Dissecting the Roles of Phosphorus Use Efficiency, Organic Acid Anions, and Aluminum-Responsive Genes under Aluminum Toxicity and Phosphorus Deficiency in Ryegrass Plants
Abstract
:1. Introduction
2. Results
2.1. Phosphorus and Aluminium Concentrations and Contents
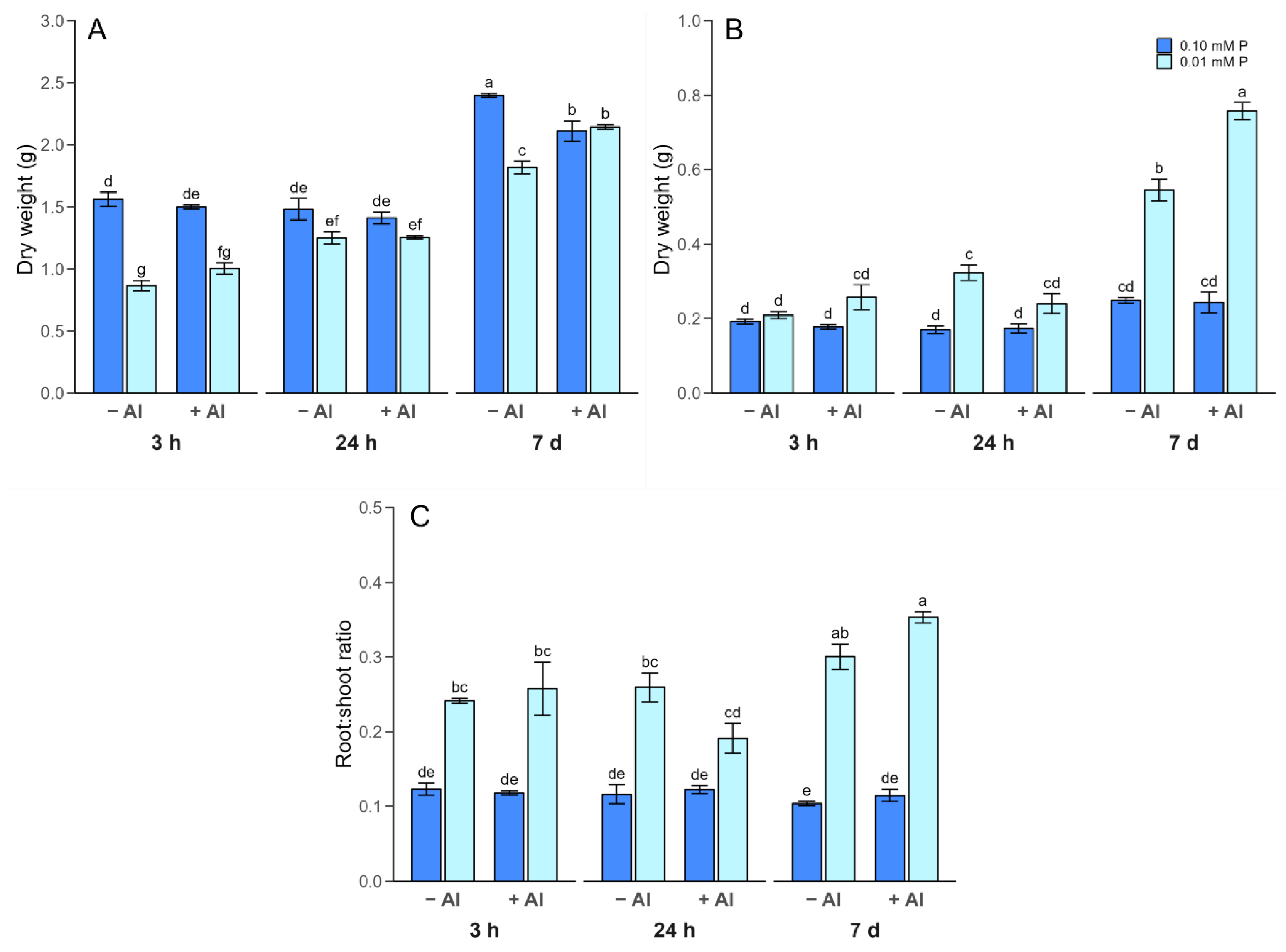
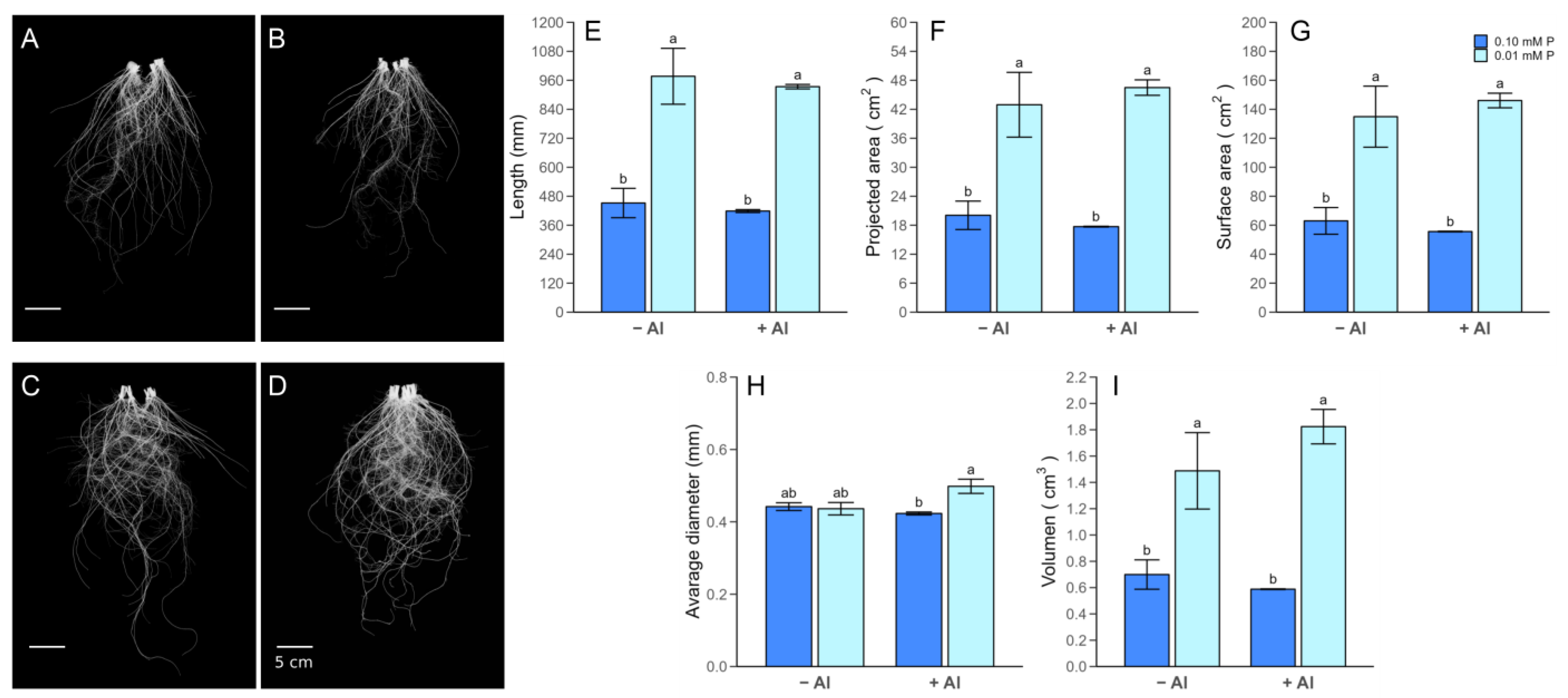
2.2. Plant Growth Parameters
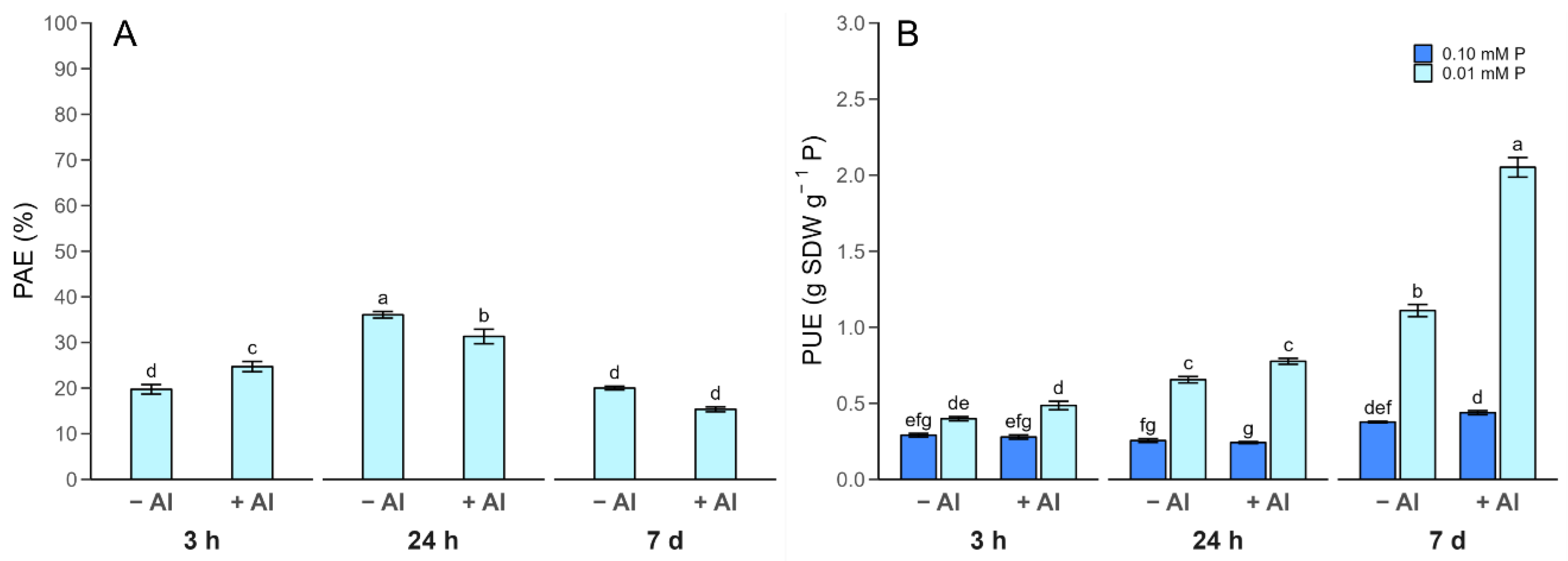
2.3. Phosphorus Use Efficiency
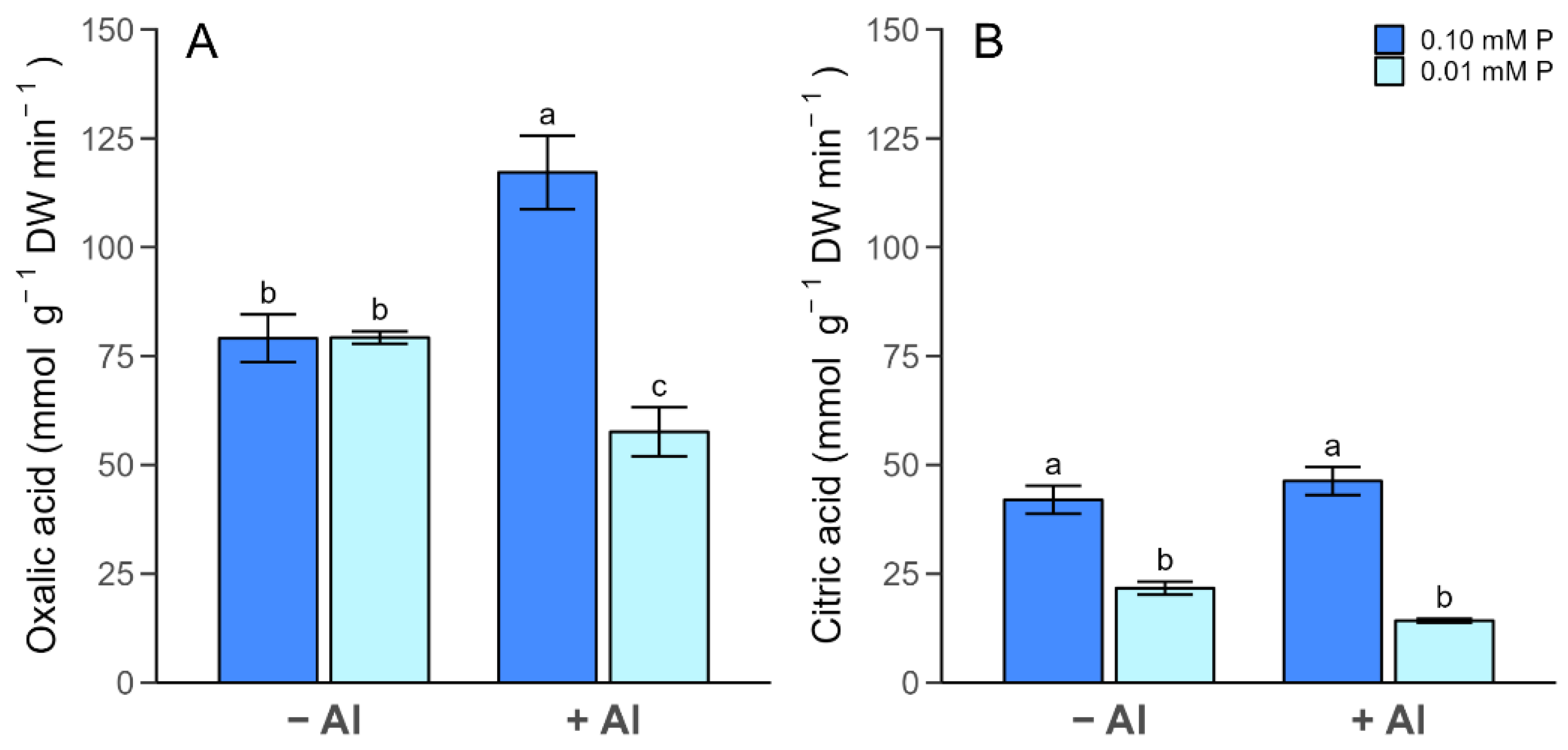
2.4. Organic Acid in Root Exudates
2.5. Gene Expression Analyses
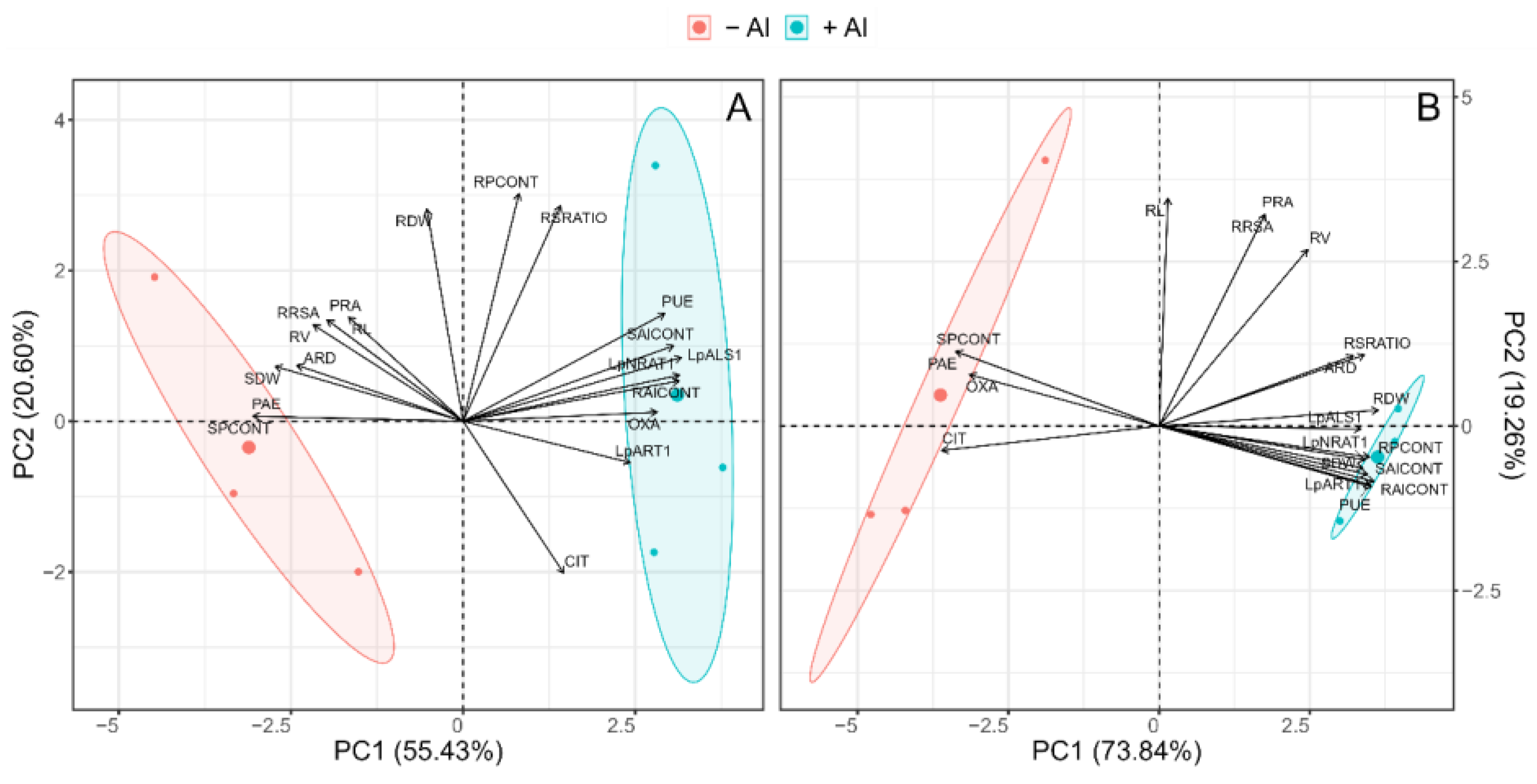
2.6. Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Phosphorus and Al Determinations
4.3. Plant Growth Traits
4.4. Determination of Root Exudates
4.5. Expression Analyses of Al-Responsive Genes
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- von Uexküll, H.R.; Mutert, E. Global Extent, Development and Economic Impact of Acid Soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Kochian, L.V.; Hoekenga, O.A.; Piñeros, M.A. How Do Crop Plants Tolerate Acid Soils? Mechanisms of Aluminum Tolerance and Phosphorous Efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef]
- Kochian, L.V.; Pi, M.A.; Liu, J. Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus Acquisition and Use: Critical Adaptations by Plants for Securing a Nonrenewable Resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef] [PubMed]
- López-Arredondo, D.L.; Leyva-González, M.A.; González-Morales, S.I.; López-Bucio, J.; Herrera-Estrella, L. Phosphate Nutrition: Improving Low-Phosphate Tolerance in Crops. Annu. Rev. Plant Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Long, Y.; Huang, J.; Xia, J. Molecular Mechanisms for Coping with Al Toxicity in Plants. Int. J. Mol. Sci. 2019, 20, 1551. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, D.K.; Singh, S.; Sharma, S.; Dubey, N.K.; Chauhan, D.K.; Vaculík, M. Toxicity of Aluminium on Various Levels of Plant Cells and Organism: A Review. Environ. Exp. Bot. 2017, 137, 177–193. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Chen, R.F.; Shen, R.F. Coadaptation of Plants to Multiple Stresses in Acidic Soils. Soil Sci. 2014, 179, 503–513. [Google Scholar] [CrossRef]
- Han, Y.; White, P.J.; Cheng, L. Mechanisms for Improving Phosphorus Utilization Efficiency in Plants. Ann. Bot. 2022, 129, 247–258. [Google Scholar] [CrossRef]
- Soumya, P.R.; Vengavasi, K.; Pandey, R. Adaptive Strategies of Plants to Conserve Internal Phosphorus under P Deficient Condition to Improve P Utilization Efficiency. Physiol. Mol. Biol. Plants 2022, 28, 1981–1993. [Google Scholar] [CrossRef]
- Heuer, S.; Gaxiola, R.; Schilling, R.; Herrera-Estrella, L.; López-Arredondo, D.; Wissuwa, M.; Delhaize, E.; Rouached, H. Improving Phosphorus Use Efficiency: A Complex Trait with Emerging Opportunities. Plant J. 2017, 90, 868–885. [Google Scholar] [CrossRef]
- Dissanayaka, D.M.S.B.; Ghahremani, M.; Siebers, M.; Wasaki, J.; Plaxton, W.C. Recent Insights into the Metabolic Adaptations of Phosphorus-Deprived Plants. J. Exp. Bot. 2021, 72, 199–223. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in Plant: Benefits, Toxicity and Tolerance Mechanisms. Front. Plant Sci. 2023, 13, 1085998. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Busoms, S. How Plants Handle Trivalent (+ 3) Elements. Int. J. Mol. Sci. 2019, 20, 3984. [Google Scholar] [CrossRef]
- Chen, Z.C.; Liao, H. Organic Acid Anions: An Effective Defensive Weapon for Plants against Aluminum Toxicity and Phosphorus Deficiency in Acidic Soils. J. Genet. Genom. 2016, 43, 631–638. [Google Scholar] [CrossRef]
- Chen, W.; Tang, L.; Wang, J.; Zhu, H.; Jin, J.; Yang, J.; Fan, W. Research Advances in the Mutual Mechanisms Regulating Response of Plant Roots to Phosphate Deficiency and Aluminum Toxicity. Int. J. Mol. Sci. 2022, 23, 1137. [Google Scholar] [CrossRef]
- Barros, V.A.; Chandnani, R.; De Sousa, S.M.; Maciel, L.S. Root Adaptation via Common Genetic Factors Conditioning Tolerance to Multiple Stresses for Crops Cultivated on Acidic Tropical Soils. Front. Plant Sci. 2020, 11, 565339. [Google Scholar] [CrossRef] [PubMed]
- Godon, C.; Mercier, C.; Wang, X.; David, P.; Richaud, P.; Nussaume, L.; Liu, D.; Desnos, T. Under Phosphate Starvation Conditions, Fe and Al Trigger Accumulation of the Transcription Factor STOP1 in the Nucleus of Arabidopsis Root Cells. Plant J. 2019, 99, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Rivera, J.O.; Oropeza-Aburto, A.; Herrera-Estrella, L. Dissection of Root Transcriptional Responses to Low PH, Aluminum Toxicity and Iron Excess under Pi-Limiting Conditions in Arabidopsis Wild-Type and Stop1 Seedlings. Front. Plant Sci. 2020, 11, 543941. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tian, J.; Zhang, H.; Liao, H. Phytohormone Regulation of Root Growth Triggered by P Deficiency or Al Toxicity. J. Exp. Bot. 2016, 67, 3655–3664. [Google Scholar] [CrossRef]
- Hajiboland, R.; Panda, C.K.; Lastochkina, O.; Gavassi, M.A.; Habermann, G.; Pereira, J.F. Aluminum Toxicity in Plants: Present and Future; Springer: New York, NY, USA, 2022; Volume 42, ISBN 0034402210866. [Google Scholar]
- Yan, L.; Riaz, M.; Liu, J.; Yu, M.; Cuncang, J. The Aluminum Tolerance and Detoxification Mechanisms in Plants; Recent Advances and Prospects. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1491–1527. [Google Scholar] [CrossRef]
- Xia, J.; Yamaji, N.; Kasai, T.; Ma, J.F. Plasma Membrane-Localized Transporter for Aluminum in Rice. Proc. Natl. Acad. Sci. USA 2010, 107, 18381–18385. [Google Scholar] [CrossRef]
- Lu, M.; Yang, G.; Li, P.; Wang, Z.; Fu, S.; Zhang, X. Bioinformatic and Functional Analysis of a Key Determinant Underlying the Substrate Selectivity of the Al Transporter, Nrat1. Front. Plant Sci. 2018, 9, 344923. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Yamaji, N.; Chen, Z.; Ma, J.F. A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J. 2012, 69, 857–867. [Google Scholar] [CrossRef]
- Magalhaes, J.V.; Piñeros, M.A.; Maciel, L.S.; Kochian, L.V.; Ryan, P. Emerging Pleiotropic Mechanisms Underlying Aluminum Resistance and Phosphorus Acquisition on Acidic Soils. Front. Plant Sci. 2018, 9, 370143. [Google Scholar] [CrossRef]
- Bernardino, K.C.; Pastina, M.M.; Menezes, C.B.; de Sousa, S.M.; Maciel, L.S.; Carvalho, G., Jr.; Guimarães, C.T.; Barros, B.A.; da Costa e Silva, L.; Carneiro, P.C.S.; et al. The Genetic Architecture of Phosphorus Efficiency in Sorghum Involves Pleiotropic QTL for Root Morphology and Grain Yield under Low Phosphorus Availability in the Soil. BMC Plant Biol. 2019, 19, 87. [Google Scholar] [CrossRef]
- Cardoso, T.B.; Pinto, R.T.; Paiva, L.V. Analysis of Gene Co-Expression Networks of Phosphate Starvation and Aluminium Toxicity Responses in Populus spp. PLoS ONE 2019, 14, e0223217. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ai, S.; Liao, H. Deciphering Interactions between Phosphorus Status and Toxic Metal Exposure in Plants and Rhizospheres to Improve Crops Reared on Acid Soil. Cells 2023, 12, 441. [Google Scholar] [CrossRef]
- Oenema, O.; De Klein, C.; Alfaro, M. Intensification of Grassland and Forage Use: Driving Forces and Constraints. Crop Pasture Sci. 2014, 65, 524–537. [Google Scholar] [CrossRef]
- Mora, M.L.; Alfaro, M.A.; Jarvis, S.C.; Demanet, R.; Cartes, P. Soil Aluminium Availability in Andisols of Southern Chile and Its Effect on Forage Production and Animal Metabolism. Soil Use Manag. 2006, 22, 95–101. [Google Scholar] [CrossRef]
- de la Luz Mora, M.; Demanet, R.; Acuña, J.J.; Viscardi, S.; Jorquera, M.; Rengel, Z.; Durán, P. Aluminum-Tolerant Bacteria Improve the Plant Growth and Phosphorus Content in Ryegrass Grown in a Volcanic Soil Amended with Cattle Dung Manure. Appl. Soil Ecol. 2017, 115, 19–26. [Google Scholar] [CrossRef]
- Parra-Almuna, L.; Diaz-Cortez, A.; Ferrol, N.; de la Luz Mora, M. Aluminium Toxicity and Phosphate Deficiency Activates Antioxidant Systems and Up-Regulates Expression of Phosphate Transporters Gene in Ryegrass (Lolium perenne L.) Plants. Plant Physiol. Biochem. 2018, 130, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, R.; Shu, K.; Lv, W.; Wang, S.; Wang, C. Aluminum Stress Signaling, Response, and Adaptive Mechanisms in Plants. Plant Signal. Behav. 2022, 17, 2057060. [Google Scholar] [CrossRef]
- Inostroza-Blancheteau, C.; Reyes-Díaz, M.; Alberdi, M.; Godoy, K.; Rojas-Lillo, Y.; Cartes, P.; Mora, M.D.L.L. Influence of selenite on selenium uptake, differential antioxidant performance and gene expression of sulfate transporters in wheat genotypes. Plant Soil 2013, 369, 47–59. [Google Scholar] [CrossRef]
- Pontigo, S.; Parra-Almuna, L.; Luengo-Escobar, A.; Poblete-Grant, P.; Nunes-Nesi, A.; Mora, M.D.L.L.; Cartes, P. Biochemical and Molecular Responses Underlying the Contrasting Phosphorus Use Efficiency in Ryegrass Cultivars. Plants 2023, 12, 1224. [Google Scholar] [CrossRef] [PubMed]
- Rosas, A.; Rengel, Z.; Mora, M.D.L.L. Manganese Supply and pH Influence Growth, Carboxylate Exudation and Peroxidase Activity of Ryegrass and White Clover. J. Plant Nutr. 2007, 30, 253–270. [Google Scholar] [CrossRef]
- Mora, M.D.L.L.; Rosas, A.; Ribera, A.; Rengel, Z. Differential Tolerance to Mn Toxicity in Perennial Ryegrass Genotypes: Involvement of antioxidative enzymes and root exudation of carboxylates. Plant Soil 2009, 320, 79–89. [Google Scholar] [CrossRef]
- Yamaji, N.; Huang, C.F.; Nagao, S.; Yano, M.; Sato, Y.; Nagamura, Y.; Ma, J.F. A Zinc Finger Transcription Factor ART1 Regulates Multiple Genes Implicated in Aluminum Tolerance in Rice. Plant Cell 2009, 21, 3339–3349. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Chen, Z.C.; Shen, R.F. Molecular Mechanisms of Al Tolerance in Gramineous Plants. Plant Soil 2014, 381, 1–12. [Google Scholar] [CrossRef]
- Sun, L.M.; Che, J.; Ma, J.F.; Shen, R.F. Expression Level of Transcription Factor Art1 Is Responsible for Differential Aluminum Tolerance in Indica Rice. Plants 2021, 10, 634. [Google Scholar] [CrossRef]
- Mora-Macías, J.; Ojeda-Rivera, J.O.; Gutiérrez-Alanís, D.; Yong-Villalobos, L.; Oropeza-Aburto, A.; Raya-González, J.; Jiménez-Domínguez, G.; Chávez-Calvillo, G.; Rellán-Álvarez, R.; Herrera-Estrell, L. Malate-Dependent Fe Accumulation Is a Critical Checkpoint in the Root Developmental Response to Low Phosphate. Proc. Natl. Acad. Sci. USA 2017, 114, E3563–E3572. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Niu, Y.; Wang, Y.; Chen, T.; Naveed, S.A.; Zhang, J.; Xu, J.; Li, Z. Genome-Wide Association Mapping of Aluminum Toxicity Tolerance and Fine Mapping of a Candidate Gene for Nrat1 in Rice. PLoS ONE 2018, 13, e0198589. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.K.; Shandilya, Z.M.; Sarma, P.; Bora, R.K.; Regon, P.; Vemireddy, L.N.R.; Tanti, B. Concurrent Effect of Aluminum Toxicity and Phosphorus Deficiency in the Root Growth of Aluminum Tolerant and Sensitive Rice Cultivars. Acta Physiol. Plant 2023, 45, 33. [Google Scholar] [CrossRef]
- Taylor, G.J.; Foy, C.D. Mechanisms of Aluminum Tolerance in Triticum aestivum L. (Wheat). I. Differential Ph Induced by Winter Cultivars in Nutrient Solutions. Am. J. Bot. 1985, 72, 695–701. [Google Scholar] [CrossRef]
- Sadzawka, A.; Grez, R.; Carrasco, A.; Mora, M. Métodos de Análisis de Tejidos Vegetales; CNA comisión de normalización y acreditación, Sociedad Chilena de las Ciencias del Suelo: Santiago, Chile, 2004; p. 53. [Google Scholar]
- Parada, J.; Valenzuela, T.; Gómez, F.; Tereucán, G.; García, S.; Cornejo, P.; Winterhalter, P.; Ruiz, A. Effect of Fertilization and Arbuscular Mycorrhizal Fungal Inoculation on Antioxidant Profiles and Activities in Fragaria Ananassa Fruit. J. Sci. Food Agric. 2019, 99, 1397–1404. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2–∆∆CT. Method 2001, 408, 402–408. [Google Scholar] [CrossRef]

| Treatment (mM) | Time | P Concentration (g kg−1) | P Content (mg pot−1) | ||
| Shoot | Root | Shoot | Root | ||
| 0 Al–0.01 P | 3 h | 2.17 ± 0.12 e | 2.61 ± 0.01 d | 1.66 ± 0.09 d | 0.55 ± 0.03 f |
| 24 h | 1.91 ± 0.03 efg | 1.97 ± 0.08 e | 2.69 ± 0.05 d | 0.64 ± 0.03 f | |
| 7 d | 1.64 ± 0.01 fg | 1.1 ± 0.11 f | 2.77 ± 0.20 d | 0.64 ± 0.02 f | |
| 0.2 Al–0.01 P | 3 h | 2.07 ± 0.06 ef | 2.68 ± 0.11 d | 2.08 ± 0.09 d | 0.68 ± 0.07 ef |
| 24 h | 1.62 ± 0.03 g | 2.25 ± 0.09 de | 2.33 ± 0.12 d | 0.54 ± 0.05 f | |
| 7 d | 1.05 ± 0.04 h | 1.29 ± 0.05 f | 2.28 ± 0.08 d | 0.98 ± 0.05 de | |
| 0 Al–0.1 P | 3 h | 5.38 ± 0.14 c | 6.36 ± 0.07 bc | 8.40 ± 0.38 c | 1.22 ± 0.03 cd |
| 24 h | 5.79 ± 0.06 bc | 6.24 ± 0.06 c | 7.46 ± 0.19 c | 1.06 ± 0.06 cd | |
| 7 d | 6.37 ± 0.04 a | 6.15 ± 0.02 c | 14.86 ± 0.44 a | 1.53 ± 0.05 ab | |
| 0.2 Al–0.1 P | 3 h | 5.41 ± 0.21 bc | 7.69 ± 0.04 a | 8.11 ± 0.23 c | 1.37 ± 0.05 abc |
| 24 h | 5.83 ± 0.05 b | 7.58 ± 0.06 a | 7.68 ± 0.65 c | 1.31 ± 0.09 bc | |
| 7 d | 4.81 ± 0.05 d | 6.74 ± 0.24 b | 10.16 ± 0.50 b | 1.63 ± 0.12 a | |
| Treatment (mM) | Time | Al concentration (g kg−1) | Al content (mg pot−1) | ||
| Shoot | Root | Shoot | Root | ||
| 0 Al–0.01 P | 3 h | 0.07 ± 0.00 de | 0.09 ± 0.00 e | 0.06 ± 0.00 ef | 0.02 ± 0.00 e |
| 24 h | 0.08 ± 0.00 cd | 0.09 ± 0.00 e | 0.09 ± 0.01 de | 0.03 ± 0.00 e | |
| 7 d | 0.09 ± 0.00 c | 0.13 ± 0.00 e | 0.10 ± 0.00 cd | 0.08 ± 0.01 de | |
| 0.2 Al–0.01 P | 3 h | 0.07 ± 0.00 de | 1.72 ± 0.06 d | 0.07 ± 0.00 def | 0.44 ± 0.06 bc |
| 24 h | 0.09 ± 0.00 c | 3.11 ± 0.30 c | 0.14 ± 0.01 c | 0.75 ± 0.12 bc | |
| 7 d | 0.12 ± 0.00 b | 5.62 ± 0.35 a | 0.26 ± 0.00 b | 4.12 ± 0.18 a | |
| 0 Al–0.1 P | 3 h | 0.02 ± 0.00 f | 0.06 ± 0.00 e | 0.04 ± 0.00 f | 0.01 ± 0.00 e |
| 24 h | 0.06 ± 0.00 e | 0.12 ± 0.00 e | 0.08 ± 0.00 def | 0.01 ± 0.00 e | |
| 7 d | 0.11 ± 0.00 b | 0.12 ± 0.00 e | 0.11 ± 0.00 cd | 0.03 ± 0.00 e | |
| 0.2 Al–0.1 P | 3 h | 0.03 ± 0.00 f | 1.75 ± 0.12 d | 0.04 ± 0.00 f | 0.44 ± 0.05 bc |
| 24 h | 0.09 ± 0.00 c | 4.41 ± 0.09 b | 0.10 ± 0.01 cd | 0.41 ± 0.02 cd | |
| 7 d | 0.18 ± 0.00 a | 3.22 ± 0.36 c | 0.38 ± 0.02 a | 0.77 ± 0.01 b | |
| Gene Name * | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| LpNRAT1 | ATGTTCACCATGGCAGGATGCT | ACTAGGGCAGAGTGCAAGAACAAG |
| LpALS1 | ACGCAGTGCTTCTGAAAGGT | CAGTTTGCACAGCTCTTCGG |
| LpART1 | ACCCCTCGGACTGATCTTCT | AGATAAGGTGGCTCACGCAG |
| eEF1α(h) | ATG TCT GTT GAG CAG CCT TC | GCG GAG TAT ATA AAG GGG TAGC |
| eEF1α(s) | CCG TTT TGT CGA GTT TGG T | AGC AAC TGT AAC CGA ACA TAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra-Almuna, L.; Pontigo, S.; Ruiz, A.; González, F.; Ferrol, N.; Mora, M.d.l.L.; Cartes, P. Dissecting the Roles of Phosphorus Use Efficiency, Organic Acid Anions, and Aluminum-Responsive Genes under Aluminum Toxicity and Phosphorus Deficiency in Ryegrass Plants. Plants 2024, 13, 929. https://doi.org/10.3390/plants13070929
Parra-Almuna L, Pontigo S, Ruiz A, González F, Ferrol N, Mora MdlL, Cartes P. Dissecting the Roles of Phosphorus Use Efficiency, Organic Acid Anions, and Aluminum-Responsive Genes under Aluminum Toxicity and Phosphorus Deficiency in Ryegrass Plants. Plants. 2024; 13(7):929. https://doi.org/10.3390/plants13070929
Chicago/Turabian StyleParra-Almuna, Leyla, Sofía Pontigo, Antonieta Ruiz, Felipe González, Nuria Ferrol, María de la Luz Mora, and Paula Cartes. 2024. "Dissecting the Roles of Phosphorus Use Efficiency, Organic Acid Anions, and Aluminum-Responsive Genes under Aluminum Toxicity and Phosphorus Deficiency in Ryegrass Plants" Plants 13, no. 7: 929. https://doi.org/10.3390/plants13070929






