RI, MS | tr | tr |
| 13. | 12.82 | cis-Thujone | 1111 | 1101 | RI, MS | tr | tr |
| 14. | 13.25 | trans-Thujone | 1121 | 1112 | RI, MS | - | tr |
| 15. | 13.92 | cis-Limonene oxide | 1137 | 1132 | RI, MS | tr | tr |
| 16. | 14.12 | trans-Limonene oxide | 1142 | 1137 | RI, MS | tr | tr |
| 17. | 14.41 | Camphor | 1149 | 1141 | RI, MS, Co-I | tr | tr |
| 18. | 14.58 | Myrcenone | 1153 | 1145 | RI, MS | tr | - |
| 19. | 16.13 | Dill ether | 1190 | 1184 | RI, MS | tr | 0.2 ± 0.00 |
| 20. | 16.70 | cis-Dihydrocarvone | 1201 | 1191 | RI, MS | 0.7 ± 0.01 | 1.0 ± 0.02 |
| 21. | 17.02 | trans-Dihydrocarvone | 1210 | 1200 | RI, MS | 6.8 ± 0.08 | 8.4 ± 0.13 |
| 22. | 18.91 | Carvone | 1249 | 1239 | RI, MS | 46.1 ± 0.06 | 49.8 ± 0.62 |
| 23. | 20.60 | (E)-Anethole | 1292 | 1282 | RI, MS, Co-I | tr | tr |
| Total identified (%) | 100.0 | 100.0 | |||||
| Grouped components (%) | |||||||
| Monoterpene hydrocarbons (1–12) | 46.4 ± 0.06 | 40.6 ± 0.47 | |||||
| Oxygen-containing monoterpenes (13–18, 20–22) | 53.6 ± 0.15 | 59.3 ± 0.77 | |||||
| Phenylpropanoids (23) | tr | tr | |||||
| Others (19) | tr | 0.2 ± 0.00 | |||||
| Dill Origin (Country) | Main Components of Dill Seed | Method of Isolation | Reference |
|---|---|---|---|
| Egypt | Carvone (62.48%), dillapiole (19.51%) and limonene (14.61%) | Liquid chromatography (GLC) analysis | Said-Al Ahl et al., 2015 [4] |
| Tajikistan | Carvone (51.7%), trans-dihydrocarvone (14.7%), dill ether (13.2%), α-phellandrene (8.1%) and limonene (6.9%) | GC/MS using an Agilent 6890 GC with Agilent 5973 mass selective detector | Sharopov et al., 2013 [46] |
| Iran | Carvone (57.3%) and limonene (33.2%) | GC and GC/MS | Sefidkon, 2001 [47] |
| Bulgaria | Limonene (43.7%), carvone (41.2%), dihydrocarvone (3.1%) and myristicin (11.70%) | GC and GC/MS | Kruger and Hammer [48] |
| Algeria | Carvone (34.33%), α-phellandrene (22.03%) and dill ether (18.84%) | GC and GC/MS | Benlembarek et al., 2022 [49] |
| Romania | Carvone (75.2%) and limonene (21.56%) | GC/MS analyses | Rădulescu et al., 2010 [38] |
| Saudi Arabia | Apiol (33.3%), limonene (30.8%) and carvone (17.70%) | GC/MS analyses | Aati et al., 2022 [11] |
| India | Carvone (41.15%), limonene (23.11%) and camphor (9.25%) | GC/MS analyses | Chahal et al., 2016 [50] |
| Uzbekistan | Carvone (73.61%), limonene (14.69%) and cis-dihydrocarvone (5.87%) | GC/MS | Yili et al., 2016 [39] |
| Method of Plant Production | Escherichia coli | Proteus vulgaris | Bacillus subtilis | Staphylococcus aureus | Klebsiella pneumoniae | Candida albicans |
|---|---|---|---|---|---|---|
| Inhibition Zone (mm) | ||||||
| Non-shaded plants | 18.0 b | 11.3 b | 12.3 b | n.z. | 11.0 c | n.z. |
| Shaded plants | 15.0 c | 11.5 b | 12.0 b | n.z. | 13.0 b | n.z. |
| Positive control (Ceftriaxone 30 μg for bacteria and Nystatin 50 μg for yeast) | 32.0 a | 30.0 a | 24.0 a | 25.0 | 20.0 a | 17.0 |
| Shading | *** | * | * | - | *** | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milenković, L.; Ilić, Z.S.; Stanojević, L.; Danilović, B.; Šunić, L.; Kevrešan, Ž.; Stanojević, J.; Cvetković, D. Chemical Composition and Bioactivity of Dill Seed (Anethum graveolens L.) Essential Oil from Plants Grown under Shading. Plants 2024, 13, 886. https://doi.org/10.3390/plants13060886
Milenković L, Ilić ZS, Stanojević L, Danilović B, Šunić L, Kevrešan Ž, Stanojević J, Cvetković D. Chemical Composition and Bioactivity of Dill Seed (Anethum graveolens L.) Essential Oil from Plants Grown under Shading. Plants. 2024; 13(6):886. https://doi.org/10.3390/plants13060886
Chicago/Turabian StyleMilenković, Lidija, Zoran S. Ilić, Ljiljana Stanojević, Bojana Danilović, Ljubomir Šunić, Žarko Kevrešan, Jelena Stanojević, and Dragan Cvetković. 2024. "Chemical Composition and Bioactivity of Dill Seed (Anethum graveolens L.) Essential Oil from Plants Grown under Shading" Plants 13, no. 6: 886. https://doi.org/10.3390/plants13060886






