Evidence for Reductions in Physical and Chemical Plant Defense Traits in Island Flora
Abstract
:1. Introduction
2. Results
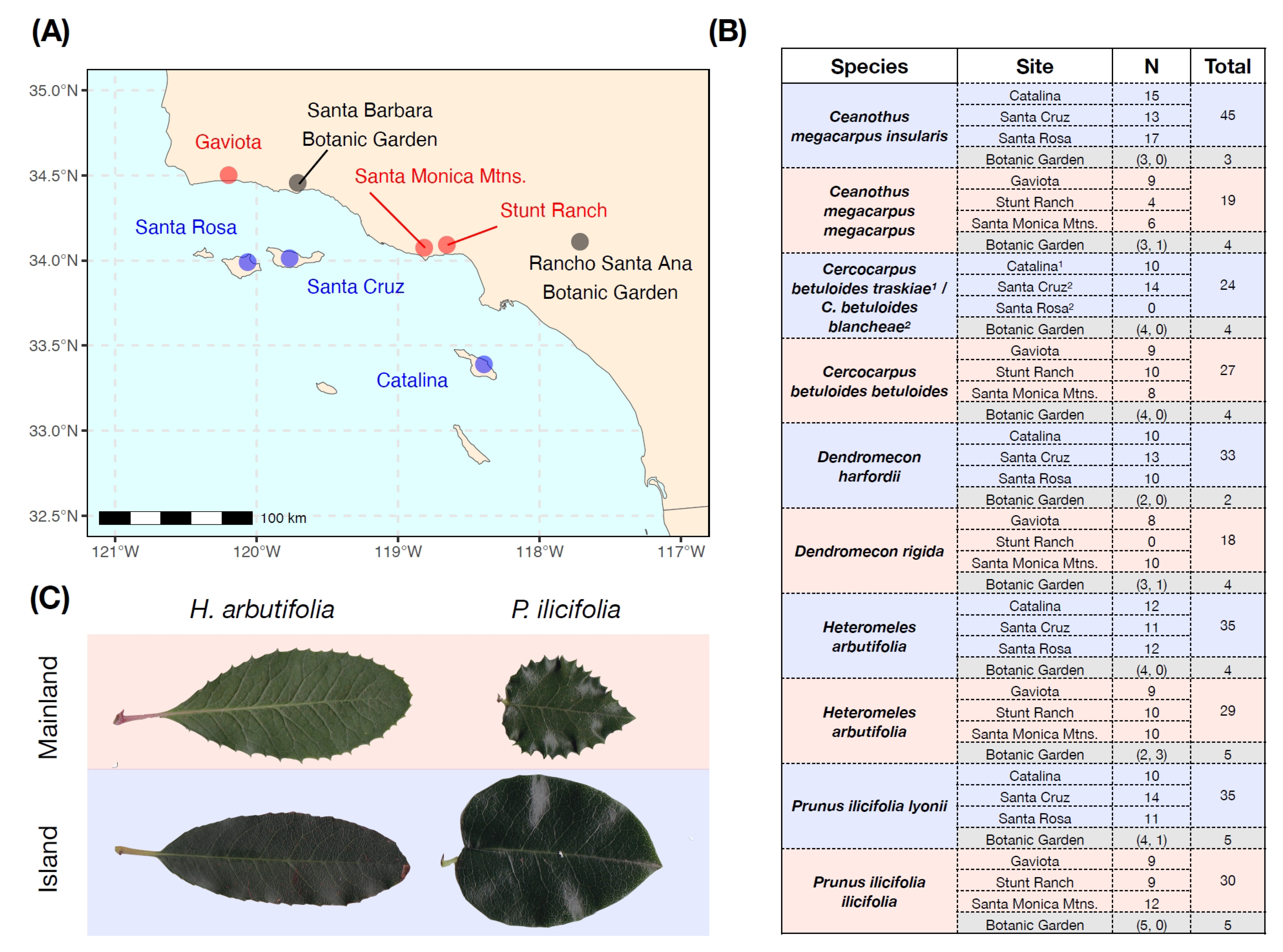
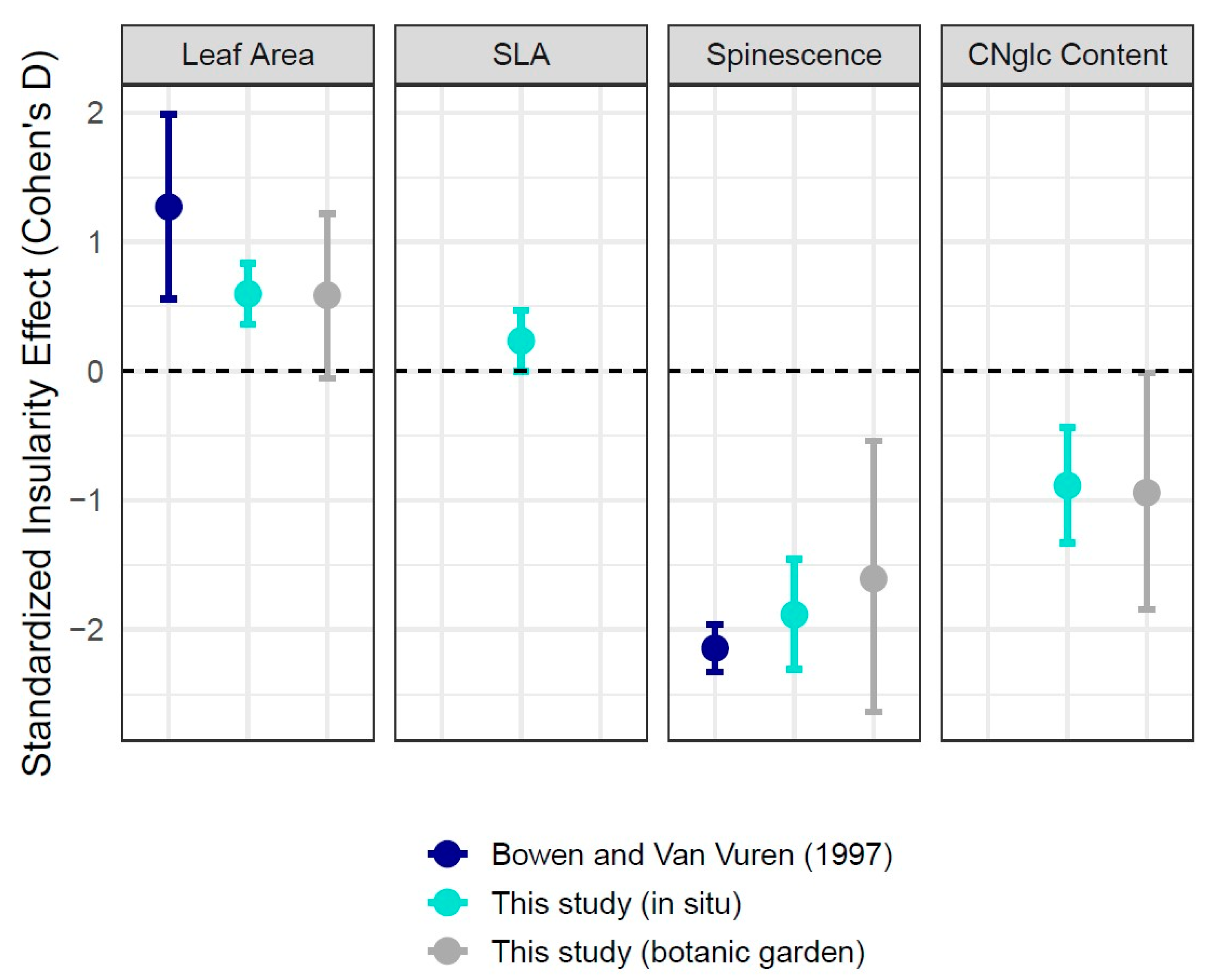
2.1. Chaparral Shrubs–Field Sampling
2.2. Chaparral Shrubs–Botanic Garden Sampling
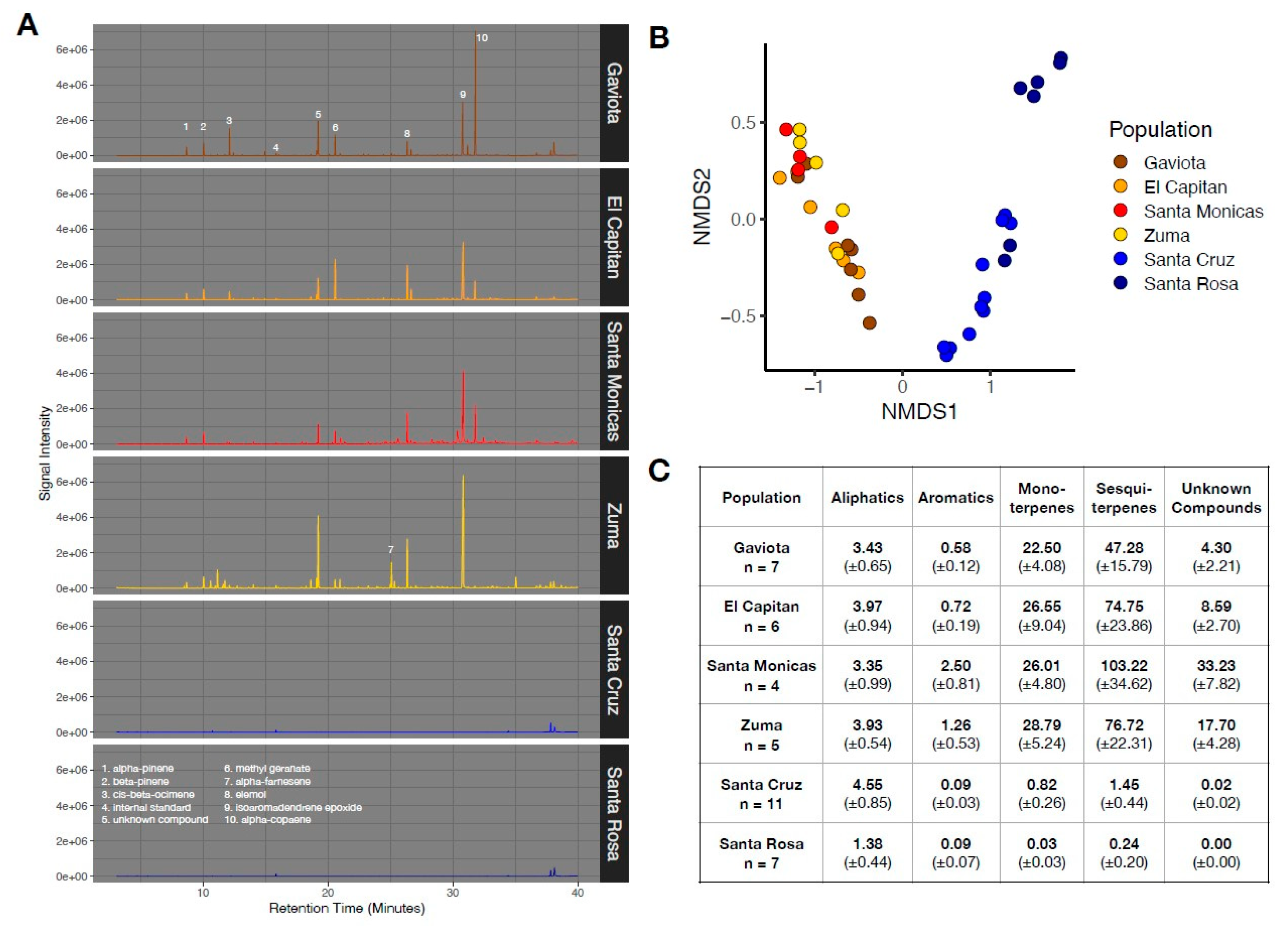
2.3. Stachys Common Garden
3. Discussion
4. Methods
4.1. Background–California Channel Islands
4.2. Study 1: Chaparral Shrub Sampling
4.3. Study 1: Abiotic Variation between Sites
4.4. Study 1: Statistical Analyses
4.5. Study 2: Stachys bullata–Background
4.6. Study 2: Stachys bullata Common Garden Experiment
4.7. Study 2: Stachys Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coley, P.D.; Bryant, J.P.; Chapin, F.S. Resource Availability and Plant Antiherbivore Defense. Science 1985, 230, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Herms, D.A.; Mattson, W.J. The Dilemma of Plants: To Grow or Defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Pennings, S.C.; Silliman, B.R. Linking Biogeography and Community Ecology: Latitudinal Variation in Plant–herbivore Interaction Strength. Ecology 2005, 86, 2310–2319. [Google Scholar] [CrossRef]
- Hahn, P.G.; Agrawal, A.A.; Sussman, K.I.; Maron, J.L. Population Variation, Environmental Gradients, and the Evolutionary Ecology of Plant Defense against Herbivory. Am. Nat. 2019, 193, 20–34. [Google Scholar] [CrossRef]
- Levin, D.A. Alkaloid-Bearing Plants: An Ecogeographic Perspective. Am. Nat. 1976, 110, 261–284. [Google Scholar] [CrossRef]
- Schemske, D.W.; Mittelbach, G.G.; Cornell, H.V.; Sobel, J.M.; Roy, K. Is There a Latitudinal Gradient in the Importance of Biotic Interactions? Annu. Rev. Ecol. Evol. Syst. 2009, 40, 245–269. [Google Scholar] [CrossRef]
- Rasmann, S.; Agrawal, A.A. Latitudinal Patterns in Plant Defense: Evolution of Cardenolides, Their Toxicity and Induction Following Herbivory. Ecol. Lett. 2011, 14, 476–483. [Google Scholar] [CrossRef]
- Pellissier, L.; Roger, A.; Bilat, J.; Rasmann, S. High elevation Plantago lanceolata plants Are Less Resistant to Herbivory than Their Low Elevation Conspecifics: Is It Just Temperature? Ecography 2014, 37, 950–959. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography: Ecology, Evolution, and Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Carlquist, S. Natural History of Islands: Island Biology; Columbia University Press: New York, NY, USA, 1974. [Google Scholar]
- Burns, K.C. Evolution in Isolation: The Search for an Island Syndrome in Plants; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Ottaviani, G.; Keppel, G.; Götzenberger, L.; Harrison, S.; Opedal, Ø.H.; Conti, L.; Liancourt, P.; Klimešová, J.; Silveira, F.A.O.; Jiménez-Alfaro, B.; et al. Linking Plant Functional Ecology to Island Biogeography. Trends Plant Sci. 2020, 25, 329–339. [Google Scholar] [CrossRef]
- Zizka, A.; Onstein, R.E.; Rozzi, R.; Weigelt, P.; Kreft, H.; Steinbauer, M.J.; Bruelheide, H.; Lens, F. The Evolution of Insular Woodiness. Proc. Natl. Acad. Sci. USA 2022, 119, e2208629119. [Google Scholar] [CrossRef]
- Bowen, L.; Van Vuren, D. Insular Endemic Plants Lack Defenses against Herbivores. Conserv. Biol. 1997, 11, 1249–1254. [Google Scholar] [CrossRef]
- Burns, K.C. Spinescence in the New Zealand Flora: Parallels with Australia. N. Z. J. Bot. 2016, 54, 273–289. [Google Scholar] [CrossRef]
- Burns, K.C. Are There General Patterns in Plant Defence against Megaherbivores? Biol. J. Linn. Soc. 2014, 111, 38–48. [Google Scholar] [CrossRef]
- Kavanagh, P.H. Herbivory and the Evolution of Divaricate Plants: Structural Defences Lost on an Offshore Island: Moa Herbivory and Divaricate Plants. Austral Ecol. 2015, 40, 206–211. [Google Scholar] [CrossRef]
- Watts, S.M.; Dodson, C.D.; Reichman, O.J. The Roots of Defense: Plant Resistance and Tolerance to Belowground Herbivory. PLoS ONE 2011, 6, e18463. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, A.; Miyashita, T. Deer Browsing Reduces Leaf Damage by Herbivorous Insects through an Induced Response of the Host Plant. Ecol. Res. 2002, 17, 527–533. [Google Scholar] [CrossRef]
- Atkinson, I.A. Introduced Animals and Extinctions. In Conservation for the Twenty-First Century; Western, D., Pearl, M.C., Eds.; Oxford University Press: New York, NY, USA, 1989; pp. 54–69. [Google Scholar]
- Bryant, J.P.; Tahvanainen, J.; Sulkinoja, M.; Julkunen-Tiitto, R.; Reichardt, P.; Green, T. Biogeographic Evidence for the Evolution of Chemical Defense by Boreal Birch and Willow Against Mammalian Browsing. Am. Nat. 1989, 134, 20–34. [Google Scholar] [CrossRef]
- Greenwood, R.M. Some Differences between Plants of the Chatham Islands and the New Zealand Mainland. N. Z. J. Ecol. 1992, 16, 51–52. [Google Scholar]
- Cubas, J.; Irl, S.D.H.; Villafuerte, R.; Bello-Rodríguez, V.; Rodríguez-Luengo, J.L.; Del Arco, M.; Martín-Esquivel, J.L.; González-Mancebo, J.M. Endemic Plant Species Are More Palatable to Introduced Herbivores than Non-Endemics. Proc. Biol. Sci. 2019, 286, 20190136. [Google Scholar] [CrossRef]
- Moreira, X.; Castagneyrol, B.; García-Verdugo, C.; Abdala-Roberts, L. A Meta-analysis of Insularity Effects on Herbivory and Plant Defences. J. Biogeogr. 2021, 48, 386–393. [Google Scholar] [CrossRef]
- Moreira, X.; Abdala-Roberts, L.; Castagneyrol, B.; Caujapé-Castells, J.; Cruz-Guedes, J.; Lago-Núñez, B.; Vicens-Fornés, M.; García-Verdugo, C. A Phylogenetically Controlled Test Does Not Support the Prediction of Lower Putative Anti-Herbivore Leaf Traits for Insular Woody Species. J. Biogeogr. 2022, 49, 274–285. [Google Scholar] [CrossRef]
- Moreira, X.; Abdala-Roberts, L. A Roadmap for Future Research on Insularity Effects on Plant–herbivore Interactions. Glob. Ecol. Biogeogr. 2022, 31, 602–610. [Google Scholar] [CrossRef]
- Bond, W.J.; Silander, J.A. Springs and Wire Plants: Anachronistic Defences against Madagascar’s Extinct Elephant Birds. Proc. Biol. Sci. 2007, 274, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Skaien, C.L.; Arcese, P. Spatial Variation in Herbivory, Climate and Isolation Predicts Plant Height and Fruit Phenotype in Plectritis congesta Populations on Islands. J. Ecol. 2018, 106, 2344–2352. [Google Scholar] [CrossRef]
- Monroy, P.; García-Verdugo, C. Testing the Hypothesis of Loss of Defenses on Islands across a Wide Latitudinal Gradient of Periploca laevigata Populations. Am. J. Bot. 2019, 106, 303–312. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Gourlay, G.; Constabel, C.P. Condensed Tannins Are Inducible Antioxidants and Protect Hybrid Poplar against Oxidative Stress. Tree Physiol. 2019, 39, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Vourc’h, G.; Martin, J.-L.; Duncan, P.; Escarré, J.; Clausen, T.P. Defensive Adaptations of Thuja plicata to Ungulate Browsing: A Comparative Study between Mainland and Island Populations. Oecologia 2001, 126, 84–93. [Google Scholar] [CrossRef]
- Salladay, R.A.; Ramirez, A.R. Reduced Defenses and Increased Herbivore Preference of Island Chaparral Shrubs Compared to Mainland Relatives. West. N. Am. Nat. 2018, 78, 768–776. [Google Scholar] [CrossRef]
- Junak, S. A Flora of Santa Cruz Island; Santa Barbara Botanic Garden with the California Native Plant Society: Santa Barbara, CA, USA, 1995. [Google Scholar]
- Charles-Dominique, T.; Davies, T.J.; Hempson, G.P.; Bezeng, B.S.; Daru, B.H.; Kabongo, R.M.; Maurin, O.; Muasya, A.M.; van der Bank, M.; Bond, W.J. Spiny Plants, Mammal Browsers, and the Origin of African Savannas. Proc. Natl. Acad. Sci. USA 2016, 113, E5572–E5579. [Google Scholar] [CrossRef]
- Barton, K.E. Prickles, Latex, and Tolerance in the Endemic Hawaiian Prickly Poppy (Argemone glauca): Variation between Populations, across Ontogeny, and in Response to Abiotic Factors. Oecologia 2014, 174, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Givnish, T.J.; Kriebel, R. Causes of Ecological Gradients in Leaf Margin Entirety: Evaluating the Roles of Biomechanics, Hydraulics, Vein Geometry, and Bud Packing. Am. J. Bot. 2017, 104, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.E.; Carpenter, J.K.; Flores, A.; Saez, L.; Armani, M. Spinescence and the Island Plant Defense Syndrome. In Ecology and Evolution of Plant-Herbivore Interactions on Islands; Moreira, X., Abdala-Roberts, L., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 13–29. [Google Scholar]
- Clark, L.L.; Burns, K.C. The Ontogeny of Leaf Spines: Progressive versus Retrogressive Heteroblasty in Two New Zealand Plant Species. N. Z. J. Bot. 2015, 53, 15–23. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Woodrow, I.E. Mini-Review: Constraints on Effectiveness of Cyanogenic Glycosides in Herbivore Defense. J. Chem. Ecol. 2002, 28, 1301–1313. [Google Scholar] [CrossRef]
- Moreira, X.; Castagneyrol, B.; Mata, R.; Fyllas, N.M.; Galmán, A.; García-Verdugo, C.; Larrinaga, A.R.; Abdala-Roberts, L. Effects of Insularity on Insect Leaf Herbivory and Chemical Defences in a Mediterranean Oak Species. J. Biogeogr. 2019, 46, 1226–1233. [Google Scholar] [CrossRef]
- Pardo, A.; Cáceres, Y.; Pulido, F. Intraspecific Variation in Heritable Secondary Metabolites and Defensive Strategies in a Relict Tree. J. Plant Ecol. 2016, 11, 256–265. [Google Scholar] [CrossRef]
- Santangelo, J.S.; Ness, R.W.; Cohan, B.; Fitzpatrick, C.R.; Innes, S.G.; Koch, S.; Miles, L.S.; Munim, S.; Peres-Neto, P.R.; Prashad, C.; et al. Global Urban Environmental Change Drives Adaptation in White Clover. Science 2022, 375, 1275–1281. [Google Scholar] [CrossRef]
- Dement, W.A.; Mooney, H.A. Seasonal Variation in the Production of Tannins and Cyanogenic Glucosides in the Chaparral Shrub, Heteromeles arbutifolia. Oecologia 1974, 15, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Goodger, J.Q.D.; Gleadow, R.M.; Woodrow, I.E. Growth Cost and Ontogenetic Expression Patterns of Defence in Cyanogenic Eucalyptus Spp. Trees 2006, 20, 757–765. [Google Scholar] [CrossRef]
- Lens, F.; Davin, N.; Smets, E.; del Arco, M. Insular Woodiness on the Canary Islands: A Remarkable Case of Convergent Evolution. Int. J. Plant Sci. 2013, 174, 992–1013. [Google Scholar] [CrossRef]
- Lehndal, L.; Ågren, J. Herbivory Differentially Affects Plant Fitness in Three Populations of the Perennial Herb Lythrum salicaria along a Latitudinal Gradient. PLoS ONE 2015, 10, e0135939. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, L.D.L.; Berner, L.T.; Badgley, G.; Sethi, M.L.; Law, B.E.; HilleRisLambers, J. Within-Species Patterns Challenge Our Understanding of the Leaf Economics Spectrum. Ecol. Lett. 2018, 21, 734–744. [Google Scholar] [CrossRef] [PubMed]
- García-Verdugo, C.; Moreira, X.; Caujapé-Castells, J.; Flexas, J. Leaf Traits Linked to Herbivory in Lineages with Mediterranean-Macaronesian Distributions: Does an Island Syndrome in Plant Defence Exist? In Ecology and Evolution of Plant-Herbivore Interactions on Islands; Moreira, X., Abdala-Roberts, L., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 55–67. [Google Scholar]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global Climatic Drivers of Leaf Size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, C.; Albert, V.A. Origin of the Hawaiian Endemic Mints within North American Stachys (Lamiaceae). Am. J. Bot. 2002, 89, 1709–1724. [Google Scholar] [CrossRef]
- Roy, T.; Cole, L.W.; Chang, T.-H.; Lindqvist, C. Untangling Reticulate Evolutionary Relationships among New World and Hawaiian Mints (Stachydeae, Lamiaceae). Mol. Phylogenet. Evol. 2015, 89, 46–62. [Google Scholar] [CrossRef]
- Wagner, W.L. Nomenclator and Review of Phyllostegia (Lamiaceae). Novon 1999, 9, 265–279. [Google Scholar] [CrossRef]
- Juvik, S.P.; Juvik, J.O.; Paradise, T.R. The Biotic Environment. In Atlas of Hawaii, Third Edition; University of Hawaiʻi Press: Honolulu, HI, USA, 2009; pp. 101–158. [Google Scholar]
- Sardans, J.; Llusià, J.; Niinemets, U.; Owen, S.; Peñuelas, J. Foliar Mono- and Sesquiterpene Contents in Relation to Leaf Economic Spectrum in Native and Alien Species in Oahu (Hawai’i). J. Chem. Ecol. 2010, 36, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Pearse, I.S.; Hipp, A.L. Global Patterns of Leaf Defenses in Oak Species. Evolution 2012, 66, 2272–2286. [Google Scholar] [CrossRef]
- García-Verdugo, C.; Monroy, P.; Pugnaire, F.I.; Jura-Morawiec, J.; Moreira, X.; Flexas, J. Leaf Functional Traits and Insular Colonization: Subtropical Islands as a Melting Pot of Trait Diversity in a Widespread Plant Lineage. J. Biogeogr. 2020, 47, 2362–2376. [Google Scholar] [CrossRef]
- Sorlien, C.C.; Halvorson, W.L.; Maender, G.J. Faulting and Uplift of the Northern Channel Islands, California. In Proceedings of the Fourth California Islands Symposium: Update on the Status of Resources; Halvorson, W.L., Maender, G.J., Eds.; Santa Barbara Museum of Natural History: Santa Barbara, CA, USA, 1994; pp. 281–296. [Google Scholar]
- Kennett, D.J.; Kennett, J.P.; West, G.J.; Erlandson, J.M.; Johnson, J.R.; Hendy, I.L.; West, A.; Culleton, B.J.; Jones, T.L.; Stafford, T.W. Wildfire and Abrupt Ecosystem Disruption on California’s Northern Channel Islands at the Ållerød–Younger Dryas Boundary (13.0–12.9ka). Quat. Sci. Rev. 2008, 27, 2530–2545. [Google Scholar] [CrossRef]
- Guilliams, C.M.; Carter, B.; Ceceña-Sanchez, M.L.; Delgadillo, J.; Holzman, B.; Junak, S.; Knapp, D.; Luno-Mendoza, L.; Vanderplank, S. The Remarkable Flora of the California Islands. Fremontia 2017, 45, 5–11. [Google Scholar]
- Agenbroad, L.D. Giants and Pygmies: Mammoths of Santa Rosa Island, California (USA). Quat. Int. 2012, 255, 2–8. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.R.; Pratt, R.B.; Jacobsen, A.L.; Davis, S.D. Exotic Deer Diminish Post-Fire Resilience of Native Shrub Communities on Santa Catalina Island, Southern California. Plant Ecol. 2012, 213, 1037–1047. [Google Scholar] [CrossRef]
- Dvorak, T.M.; Catalano, A.E. Exclusion of Introduced Deer Increases Size and Seed Production Success in an Island-Endemic Plant Species. Ecol. Evol. 2016, 6, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.T.; Still, C.J.; Ebert, C.M.; Baguskas, S.A.; Park Williams, A. Fog Drip Maintains Dry Season Ecological Function in a California Coastal Pine Forest. Ecosphere 2016, 7, e01364. [Google Scholar] [CrossRef]
- Ramirez, A.R.; De Guzman, M.E.; Dawson, T.E.; Ackerly, D.D. Plant Hydraulic Traits Reveal Islands as Refugia from Worsening Drought. Conserv Physiol 2020, 8, coz115. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas: New Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.8.8. 2023. Available online: https://github.com/rvlenth/emmeans (accessed on 10 March 2024).
- Ben-Shachar, M.S.; Lüdecke, D.; Makowski, D. Effectsize: Estimation of Effect Size Indices and Standardized Parameters. J. Open Source Softw. 2020, 5, 2815. [Google Scholar] [CrossRef]
- Boachon, B.; Buell, C.R.; Crisovan, E.; Dudareva, N.; Garcia, N.; Godden, G.; Henry, L.; Kamileen, M.O.; Kates, H.R.; Kilgore, M.B.; et al. Phylogenomic Mining of the Mints Reveals Multiple Mechanisms Contributing to the Evolution of Chemical Diversity in Lamiaceae. Mol. Plant 2018, 11, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Schnitzler, J.-P. Abiotic Stresses and Induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Raguso, R.A. Why Do Plants Produce so Many Terpenoid Compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef]
- Pratt, J.D.; Keefover-Ring, K.; Liu, L.Y.; Mooney, K.A. Genetically Based Latitudinal Variation in Artemisia californica Secondary Chemistry. Oikos 2014, 123, 953–963. [Google Scholar] [CrossRef]



| Field Sampling | |||||
|---|---|---|---|---|---|
| Trait | Species Pair | Island Mean | Mainland Mean | t | p |
| Leaf area | Ceanothus | 1.99 | 1.07 | 5.058 | <0.001 |
| Cercocarpus | 4.06 | 2.91 | 2.558 | 0.018 | |
| Dendromecon | 5.24 | 2.37 | 6.123 | <0.001 | |
| Heteromeles | 8.67 | 7.06 | 1.793 | 0.095 | |
| Prunus | 11.51 | 4.58 | 7.817 | <0.001 | |
| Specific leaf area (SLA) | Ceanothus | 4.76 | 3.11 | 2.554 | 0.015 |
| Cercocarpus | 7.84 | 7.17 | 1.064 | 0.296 | |
| Dendromecon | 7.18 | 7.50 | −0.485 | 0.631 | |
| Heteromeles | 4.59 | 4.15 | 0.791 | 0.439 | |
| Prunus | 8.29 | 6.30 | 3.403 | 0.002 | |
| Marginal leaf spinescence | Heteromeles | 1.86 | 3.54 | −2.406 | 0.060 |
| Prunus | 1.79 | 7.73 | −8.484 | <0.001 | |
| Cyanogenic glycoside content | Heteromeles | 0.48 | 1.20 | −2.635 | 0.037 |
| Prunus | 1.55 | 2.91 | −1.796 | 0.122 | |
| Botanic Garden Sampling | |||||
|---|---|---|---|---|---|
| Trait | Species Pair | Island Mean | Mainland Mean | t | p |
| Leaf Area | Ceanothus | 1.46 | 0.98 | 0.992 | 0.331 |
| Cercocarpus | 2.63 | 2.32 | 0.327 | 0.746 | |
| Dendromecon | 6.10 | 7.93 | −0.562 | 0.579 | |
| Heteromeles | 11.23 | 8.61 | 0.550 | 0.587 | |
| Prunus | 19.92 | 5.72 | 3.616 | 0.001 | |
| Marginal leaf spinescence | Heteromeles | 1.35 | 1.68 | −0.305 | 0.766 |
| Prunus | 0.00 | 3.17 | −10.885 | <0.001 | |
| Cyanogenic glycoside content | Heteromeles | 0.42 | 0.93 | −1.841 | 0.085 |
| Prunus | 1.34 | 3.48 | −2.000 | 0.058 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freedman, M.G.; Long, R.W.; Ramírez, S.R.; Strauss, S.Y. Evidence for Reductions in Physical and Chemical Plant Defense Traits in Island Flora. Plants 2024, 13, 1026. https://doi.org/10.3390/plants13071026
Freedman MG, Long RW, Ramírez SR, Strauss SY. Evidence for Reductions in Physical and Chemical Plant Defense Traits in Island Flora. Plants. 2024; 13(7):1026. https://doi.org/10.3390/plants13071026
Chicago/Turabian StyleFreedman, Micah G., Randall W. Long, Santiago R. Ramírez, and Sharon Y. Strauss. 2024. "Evidence for Reductions in Physical and Chemical Plant Defense Traits in Island Flora" Plants 13, no. 7: 1026. https://doi.org/10.3390/plants13071026





