Phytochemical Profiling and Biological Activities of Rhododendron Subsect. Ledum: Discovering the Medicinal Potential of Labrador Tea Species in the Northern Hemisphere
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nomenclature and Taxonomy
2.2. Distribution of the Subsect. Ledum Species
2.3. Phytochemical Composition of Rhododendron Subsect. Ledum Species
2.3.1. Principal Components of Essential Oil in R. tomentosum, R. goenlandicum and R. columbianum Species
| Components | Plant Part | Extraction Method | Species | Detection Method | Country | Reference | Bio-Activity |
|---|---|---|---|---|---|---|---|
| Palustrol (15.9–53.5%), | Plant shoots | hydrodistillation | R. tomentosum | Gas chromatography—mass spectrometry methods | Estonia | [8] | _ |
| Ledol (11.8–18.3%), | |||||||
| γ-terpineol (0–31.2%), | |||||||
| p-cymene (0.1–13.9%) | |||||||
| Ledol (36.5%), | Plant shoots | hydrodistillation | R. tomentosum | Gas chromatography—mass spectrometry methods | Lithuania | [9] | _ |
| Palustrol (21.0%), | |||||||
| Ascadirole (4.0%), | |||||||
| Lepanone (3.0%), | |||||||
| Lepanol (2.8%), | |||||||
| P-cymene (2.2%), | |||||||
| Myrcene (1.9%) | |||||||
| Palustrol (38.3%), | Plant seeds | hydrodistillation | R. tomentosum | Gas chromatography—mass spectrometry methods | Lithuania | [9] | _ |
| Ledol (27.0%), | |||||||
| P-Cymene (1.7%), Lepalol (1.6%), | |||||||
| Geraniol (1.2%) | |||||||
| Palustrol (24.6–33.5%) | Plant shoots and inflorescences | hydrodistillation | R. tomentosum | Gas chromatography—mass spectrometry methods | Lithuania | [9] | Antioxidant activity, Antifungal activity against Candida Parapsilosis |
| Ledol (18.0–29.0%) | |||||||
| Ascadirole (7.0–14.0%), | |||||||
| Myrcene (7.2–10.1%) | |||||||
| Lepanol (3.3–7.9%) | |||||||
| cyclocolorenone isomers (4.1%) | |||||||
| β-myrcene (31%) | Plant stems and leaves | Hydrodistillation | R. tomentosum | Gas chromatography—mass spectrometry methods | Finland | [24] | _ |
| Palustrol (38.8%) | |||||||
| Ledol (15.9 %) | |||||||
| Sabinene (0.05–35.0%) | Plant stems and leaves | hydrodiffusion | R. groenlandicum | Gas chromatography—flame ionisation detector (GC-FID) | Canada | [30] | _ |
| β-pinene (0.05–8,4%) | |||||||
| p-cymene (0.2–3.4%) | |||||||
| Limonene (0.3–67.0%) | |||||||
| Camphene (1.3%) | |||||||
| a-terpinene (2.3%) | |||||||
| Terpinolene (1.5%) | |||||||
| Terpinen-4-Ol (0.5–5.1%) | |||||||
| Myrtenal (0.3–3.8%) | |||||||
| Bornyl acetate (0.3–8.4%) | |||||||
| Sabinene (11.93%) | Plant leaves | - | R. groenlandicum | Gas chromatography–mass spectrometry (GC–MS) and gas chromatography/flame-ionization detection (GC/FID) | Canada | [31] | Antibacterial activity |
| β-Selinene (10.95%) | |||||||
| Germacrene B (9.75%) | |||||||
| α-Selinene (8.89%) | |||||||
| Germacrone (8.51%) | |||||||
| Ascaridole (67.7%) | Plant leaves | hydrodistillation | Rhododendron tomentosum ssp. subarcticum | Gas chromatography–mass spectrometry (GC–MS) and gas chromatography/flame-ionization detection (GC/FID) | Canada | [25] | Antiparasitic activity |
| p-cymene (21.1%) | |||||||
| Terpinen-4-ol (2.5%) | |||||||
| β-pinene (1.2%) | |||||||
| Sabinene (17.9%) | Plant stems and leaves | hydrodistillation | R. tomentosum | Gas chromatography–mass spectrometry (GC–MS) | Korea | [39] | Antioxidant activity, antimicrobial activity, |
| Terpinen-ol (7.61%) | |||||||
| Myrtenal (7.44%) | |||||||
| β-selinene (6.5%) | |||||||
| Myrtenol (3.53%) | |||||||
| p-cymene (25.5%) | Plant stems and leaves | hydrodistillation | R. tomentosum | Gas chromatography–mass spectrometry (GC–MS) | Poland | [37] | Insecticidal activity |
| Isoascaridole (20.5%) | |||||||
| is-ascaridole (14.8%) | |||||||
| Geranyl acetate (4.2%) |
2.3.2. Phenolic Compounds in R. tomentosum, R. goenlandicum and R. columbianum Species
2.3.3. Triterpenic Compounds in R. tomentosum, R. goenlandicum and R. columbianum Species
2.3.4. Other Compounds in R. tomentosum, R. goenlandicum and R. columbianum Species
2.4. Biological Activities of Rhododendron Subsect. Ledum Species
| Region | Part Used | Ethnopharmacological Uses | Preparation | References |
|---|---|---|---|---|
| Asia and northern europe | herb (leaves) | Arthrosis, Rheumatism, bronchitis, lung diseases, bug bites, pain relief, wounds, itch, eruptions, cold and fever, cough, sore throat, dyspepsia, dysentery, gout, leprosy and whitlow | – | [50,55] |
| Estonia | herb (leaves) | coughs, tuberculosis, cold, rheumatic diseases | – | [56,57] |
| Estonia | dried branches | repellent against bedbugs, clothing moths, fleas | – | [57] |
| Sweden, norway, | herb (leaves) | against lice | – | [57] |
| Finland | ||||
| Norway, denmark | herb (leaves) | cold, whooping cough, for rheymatism as pain reliever, for high blood pressure, bladder catarrh and diphtheria | – | [58] |
| Russia | herb (leaves) | bronchitis, tuberculosis, cough, asthma, spastic enterocolitis | the infusion | [5] |
| herb (leaves) | as anthelmintic, fever, urethritis, metrorrhagia, women’s diseases and gastritis. | the decoction | [5] | |
| herb (leaves) | eczema, scabies, insect stings, bruises, wounds, boils, hematomas, ringworm, chicken pox, blepharitis and conjuctivitis. | the oinment on the base of linseed oil or animal fats | [5] | |
| herb (leaves) | Rhinitis | drops | [5] | |
| herb (leaves) | Hypnotic and sedative effect | by smoking | [5] | |
| Yakutia (russia) and bulgaria | herb (leaves) | as abortifacient | – | [5] |
| Poland | herb (leaves) | Toothache and painful gums | mouth rinsing solution | [59] |
| China | Leaves | Infection and inflammation | – | [50] |
| Korea | Leaves | Female disorders | – | [50] |
| Tibet (china) | herb (leaves) | tuberculosis, bronchitis, endometris, jaundice and liver disease | the infusion and decoction | [5] |
| herb (leaves) | gynecological diseases | bath form | [5] | |
| herb (leaves) | diarrhea | in the form as ash | [5] | |
2.4.1. Antioxidant Properties
2.4.2. Anti-Inflammatory Properties
2.4.3. Antimicrobial and Antiviral Properties
2.4.4. Antidiabetic Properties
2.4.5. Anti-Cancer Properties
2.4.6. Other
2.5. Rhododendron Subsect. Ledum Species Toxicity
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harmaja, H. Taxonomic notes on Rhododendron subsection Ledum (Ledum, Ericaceae), with a key to its species. Ann. Bot. Fenn. 1991, 28, 171–173. [Google Scholar]
- Harmaja, H. New Names and Nomenclatural Combinations in Rhododendron (Ericaceae). Ann. Bot. Fenn. 1990, 27, 203–204. [Google Scholar]
- Khan, G.; Nolzen, J.; Schepker, H.; Albach, D.C. Incongruent phylogenies and their implications for the study of diversification, taxonomy, and genome size evolution of Rhododendron. Am. J. Bot. 2021, 108, 1957–1981. [Google Scholar] [CrossRef]
- Hart, A.; Kron, K.; Gillespie, E. Molecular phylogenetic analysis of the north-temperate Labrador teas (Ericaceae: Rhododendron subsect. Ledum) suggests a complex genetic history. J. Bot. Res. Inst. Tex. 2017, 11, 53–65. [Google Scholar] [CrossRef]
- Dampc, A.; Luczkiewicz, M. Rhododendron tomentosum (Ledum palustre). A review of traditional use based on current research. Fitoterapia 2013, 85, 130–143. [Google Scholar] [CrossRef]
- Dampc, A.; Luczkiewicz, M. Labrador tea—The aromatic beverage and spice: A review of origin, processing and safety. J. Sci. Food Agric. 2015, 95, 1577–1583. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef] [PubMed]
- Raal, A.; Orav, A.; Gretchushnikova, T. Composition of the essential oil of the Rhododendron tomentosum Harmaja from Estonia. Nat. Prod. Res. 2014, 28, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Judzentiene, A.; Butkiene, R.; Budiene, J.; Tomi, F.; Casanova, J. Composition of seed essential oils of Rhododendron tomentosum. Nat. Prod. Commun. 2012, 7, 227–230. [Google Scholar] [CrossRef]
- Zidorn, C. Plant Chemophenetics—A New Term for Plant Chemosystematics/Plant Chemotaxonomy in the Macro-Molecular Era. Phytochemistry 2019, 163, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Kristian Theqvist. Taxonomy of Species in Rhododendron Subsection Ledum. 2013. Available online: https://www.rhodogarden.com/cross/ledum_taxonomy.html (accessed on 15 November 2023).
- United States Department of Agriculture. Ledum groenlandicum Oeder. Available online: https://plants.usda.gov/home/plantProfile?symbol=LEGR (accessed on 24 November 2023).
- Burke Herbarium Image Collection. Rhododendron Columbianum. Available online: https://burkeherbarium.org/imagecollection/taxon.php?Taxon=Rhododendron%20columbianum (accessed on 24 November 2023).
- Hébert, F.; Thiffault, N. The Biology of Canadian Weeds. 146. Rhododendron groenlandicum (Oeder) Kron and Judd. Can. J. Plant Sci. 2011, 91, 725–738. [Google Scholar] [CrossRef]
- Royal Botanic Gardens Kew. Rhododendron tomentosum Harmaja. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:961622-1 (accessed on 12 September 2023).
- Anderson, M. Plant Guide for Bog Labrador Tea (Ledum groenlandicum); USDA-Natural Resources Conservation Service, National Plant Data Team: Washington, DC, USA, 2011. [Google Scholar]
- Walter, S.; Kathleen, A. Rhododendron columbianum. In Flora of North America North of Mexico; Oxford University Press: New York, NY, USA; Oxford, UK, 2009; Volume 8. [Google Scholar]
- Baldwin, T.A.; Oberbauer, S.F. Essential oil content of Rhododendron tomentosum responds strongly to manipulation of ecosystem resources in Arctic Alaska. Arct. Sci. 2022, 8, 916–934. [Google Scholar] [CrossRef]
- Spiridonov, N.; Konovalov, D.; Arhipov, V. Cytotoxicity of some Russian ethnomedicinal plants and plant compouds. Phytother. Res. 2005, 19, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, N.; Rybalko, K. A study of Ledum Chemical constitution palustre. Chem. Nat. Compd. 1980, 16, 131–135. [Google Scholar] [CrossRef]
- Dubois, M.; Wierer, M.; Wagner, H. Palustroside: A new coumarin glucoside ester from Ledum palustre. Planta Med. 1990, 56, 664–665. [Google Scholar] [CrossRef]
- Shotyk, W.; Javed, M.B.; Noernberg, T. Trace elements in Labrador Tea (Rhododendron groenlandicum): How predominant sources to the plants impact the chemical composition of hot water extracts. Environ. Res. 2020, 183, 109272. [Google Scholar] [CrossRef]
- Judzentiene, A.; Budiene, J.; Svediene, J.; Garjonyte, R. Toxic, Radical Scavenging, and Antifungal Activity of Rhododendron tomentosum H. Essential Oils. Molecules 2020, 25, 1676. [Google Scholar] [CrossRef]
- Korpinen, R.I.; Välimaa, A.-L.; Liimatainen, J.; Kunnas, S. Essential Oils and Supercritical CO2 Extracts of Arctic Angelica (Angelica archangelica L.), Marsh Labrador Tea (Rhododendron tomentosum) and Common Tansy (Tanacetum vulgare)—Chemical Compositions and Antimicrobial Activities. Molecules 2021, 26, 7121. [Google Scholar] [CrossRef]
- Séguin, J.C.; Gagnon, D.; Bélanger, S.; Richard, D.; Fernandez, X.; Boudreau, S.; Voyer, N. Chemical Composition and Antiplasmodial Activity of the Essential Oil of Rhododendron subarcticum Leaves from Nunavik, Québec, Canada. ACS Omega 2023, 8, 16729–16737. [Google Scholar] [CrossRef]
- Govaerts, R.; Lughadha, N.; Black, N. The World Checklist of Vascular Plants, a continuously updated resource for exploring global plant diversity. Sci. Data 2021, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Jesionek, A.; Kokotkiewicz, A.; Mikosik-Roczynska, A.; Ciesielska-Figlon, K.; Luczkiewicz, P.; Bucinski, A.; Daca, A.; Witkowski, J.M.; Bryl, E.; Zabiegala, B.; et al. Chemical variability of rhododendron tomentosum (Ledum palustre) essential oils and their pro-apoptotic effect on lymphocytes and rheumatoid arthritis synoviocytes. Fitoterapia 2019, 139, 104402. [Google Scholar] [CrossRef] [PubMed]
- Jesionek, A.; Kokotkiewicz, A.; Wlodarska, P.; Filipowicz, N.; Bogdan, A.; Ochocka, R.; Szreniawa-Sztajnert, A.; Zabiegala, B.; Bucinski, A.; Luczkiewicz, M. In vitro propagation of Rhododendron tomentosum—An endangered essential oil bearing plant from peatland. Acta Biol. Crac. Ser. Bot. 2016, 56, 29–43. [Google Scholar] [CrossRef]
- Jesionek, A.; Kokotkiewicz, A.; Wlodarska, P.; Zabiegala, B.; Bucinski, A.; Luczkiewicz, M. Bioreactor shoot cultures of Rhododendron tomentosum (Ledum palustre) for a large-scale production of bioactive volatile compounds. Plant Cell Tiss. Organ. Cult. 2017, 131, 51–64. [Google Scholar] [CrossRef]
- Collin, G. Aromas from Quebec. IV. Chemical composition of the essential oil of Ledum groenlandicum: A review. Am. J. Essent. Oils Nat. Prod. 2015, 2, 06–11. [Google Scholar]
- Lagha, B.A.; Vaillancourt, K.; Maquera Huacho, P.; Grenier, D. Effects of Labrador Tea, Peppermint, and Winter Savory Essential Oils on Fusobacterium nucleatum. Antibiotics 2020, 9, 794. [Google Scholar]
- Marion, E. Valorisation d’extraits de fleurs de Rhododendron groenlandicum pour des Applications Cosmétiques. Master’s Thesis, University of Quebec at Chicoutimi, Chicoutimi, QC, Canada, 2018; pp. 1–70. [Google Scholar]
- Ehlers, B.K.; Thompson, J. Do co-occurring plant species adapt to one another? The response of Bromus erectus to the presence of different Thymus vulgaris chemotypes. Oecologia 2004, 141, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Tam, T.W.; Liu, R.; Saleem, A.; Arnason, J.T.; Krantis, A.; Haddad, P.S.; Foster, B.C. The effect of Cree traditional medicinal teas on the activity of human cytochrome P450-mediated metabolism. J. Ethnopharmacol. 2014, 155, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Dufour, D.; Pichette, A.; Mshvildadze, V.; Bradette-Hébert, M.; Lavoie, S.; Longtin, A.; Laprise, C.; Legault, J. Antioxidant, anti-inflammatory and anticancer activities of methanolic extracts from Ledum groenlandicum Retzius. J. Ethnopharmacol. 2007, 111, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Ouchfoun, M.; Saleem, A.; Jose, A.; Analco, G.; Walshe-Roussel, B.; Musallam, L.; Rapinski, M.; Cuerrier, A.; Martineau, L.C.; et al. A combination of (þ)-catechin and ()-epicatechin underlies the in vitro adipogenic action of Labrador tea (Rhododendron groenlandicum), an antidiabetic medicinal plant of the Eastern James Bay Cree pharmacopeia. J. Ethnopharmacol. 2016, 178, 251–257. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Cianfaglione, K.; Sender, J.; Danuta, U.; Maślanko, W.; Canale, A.; Barboni, L.; Petrelli, R.; Zeppa, L.; et al. Ascaridole-rich essential oil from marsh rosemary (Ledum palustre) growing in Poland exerts insecticidal activity on mosquitoes, moths and flies without serious effects on non-target organisms and human cells. Food Chem. Toxicol. 2020, 138, 111184. [Google Scholar] [CrossRef]
- Royal Botanic Gardens Kew. Rhododendron Columbianum (Piper) Harmaja. Available online: https://powo.science.kew.org/taxon/947975-1?_gl=1*55sizk*_ga*NzY5OTQ1OTQuMTY5MjU1NTMyMA..*_ga_ZVV2HHW7P6*MTY5MzczMDU2Mi4xMS4xLjE2OTM3MzA1NzEuMC4wLjA.#synonyms (accessed on 12 September 2023).
- Kim, D.M.; Nam, B.W. Extracts and essential oil of Ledum palustre L. leaves and their antioxidant and antimicrobial activities. J. Food Sci. Nutr. 2006, 11, 100–104. [Google Scholar] [CrossRef]
- Jiang, S.; Ding, Q.; Wu, Y.; Zhong, Z.; Ouyang, J. Comparison of the Chemical Compounds and Antioxidant Activities of Essential Oil and Ethanol Extract from Rhododendron tomentosum Harmaja. J. Essent. Oil Bear. Plants 2017, 20, 927–936. [Google Scholar] [CrossRef]
- Black, P.; Saleem, A.; Dunford, A.; Guerrero-Analco, J.; Walshe-Roussel, B.; Haddad, P. Seasonal variation of phenolic constituents and medicinal activities of Northern Labrador tea, Rhododendron tomentosum ssp. subarcticum, an Inuit and cree First Nations traditional medicine. Planta Med. 2011, 77, 1655–1662. [Google Scholar]
- Thomas, M.; Lamara, M.; Asselin, H.; Fenton, N.J. Effects of industrial disturbances on the flavonoid concentration of Rhododendron groenlandicum. Botany 2023, 101, 343–356. [Google Scholar] [CrossRef]
- Rapinski, M.; Liu, R.; Saleem, A.; Arnason, J.T.; Cuerrier, A. Environmental trends in the variation of biologically active phenolic compounds in Labrador tea, Rhododendron groenlandicum, from northern Quebec, Canada. Botany 2014, 92, 783–794. [Google Scholar] [CrossRef]
- Saleem, A.; Harris, C.S.; Asim, M.; Cuerrier, A.; Martineau, L.; Haddad, P.S.; Arnason, J.T. A RP-HPLC-DAD-APCI/MSD method for the characterisation of medicinal Ericaceae used by the Eeyou Istchee Cree First Nations. Phytochem. Anal. 2010, 21, 328–339. [Google Scholar] [CrossRef]
- Zhang, K. Triterpenoids isolated from leaves of Ledum palustre. Chin. Tradit. Herb. Drugs 2018, 24, 1250–1254. [Google Scholar]
- McGill, C.M.; Tomco, P.L.; Ondrasik, R.M.; Belknap, K.C.; Dwyer, G.K.; Quinlan, D.J.; Kircher, T.A.; Andam, C.P.; Brown, T.J.; Claxton, D.F.; et al. Therapeutic effect of Northern Labrador tea extracts for acute myeloid leukemia. Phytother. Res. 2018, 32, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, W.; Liu, H.; Wu, G.; Song, M.; Yang, B.; Yang, D.; Wang, Q.; Kuang, H. Simultaneous Determination of Aesculin, Aesculetin, Fraxetin, Fraxin and Polydatin in Beagle Dog Plasma by UPLC-ESI-MS/MS and Its Application in a Pharmacokinetic Study after Oral Administration Extracts of Ledum palustre L. Molecules 2018, 23, 2285. [Google Scholar] [CrossRef]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Popescu, R.; Kopp, B. The genus Rhododendron: An ethnopharmacological and toxicological review. J. Etnopharmacol. 2013, 147, 42–62. [Google Scholar] [CrossRef]
- Jin, C.; Strembiski, W.; Kulchytska, Y.; Micetich, R.G.; Danesstalab, M. Flavonoid glycosides from Ledum palustre L. subsp. decumbens (Ait.) Hulton. DARU J. Pharm. Sci. 1999, 7, 4. [Google Scholar]
- Ryzhikova, M.A.; Gabitova, D.M.; Ryzhikova, V.O. Herbal preparation of Ledum palustre—Perspective remedy for medical treatment of bronchitis and bronchial asthma pathology. Bashkirskii Khimicheskii Zhurnal 2006, 13, 47–49. [Google Scholar]
- Narimanov, A.A. Protective efficacy of a mixture of extracts for Archangelica officinalis and Ledum palustre against fractionated gamma-irradiation of mice. Radiobiologiya 1993, 33, 280–284. [Google Scholar]
- Marles, R.J.; Clavelle, C.; Monteleone, L.; Tays, N.; Burns, D. Aboriginal Plant Use in Canadaʼs Northwest Boreal Forest; UBC Press: Vancouver, BC, Canada, 2000; p. 368. [Google Scholar]
- Harbilas, D.; Martineau, L.C.; Harris, C.S.; Adeyiwola-Spoor, D.C.; Saleem, A.; Lambert, J. Evaluation of the antidiabetic potential of selected medicinal plant extracts from the Canadian boreal forest used to treat symptoms of diabetes: Part II. Can. J. Physiol. Pharmacol. 2009, 87, 479–492. [Google Scholar] [CrossRef]
- Butkienė, R.; Šakočiūtė, V.; Latvėnaitė, D.; Mockutė, D. Composition of young and aged shoot essential oils of the wild Ledum palustre L. Chemija 2008, 19, 19–24. [Google Scholar]
- Egigu, M.C.; Ibrahim, M.A.; Yahya, A.; Holopainen, J.K. Cordeauxia edulis and Rhododendron tomentosum extracts disturb orientation and feeding behavior of Hylobius abietis and Phyllodecta laticollis. Entomol. Exp. Appl. 2011, 138, 162–174. [Google Scholar] [CrossRef]
- Sõukand, R.; Kalle, R.; Svanberg, I. Uninvited guests: Traditional insect repellents in Estonia used against the clothes moth Tineola bisselliella, human flea Pulex irritons and bedbug Cimex lectularius. J. Insect Sci. 2010, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Alm, T.; Iversen, M. Norway’s Rosmarin (Rhododendron tomentosum) in past and present tradition. In Ethnobotany in the New Europe: People, Health and Wild Plant Resources; Pardo-de-Santayana, M., Pieroni, A., Puri, R.K., Eds.; Berghahn Books: Brooklyn, NY, USA, 2010; pp. 263–270. [Google Scholar]
- Wawrzyniak, E. Leczenie ziołami. Kompendium fitoterapii; Instytut Wydawniczy Związków Zawodowych: Warszawa, Poland, 1992. [Google Scholar]
- Antropova, I.G.; Revina, A.A.; Kurakina, E.S.; Magomedbekov, E.P. Radiation Chemical Investigation of Antioxidant Activity of Biologically Important Compounds from Plant Materials. ACS Omega 2020, 5, 5976–5983. [Google Scholar] [CrossRef] [PubMed]
- Tunón, H.; Olavsdotter, C.; Bohlin, L. Evaluation of anti-inflammatory activity of some Swedish medicinal plants. Inhibition of prostaglandin biosynthesis and PAF-induced exocytosis. J. Ethnopharmacol. 1995, 48, 61–76. [Google Scholar] [CrossRef]
- Xiao, H.-T.; Wen, B.; Shen, X.-C.; Bian, Z.-X. Potential of Plant-sourced Phenols for Inflammatory Bowel Disease. Curr. Med. Chem. 2018, 25, 5191–5237. [Google Scholar] [CrossRef] [PubMed]
- Spoor, D.C.; Martineau, L.C.; Leduc, C.; Benhaddou-Andaloussi, A.; Meddah, B.; Harris, C.; Burt, A.; Fraser, M.H.; Coonishish, J.; Joly, E.; et al. Selected plant species from the Cree pharmacopoeia of northern Quebec possess anti-diabetic po- tential. Can. J. Physiol. Pharmacol. 2006, 84, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Brault, A.; Villavicencio, M.S.; Haddad, P.S. Rhododendron groenlandicum (Labrador tea), an antidiabetic plant from the traditional pharmacopoeia of the Canadian Eastern James Bay Cree, improves renal integrity in the diet-induced obese mouse model. Pharm. Biol. 2016, 54, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, R.; Egan, P.A.; Rosindell, J.; Farrell, I.W.; Stevenson, P.C. Grayanotoxin I variation across tissues and species of Rhododendron suggests pollinator-herbivore defence trade-offs. Phytochemistry 2023, 212, 113707. [Google Scholar] [CrossRef]
- Plugge, P.C.; de Zaayer, H.G. Andromedotoxin. Am. J. Pharm. 1889, 61, 6–8. [Google Scholar]

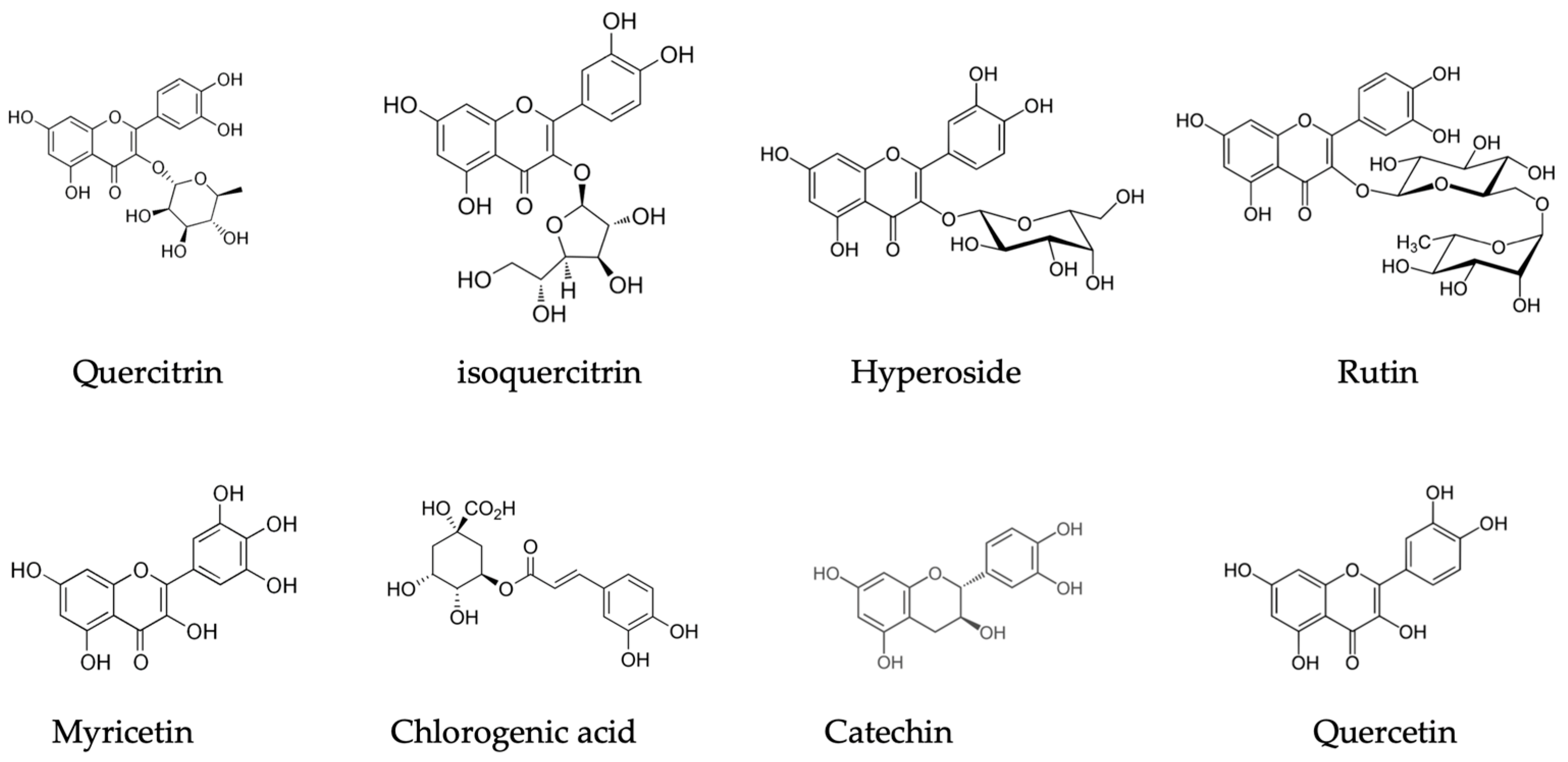
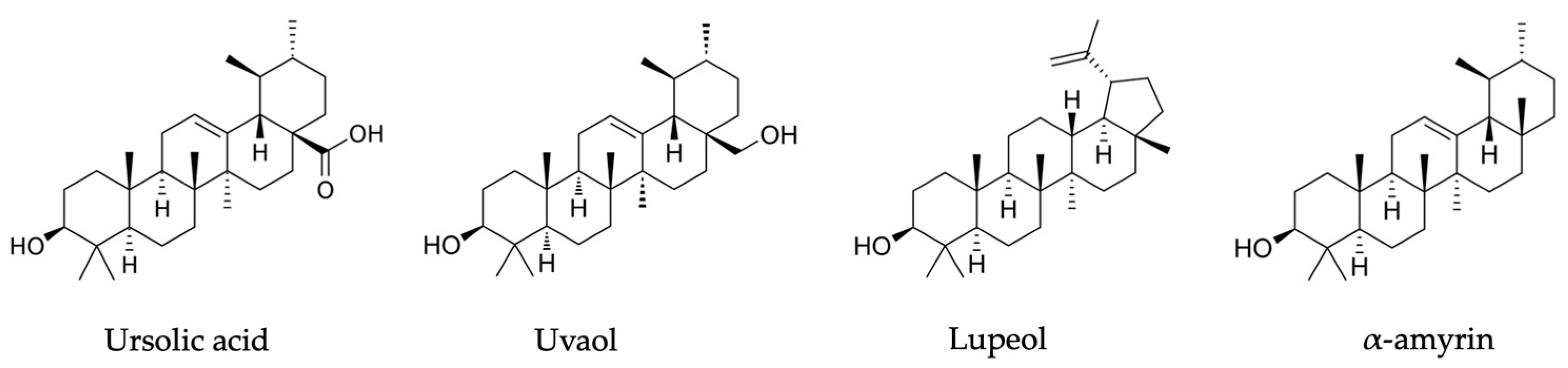
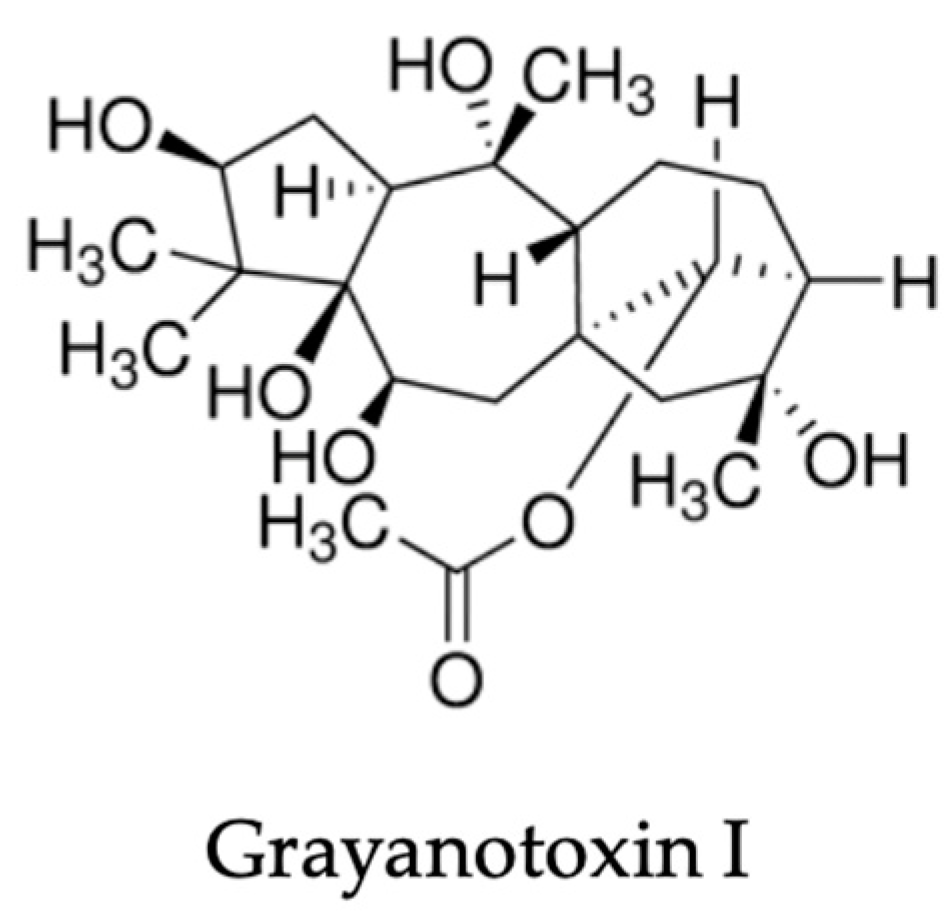
| Components | Plant Part | Extraction Method | Species | Detection Method | Country | Reference | Bio-Activity |
|---|---|---|---|---|---|---|---|
| (+)-catechin | twigs | 80% ethanol | Rhododendron tomentosum ssp. subarcticum | HPLC-DAD | Canada | [41] | Antioxidant activity; TNF-α anti-inflammatory |
| Quercetin pentoside | |||||||
| Quercetin 3-O-galactoside (4.58 mg/gDW) | |||||||
| procyanidin B2 | |||||||
| procyanidin B3 | |||||||
| Procyanidin B1 | |||||||
| Caffeic acid derivatives | |||||||
| Myricetin, quercetin, quercetin 3-O-glucoside, quercetin 3-O-rhamnoside—minor compounds | |||||||
| quercetin-3-galactoside | leaves | 80% ethanol | Rhododendron groenlandicum | HPLC-DAD | Canada | [43] | — |
| quercetin-glycoside | |||||||
| (+)-catechin | |||||||
| chlorogenic acid | |||||||
| (–)-epicatechin | |||||||
| Taxifolin glycoside | leaves | 80% ethanol | Rhododendron tomentosum ssp. subarcticum | HPLC-DAD | Canada | [44] | — |
| taxifolin | |||||||
| Catechin | |||||||
| Chlorogenic acid | |||||||
| (−)-Epicatechin | |||||||
| (+)-Catechin | |||||||
| Caffeoylquinic acid | |||||||
| Proanthocyanidin A1 | |||||||
| Quercetin-3-O-galactoside | |||||||
| Quercetin-3-O-glucoside | |||||||
| Proanthocyanidin A2 | |||||||
| Quercetin glycoside | |||||||
| Myricetin | |||||||
| (+)-Catechin | leaves | 80% ethanol | Rhododendron groenlandicum | HPLC-DAD | Canada | [44] | - |
| Chlorogenic acid | |||||||
| (−)-Epicatechin | |||||||
| Caffeoylquinic acid | |||||||
| Procyanidin B2 | |||||||
| Procyanidin A1 | |||||||
| Quercetin-3-O-galactoside | |||||||
| Quercetin-3-O-glucoside | |||||||
| Proanthocyanidin A2 | |||||||
| Quercetin glycoside | |||||||
| Myricetin | |||||||
| (+)-Catechin | leaves | 80% ethanol | Rhododendron groenlandicum | HPLC-DAD-MS | Canada | [36] | - |
| (−)-Epicatechin | |||||||
| Quercetin | |||||||
| Chlorogenic acid | |||||||
| Quercetin-3-O-galactoside | |||||||
| Quercetin-3-O-glucoside | |||||||
| Quercetin-3-O-arabinoside | |||||||
| quercetin-glycoside | |||||||
| (+)-Catechin | leaves | 80% methanol | Rhododendron groenlandicum | UHPLC-PDA | Canada | [42] | - |
| (−)-Epicatechin | |||||||
| Procyanidin A1 | |||||||
| Proanthocyanidin A2 | |||||||
| Quercetin-3-O-galactoside | |||||||
| Quercetin-3-O-glucoside | |||||||
| Quercetin glycosides | |||||||
| Quercetin | |||||||
| Myricetin derivatives | |||||||
| Myricetin | |||||||
| uvaol | leaves | chromatographic purification | Rhododendron tomentosum (Ledum palustre) | HPLC | China | [45] | - |
| lepenone | |||||||
| α-amyrenone | |||||||
| ursolic acid | |||||||
| lupeol | |||||||
| amyrin | |||||||
| α-fern-9(11)-ene-2α,3β-diol | |||||||
| fernenol | |||||||
| 6a-hydroxy-14-taraxerene-3,16,21- trione | leaves | absolute ethanol | Rhododendron tomentosum (Ledum palustre) | HR-ESI-TOFMS | China | [45] | - |
| 6a,26-dihydroxy-14-taraxerene-3,16,21-trione | |||||||
| ursolic acid | leaves | acetone | Rhododendron tomentosum | GC-MS | Alaska | [46] | anti -acute myeloid leukemia activity. |
| aesculin | leaves | ethanol | Rhododendron tomentosum | UPLC-MS; HPLC | China | [47] | anti-inflammatory, anti-oxidant, anti-tumor, anti-viral |
| aesculetin | |||||||
| fraxetin | |||||||
| fraxin | |||||||
| polydatin | |||||||
| Chlorogenic acid | leaves | decoctions with boiling water; ethanol | Rhododendron groenlandicum | HPLC-DAD | Canada | [34] | inhibition towards CYP3A4 |
| (+)-Catechin | |||||||
| (−)-Epicatechin | |||||||
| Quercetin-3-O-galactoside | |||||||
| Quercetin-3-O-rutinoside |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vengrytė, M.; Raudonė, L. Phytochemical Profiling and Biological Activities of Rhododendron Subsect. Ledum: Discovering the Medicinal Potential of Labrador Tea Species in the Northern Hemisphere. Plants 2024, 13, 901. https://doi.org/10.3390/plants13060901
Vengrytė M, Raudonė L. Phytochemical Profiling and Biological Activities of Rhododendron Subsect. Ledum: Discovering the Medicinal Potential of Labrador Tea Species in the Northern Hemisphere. Plants. 2024; 13(6):901. https://doi.org/10.3390/plants13060901
Chicago/Turabian StyleVengrytė, Martyna, and Lina Raudonė. 2024. "Phytochemical Profiling and Biological Activities of Rhododendron Subsect. Ledum: Discovering the Medicinal Potential of Labrador Tea Species in the Northern Hemisphere" Plants 13, no. 6: 901. https://doi.org/10.3390/plants13060901







