Potential of Chitosan for the Control of Powdery Mildew (Leveillula taurica (Lév.) Arnaud) in a Jalapeño Pepper (Capsicum annuum L.) Cultivar
Abstract
:1. Introduction
2. Results
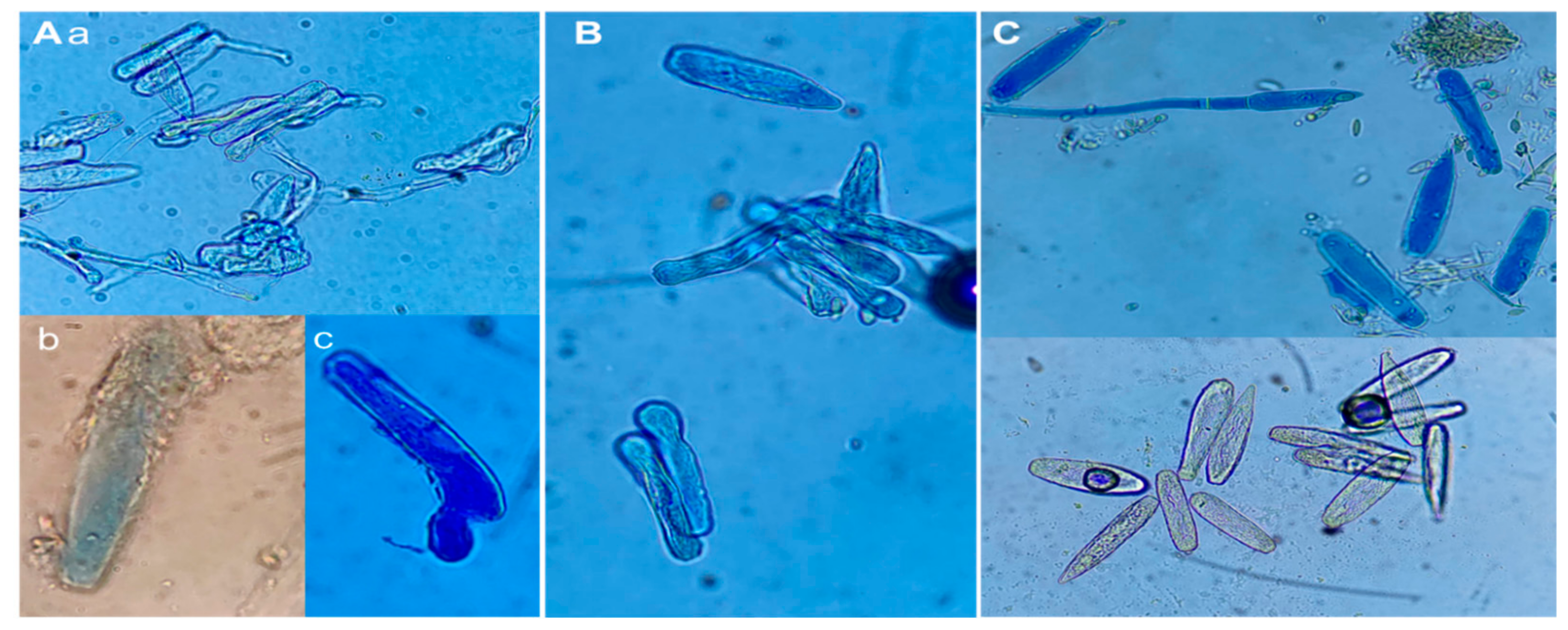
2.1. Identification of the Causal Agent of Powdery Mildew on Jalapeño Pepper Plants
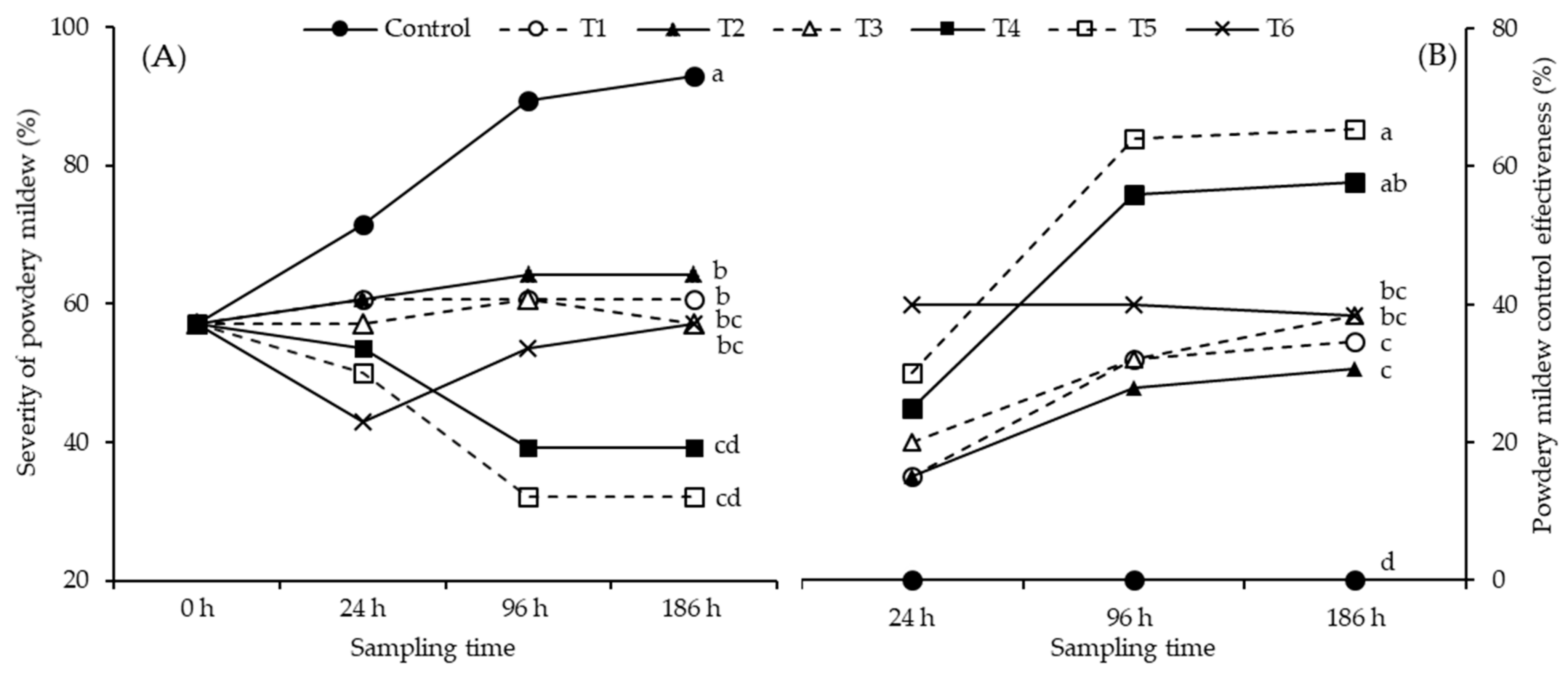
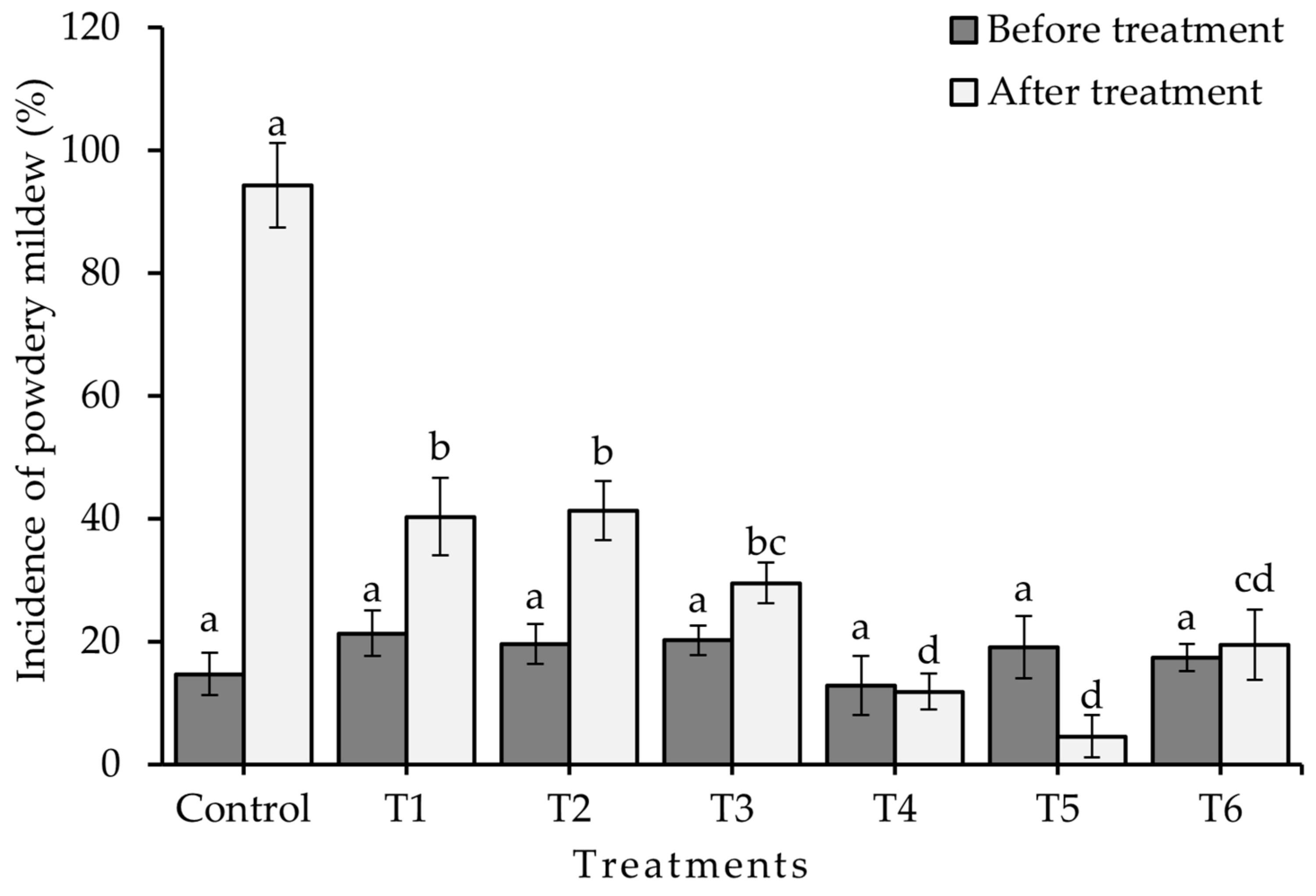
2.2. Control of Leveillula taurica on Pepper Leaves by Application of Chitosan under Laboratory Conditions
Experiment 1
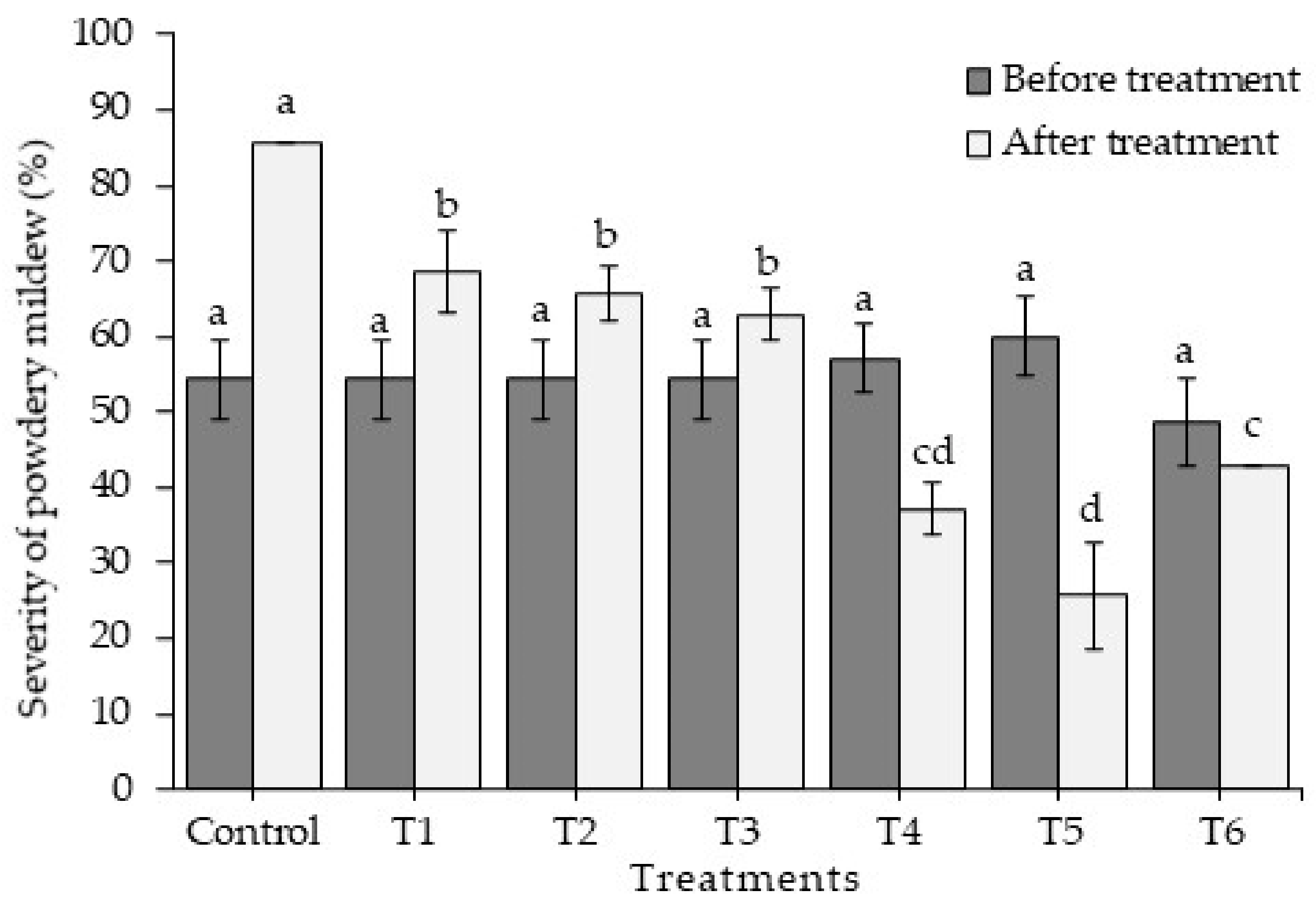
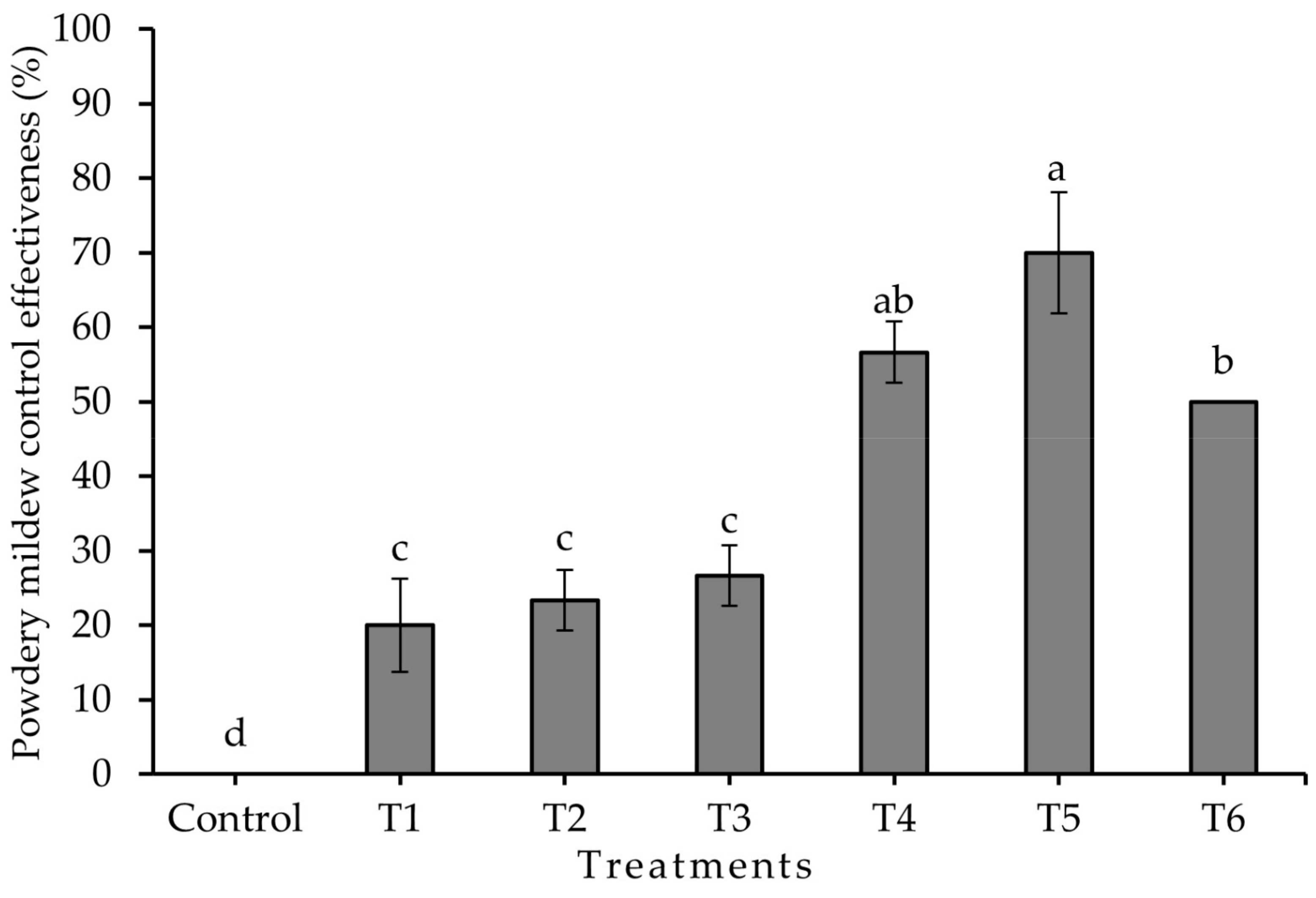
2.3. Chitosan Activity against Leveillula taurica in Greenhouse Conditions
Experiment 2
3. Discussion
4. Materials and Methods
4.1. Location of the Study
4.2. Morphological Identification of the Causal Agent of Powdery Mildew in Jalapeño Pepper
4.3. Evaluation of the Control of Powdery Mildew on Jalapeño Pepper Leaves under Laboratory Conditions by the Application of Chitosan
Experiment 1
4.4. Application of Chitosan for the Control of Powdery Mildew on Mixtecos Jalapeño Bell Pepper Plants under Greenhouse Conditions
Experiment 2
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vázquez-Espinosa, M.; González-De-Peredo, A.V.; Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. The effect of ripening on the capsaicinoids composition of Jeromin pepper (Capsicum annuum L.) at two different stages of plant maturity. Food Chem. 2023, 399, 133979. [Google Scholar] [CrossRef] [PubMed]
- Guigón-López, C.; González-González, A. Estudio regional de las enfermedades del chile (Capsicum annuum L.) y su comportamiento temporal en el sur de Chihuahua, México. Rev. Mex. Fitopatol. 2001, 18, 49–56. [Google Scholar]
- De Souza, V.L.; Café-Filho, A.C. Resistance to Leveillula taurica in the genus Capsicum. Plant Pathol. 2003, 52, 613–619. [Google Scholar] [CrossRef]
- Cerkauskas, R.F.; Ferguson, G.; Banik, M. Powdery mildew (Leveillula taurica) on greenhouse and field peppers in Ontario—Host range, cultivar response and disease management strategies. Can. J. Plant Pathol. 2011, 33, 485–498. [Google Scholar] [CrossRef]
- Félix-Gastélum, R.; Maldonado-Mendoza, I.E.; Beltran-Peña, H.; Apodaca-Sánchez, M.; Espinoza-Matías, S.; Martínez-Valenzuela, M.d.C.; Longoria-Espinoza, R.M.; Olivas-Peraza, N.G. Las cenicillas en cultivos agrícolas de Sinaloa: Situación actual sobre su identificación y líneas futuras de investigación. Rev. Mex. Fitopatol. 2017, 35, 106–129. [Google Scholar] [CrossRef]
- Karkanis, A.; Bilalis, D.; Efthimiadou, A.; Katsenios, N. Effects of field bindweed (Convolvulus arvensis L.) and powdery mildew (Leveillula taurica (Lev.) Arn.) on pepper growth and yield—Short communication. Hortic. Sci. 2012, 39, 135–138. [Google Scholar] [CrossRef]
- Jones, B.W.; Thomson, V.S. Source of inoculum, yield, and quality of tomato as affected by Leveillula taurica. Plant Dis. 1987, 71, 266–268. [Google Scholar] [CrossRef]
- Elad, Y.; Messika, Y.; Brand, M.; David, D.R.; Sztejnberg, A. Effect of microclimate on Leveillula taurica powdery mildew of sweet pepper. Ecol. Epidemiol. 2007, 97, 813–824. [Google Scholar] [CrossRef]
- Martínez-Ruiz, F.E.; Cervantes-Díaz, L.; Aíl-Catzím, C.E.; Hernández-Montiel, L.G.; Sánchez, C.L.D.T.; Rueda-Puente, E.O. Hongos fitopatógenos asociados al tomate (Solanum Lycopersicum L.) en la zona árida del noroeste de México: La importancia de su diagnóstico. Eur. Sci. J. ESJ 2016, 12, 232–256. [Google Scholar] [CrossRef]
- Sudha, A.; Lakshmanan, P. Integrated disease management of powdery mildew (Leveillula taurica (Lev.) Arn.) of chilli (Capsicum annuum L.). Arch. Phytopathol. Plant Prot. 2009, 42, 299–317. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Liu, Y. Review: Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 2013, 167, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Lima, G.; Sanzani, S.M.; Curtis, F.; Ippolito, A. Biological Control of Postharvest Diseases. Adv. Postharvest Fruit Veg. Technol. 2016, 4, 65–81. [Google Scholar]
- Bazioli, J.M.; Belinato, J.R.; Costa, J.H.; Akiyama, D.Y.; Pontes, J.G.d.M.; Kupper, K.C.; Augusto, F.; de Carvalho, J.E.; Fill, T.P. Biological Control of Citrus Postharvest Phytopathogens. Toxins 2019, 11, 460. [Google Scholar] [CrossRef]
- Dafermos, N.G.; Kasselaki, A.M.; Goumas, D.E.; Spantidakis, K.; Eyre, M.D.; Leifert, C. Integration of elicitors and less-susceptible hybrids for the control of powdery mildew in organic tomato crops. Plant Dis. 2012, 96, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Faoro, F.; Maffi, D.; Cantu, D.; Iriti, M. Chemical-induced resistance against powdery mildew in barley: The effects of chitosan and benzothiadiazole. BioControl 2007, 53, 387–401. [Google Scholar] [CrossRef]
- Palacio-MáRquez, A.; RamíRez-Estrada, C.A.; Sanchez, E.; Ojeda-Barrios, D.L.; CháVez-Mendoza, C.; Sida-Arreola, J.P.; Preciado-Rangel, P. Use of biostimulant compounds in agriculture: Chitosan as a sustainable option for plant development. Not. Sci. Biol. 2022, 14, 11124. [Google Scholar]
- Elagamey, E.; Abdellatef, M.A.; Arafat, Y. Proteomic insights of chitosan mediated inhibition of Fusarium oxysporum f. sp. cucumerinum. J. Proteom. 2022, 261, 104590. [Google Scholar] [CrossRef]
- Rautela, A.; Dwivedi, M.; Tewari, A.K.; Kumar, J. Enzymatic activity and secondary metabolite profile of Trichoderma asperellum in presence of chitosan. Indian Phytopathol. 2019, 72, 437–444. [Google Scholar] [CrossRef]
- Molina, Z.J.A.; Colina, R.M.C.; Rincón, D.; Vargas, C.J.A. Efecto del uso de quitosano en el mejoramiento del cultivo de arroz (Oryza sativa L. variedad sd20a). Rev. Investig. Agrar. Ambient. 2017, 8, 151–165. [Google Scholar] [CrossRef]
- Reyes-Pérez, J.J.; Enríquez-Acosta, E.A.; Ramírez-Arrebato, M.A.; Zuñiga, V.E.; Lara-Capistrán, L.; Hernández-Montiel, L.G. Efecto del quitosano sobre variables del crecimiento, rendimiento y contenido nutricional del tomate. Rev. Mex. Cienc. Agríc. 2020, 11, 457–465. [Google Scholar] [CrossRef]
- Salmon, S.E. On Oidiopsis taurica (Lén.), an endophytic member of the Erysiphaceae. Ann. Bot. 1906, 20, 187–200. [Google Scholar] [CrossRef]
- Mosquera, S.; Chen, L.-H.; Aegerter, B.; Miyao, E.; Salvucci, A.; Chang, T.-C.; Epstein, L.; Stergiopoulos, I. Cloning of the cytochrome b gene from the tomato powdery mildew fungus Leveillula taurica reveals high levels of allelic variation and heteroplasmy for the G143A mutation. Front. Microbiol. 2019, 10, 663. [Google Scholar] [CrossRef]
- Glawe, D.A. Synopsis of genera of Erysiphales (powdery mildew fungi) occurring in the pacific northwest. Pac. Northwest Fungi 2006, 1, 1–27. [Google Scholar] [CrossRef]
- Reis, A.; Boiteux, L.S.; Paz-Lima, M.L.; Silva, P.P.; Lopes, C.A. Powdery mildew of Allium species caused by Oidiopsis taurica in Brazil. Hortic. Bras. 2004, 22, 758–760. [Google Scholar] [CrossRef]
- Reis, A.; Lopes, A.C.; Paz, L.M.L.; Boiteux, S.L. Eryngium foetidum, Petroselinum crispun and Coriandrum sativum: New apiaceae hosts of Oidiopsis taurica in Brazil. Fitopatol. Bras. 2004, 29, 339. [Google Scholar] [CrossRef]
- Jones, R.W.; Stommel, J.R.; Wanner, L.A. First report of Leveillula taurica causing powdery mildew on pepper in Maryland. Plant Dis. 2009, 93, 1222. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Azeem, A.M.; Abdel-Moneim, T.S. First record of Oidiopsis taurica causing powdery mildew of Capparis spinosa in Egypt. Plant Dis. 2009, 93, 555. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Nonomura, T.; Bóka, K.; Matsuda, Y.; Visser, R.G.F.; Toyoda, H.; Kiss, L.; Bai, Y. Detection and quantification of Leveillula taurica growth in pepper leaves. Phytopathology 2013, 103, 623–632. [Google Scholar] [CrossRef]
- Romero, C.S. Hongos Fitopatógenos; Universidad Autónoma de Chapingo: Texcoco, México, 1993. [Google Scholar]
- Arici, E.; Özkaya, R. Evaluation of biofungicides in the control against powdery mildew disease [Leveillula taurica (Lev.) Arm.] in pepper. Kahramanmaraş Sütçü İmam Üniv. Tarım Doğa Derg. 2022, 25, 274–281. [Google Scholar] [CrossRef]
- Alharbi, A.A.; Alawlaqi, M.M. Impact of some Bacillus spp., induce resistant chemicals and cow’s skim milk on management of pepper powdery mildew disease in Saudi Arabia. Life Sci. J. 2014, 11, 1–7. [Google Scholar] [CrossRef]
- Abdel-Kader, M.M.; El-Mougy, N.S.; Aly, M.D.E.; Lashin, S.M. Integration of biological and fungicidal alternatives for controlling foliar diseases of vegetables under greenhouse conditions. Int. J. Agric. For. 2012, 2, 38–48. [Google Scholar] [CrossRef]
- Domãnguez-Serrano, D.; García-Velasco, R.; Mora-Herrera, M.E.; Salgado-Siclan, M.L. Identificación y alternativas de manejo de la cenicilla del rosal. Rev. Mex. Fitopatol. 2015, 34, 22–42. [Google Scholar] [CrossRef]
- Aleksandrowicz-Trzcińska, M.; Bogusiewicz, A.; Szkop, M.; Drozdowski, S. Effect of Chitosan on Disease Control and Growth of Scots Pine (Pinus sylvestris L.) in a Forest Nursery. Forests 2015, 6, 3165–3176. [Google Scholar] [CrossRef]
- Sharif, R.; Mujtaba, M.; Rahman, M.U.; Shalmani, A.; Ahmad, H.; Anwar, T.; Tianchan, D.; Wang, X. The Multifunctional Role of Chitosan in Horticultural Crops; A Review. Molecules 2018, 23, 872. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Teniente, L.; Durán-Flores, F.d.D.; Chapa-Oliver, A.M.; Torres-Pacheco, I.; Cruz-Hernández, A.; González-Chavira, M.M.; Ocampo-Velázquez, R.V.; Guevara-González, R.G. Oxidative and Molecular Responses in Capsicum annuum L. after Hydrogen Peroxide, Salicylic Acid and Chitosan Foliar Applications. Int. J. Mol. Sci. 2013, 14, 10178–10196. [Google Scholar] [CrossRef] [PubMed]
- Palma-Guerrero, J.; Lopez-Jimenez, J.A.; Pérez-Berná, A.J.; Huang, I.; Jansson, H.; Salinas, J.; Villalaín, J.; Read, N.D.; Lopez-Llorca, L.V. Membrane fluidity determines sensitivity of filamentous fungi to chitosan. Mol. Microbiol. 2010, 75, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Abdellatef, M.A.E.; Elagamey, E.; Kamel, S.M. Chitosan is the ideal resource for plant disease management under sustainable agriculture. In Chitin and Chitosan—Isolation, Properties, and Applications; IntechOpen: London, UK, 2023; Chapter 5. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Baños, S.; Nacional, I.P.; Ventura-Aguilar, R.I.; Correa-Pacheco, Z.; Corona-Rangel, M.L. Chitosan: A versatile antimicrobial polysaccharide for fruit and vegetables in postharvest—A review. Rev. Chapingo Ser. Hortic. 2017, XXIII, 103–121. [Google Scholar] [CrossRef]
- Singh, T.; Vesentini, D.; Singh, A.P.; Daniel, G. Effect of chitosan on physiological, morphological, and ultrastructural characteristics of wood-degrading fungi. Int. Biodeterior. Biodegrad. 2008, 62, 116–124. [Google Scholar] [CrossRef]
- Jiménez, M.R.; Arceo, M.M.T.; Loeza, L.P.D. Quitosano: Actividad antimicrobiana y mecanismos de acción. e-CUCBA 2018, 9, 17–23. [Google Scholar] [CrossRef]
- Muñoz-Leoz, B.; Ruiz-Romera, E.; Antigüedad, I.; Garbisu, C. Tebuconazole application decreases soil microbial biomass and activity. Soil Biol. Biochem. 2011, 43, 2176–2183. [Google Scholar] [CrossRef]
- Reyes-Pérez, J.J.; Rivero-Herrada, M.; Solórzano-Cedeño, A.E.; Carballo-Méndez, F.d.J.; Lucero-Vega, G.; Ruiz-Espinoza, F.H. Aplicación de ácidos húmicos, quitosano y hongos micorrízicos como influyen en el crecimiento y desarrollo de pimiento. Rev. Terra Latinoam. 2021, 39, e833. [Google Scholar] [CrossRef]
- Reyes-Pérez, J.J.; Rivero-Herrada, M.; García-Bustamante, E.L.; Beltran-Morales, F.A.; Ruiz-Espinoza, F.H. Aplicación de quitosano incrementa la emergencia, crecimiento y rendimiento del cutivo de tomate (Solanum lycopersicum L.) en condiciones de invernadero. Biotecnia 2020, 22, 156–163. [Google Scholar] [CrossRef]
- Townsend, G.R.; Heuberger, J.W. Methods for estimating losses caused by diseases in fungicide experiments. Plant Dis. Rep. 1943, 27, 340–343. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Èntomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Pérez-Torres, E.; Bernal-Cabrera, A.; Milanés-Virelles, P.; Sierra-Reyes, Y.; Leiva-Mora, M.; Marín-Guerra, S.; Monteagudo-Hernández, O. Eficiencia de Trichoderma harzianum (cepa A-34) y sus filtrados en el control de tres enfermedades fúngicas foliares en arroz. Bioagro 2018, 30, 17–26. [Google Scholar]


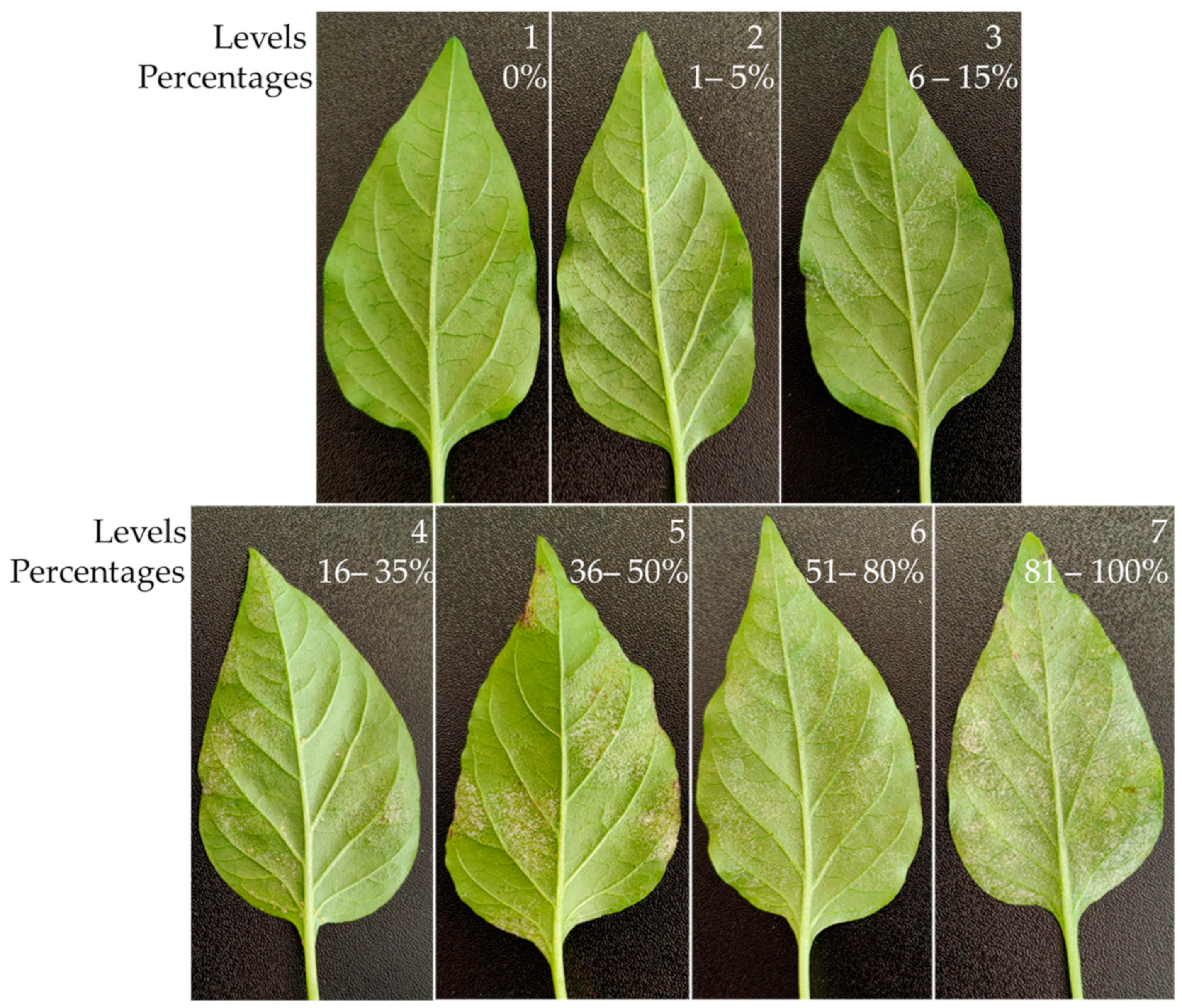
| % of Affected Area | ||
|---|---|---|
| Levels | Plant | Leaf |
| 1 | 0 | 0 |
| 2 | 1–30 | 1–15 |
| 3 | 1–30 | 16–35 |
| 4 | 1–30 | 36–50 |
| 5 | 1–30 | 51–80 |
| 6 | 1–30 | 81–100 |
| 7 | 31–60 | 1–15 |
| 8 | 31–60 | 16–35 |
| 9 | 31–60 | 36–50 |
| 10 | 31–60 | 51–80 |
| 11 | 31–60 | 81–100 |
| 12 | 61–100 | 1–15 |
| 13 | 61–100 | 16–35 |
| 14 | 61–100 | 36–50 |
| 15 | 61–100 | 51–80 |
| 16 | 61–100 | 81–100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Pérez, O.; Gallegos-Morales, G.; Espinoza-Ahumada, C.A.; Delgado-Luna, C.; Preciado-Rangel, P.; Espinosa-Palomeque, B. Potential of Chitosan for the Control of Powdery Mildew (Leveillula taurica (Lév.) Arnaud) in a Jalapeño Pepper (Capsicum annuum L.) Cultivar. Plants 2024, 13, 915. https://doi.org/10.3390/plants13070915
Jiménez-Pérez O, Gallegos-Morales G, Espinoza-Ahumada CA, Delgado-Luna C, Preciado-Rangel P, Espinosa-Palomeque B. Potential of Chitosan for the Control of Powdery Mildew (Leveillula taurica (Lév.) Arnaud) in a Jalapeño Pepper (Capsicum annuum L.) Cultivar. Plants. 2024; 13(7):915. https://doi.org/10.3390/plants13070915
Chicago/Turabian StyleJiménez-Pérez, Omar, Gabriel Gallegos-Morales, Cesar Alejandro Espinoza-Ahumada, Carolina Delgado-Luna, Pablo Preciado-Rangel, and Bernardo Espinosa-Palomeque. 2024. "Potential of Chitosan for the Control of Powdery Mildew (Leveillula taurica (Lév.) Arnaud) in a Jalapeño Pepper (Capsicum annuum L.) Cultivar" Plants 13, no. 7: 915. https://doi.org/10.3390/plants13070915








