N2 Fixation, N Transfer, and Land Equivalent Ratio (LER) in Grain Legume–Wheat Intercropping: Impact of N Supply and Plant Density
Abstract
:1. Introduction
2. Results
2.1. Gas Exchange Parameters
2.2. Photosynthetic Pigments
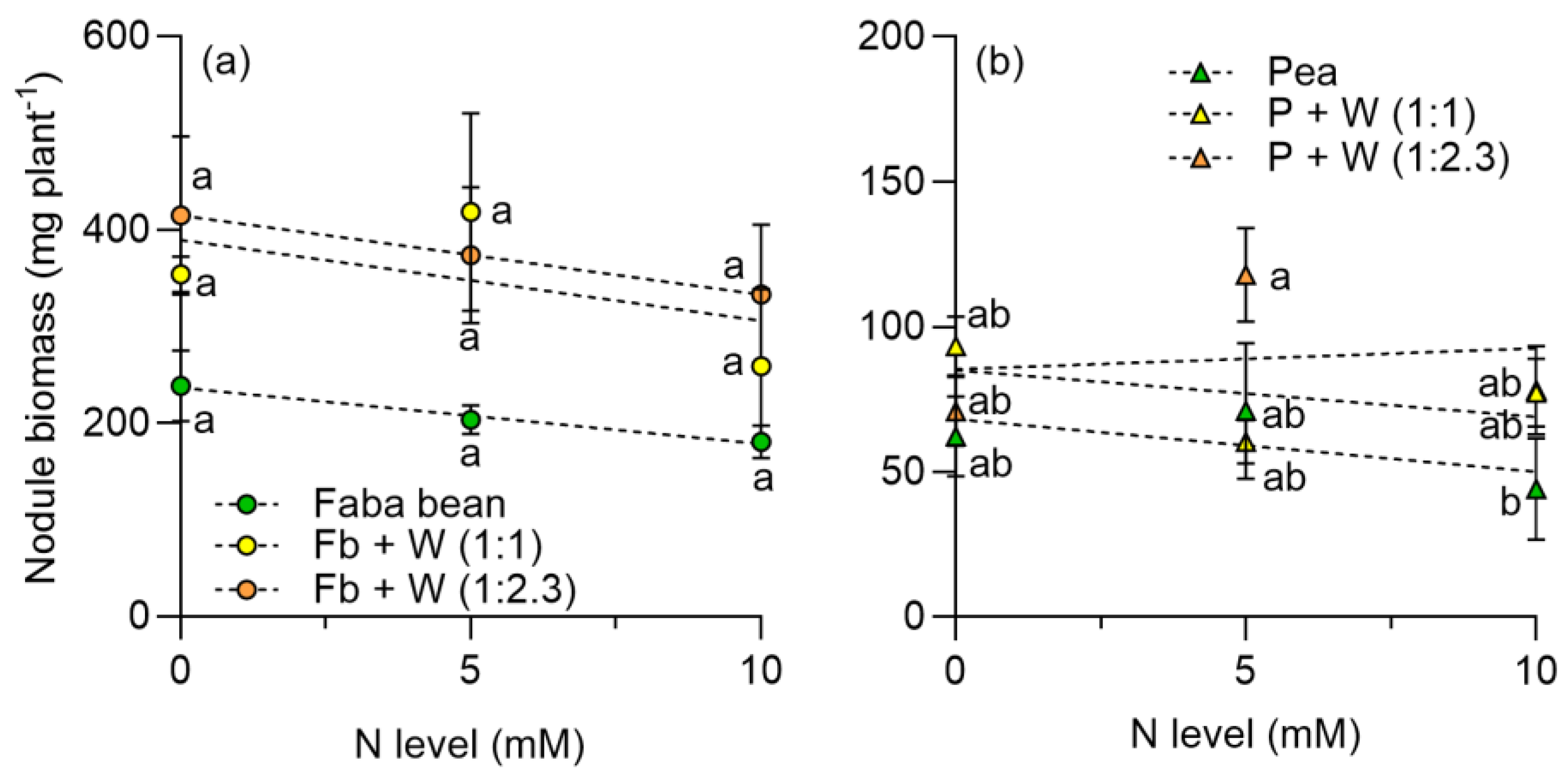
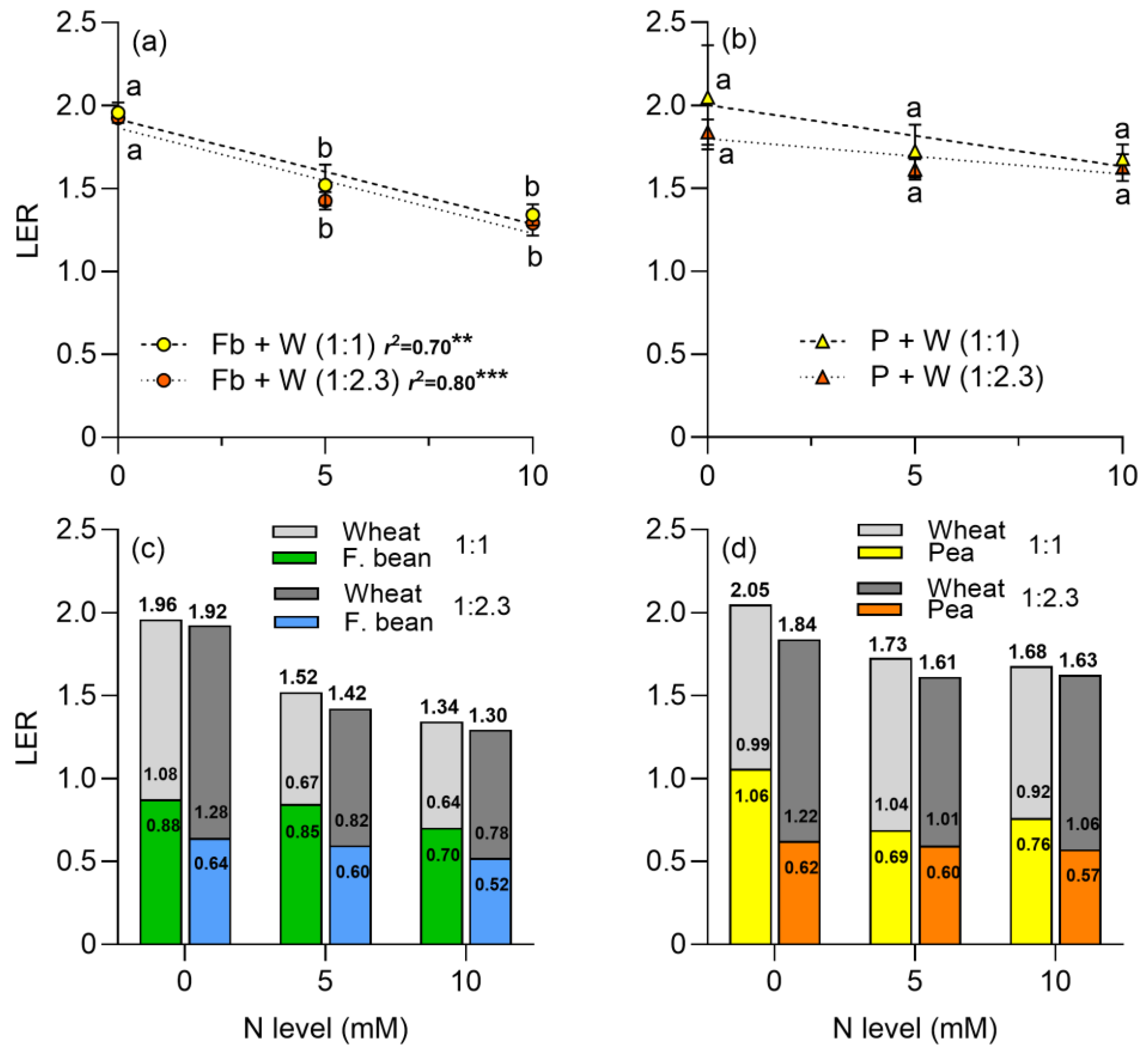
2.3. Dry Matter and Land Equivalent Ratio (LER)
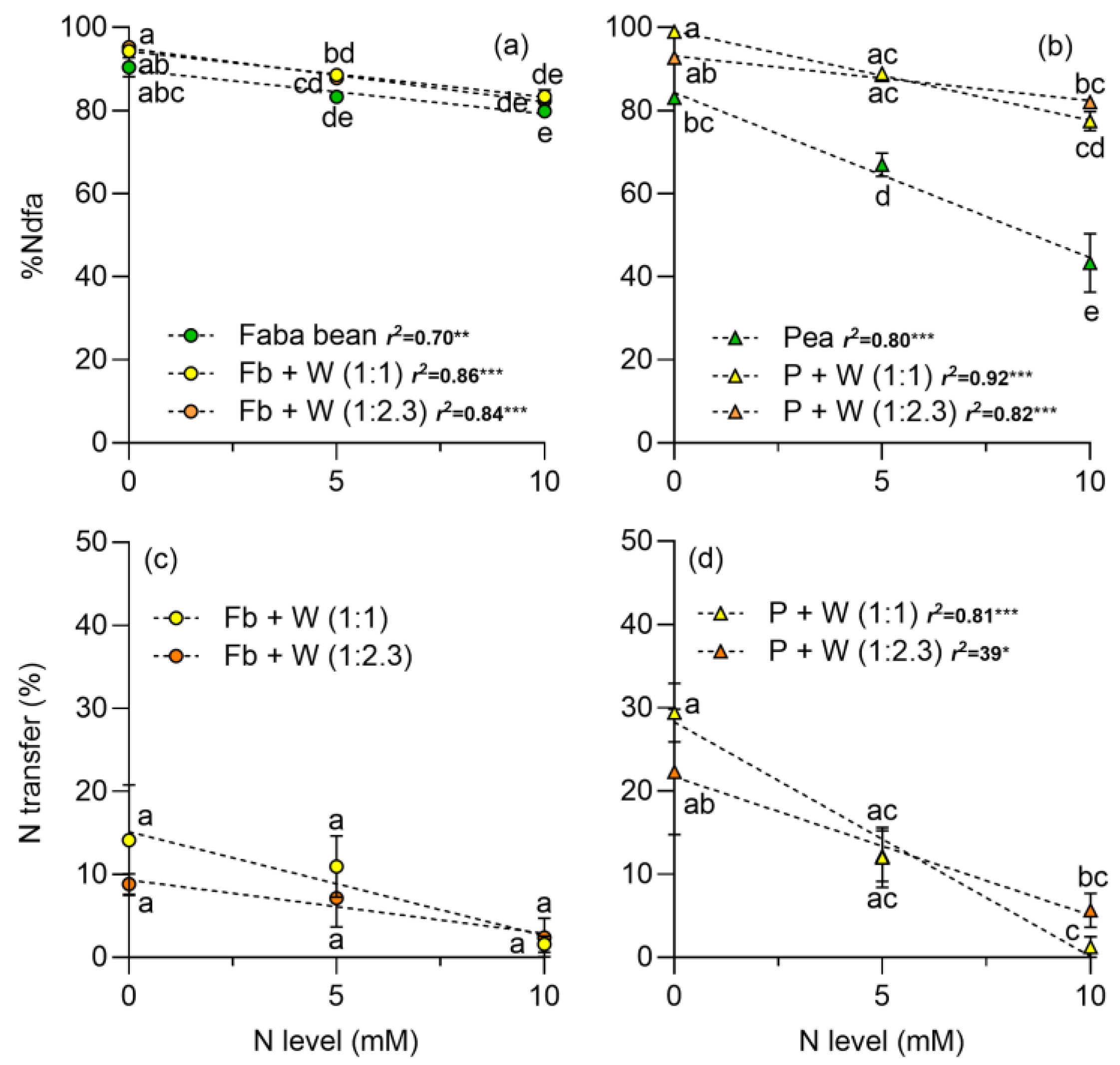
2.4. Proportion of Nitrogen Derived from Air (%Ndfa), N Transfer, and Equivalent N Uptake Ratio (LERN)
3. Discussion
3.1. Overall Aspects
3.2. Leaf Photosynthetic Traits
3.3. Dry Matter Production and Land Equivalent Ratio (LER)
3.4. N2 Fixation, N Transfer, and Land Equivalent Ratio for N (LERN)
4. Materials and Methods
4.1. Plant Growth Conditions and Experimental Design
4.2. Gas Exchange and Photosynthetic Pigment Content
4.3. Determination of Plant and Nodule Dry Matter
4.4. Determining the Proportion of N Derived from Air (%Ndfa)
4.5. Determination of the β Value
4.6. N Transfer from Legume to Cereal
4.7. Land Equivalent Ratio (LER)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vance, C.P. Legume Symbiotic Nitrogen Fixation: Agronomic Aspects. In The Rhizobiaceae: Molecular Biology of Model Plant-Associated Bacteria; Spaink, H.P., Kondorosi, A., Hooykaas, P.J.J., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 509–530. ISBN 978-94-011-5060-6. [Google Scholar]
- Gan, Y.; Liang, C.; Chai, Q.; Lemke, R.L.; Campbell, C.A.; Zentner, R.P. Improving Farming Practices Reduces the Carbon Footprint of Spring Wheat Production. Nat. Commun. 2014, 5, 5012. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Lam, H.-M.; Nguyen, H.T.; Siddique, K.H.M.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M.; et al. Neglecting Legumes Has Compromised Human Health and Sustainable Food Production. Nat. Plants 2016, 2, 16112. [Google Scholar] [CrossRef] [PubMed]
- Didinger, C.; Thompson, H.J. The Role of Pulses in Improving Human Health: A Review. Legume Sci. 2022, 4, e147. [Google Scholar] [CrossRef]
- Gdala, J. Composition, Properties, and Nutritive Value of Dietary Fibre of Legume Seeds. A Review. J. Anim. Feed. Sci. 1998, 7, 131–150. [Google Scholar] [CrossRef]
- Fustec, J.; Lesuffleur, F.; Mahieu, S.; Cliquet, J.-B. Nitrogen Rhizodeposition of Legumes. A Review. Agron. Sustain. Dev. 2010, 30, 57–66. [Google Scholar] [CrossRef]
- Monti, M.; Pellicanò, A.; Pristeri, A.; Badagliacca, G.; Preiti, G.; Gelsomino, A. Cereal/Grain Legume Intercropping in Rotation with Durum Wheat in Crop/Livestock Production Systems for Mediterranean Farming System. Field Crops Res. 2019, 240, 23–33. [Google Scholar] [CrossRef]
- Lithourgidis, A.S.; Dhima, K.V.; Vasilakoglou, I.B.; Dordas, C.A.; Yiakoulaki, M.D. Sustainable Production of Barley and Wheat by Intercropping Common Vetch. Agron. Sustain. Dev. 2007, 27, 95–99. [Google Scholar] [CrossRef]
- Stomph, T.; Dordas, C.; Baranger, A.; de Rijk, J.; Dong, B.; Evers, J.; Gu, C.; Li, L.; Simon, J.; Jensen, E.S.; et al. Chapter One-Designing Intercrops for High Yield, Yield Stability and Efficient Use of Resources: Are There Principles? In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 160, pp. 1–50. [Google Scholar]
- Bedoussac, L.; Journet, E.-P.; Hauggaard-Nielsen, H.; Naudin, C.; Corre-Hellou, G.; Jensen, E.S.; Prieur, L.; Justes, E. Ecological Principles Underlying the Increase of Productivity Achieved by Cereal-Grain Legume Intercrops in Organic Farming. A Review. Agron. Sustain. Dev. 2015, 35, 911–935. [Google Scholar] [CrossRef]
- Yu, Y.; Stomph, T.-J.; Makowski, D.; Zhang, L.; van der Werf, W. A Meta-Analysis of Relative Crop Yields in Cereal/Legume Mixtures Suggests Options for Management. Field Crop Res. 2016, 198, 269–279. [Google Scholar] [CrossRef]
- Yu, L.; Tang, Y.; Wang, Z.; Gou, Y.; Wang, J. Nitrogen-Cycling Genes and Rhizosphere Microbial Community with Reduced Nitrogen Application in Maize/Soybean Strip Intercropping. Nutr. Cycl. Agroecosyst. 2019, 113, 35–49. [Google Scholar] [CrossRef]
- Kermah, M.; Franke, A.C.; Adjei-Nsiah, S.; Ahiabor, B.D.K.; Abaidoo, R.C.; Giller, K.E. Maize-Grain Legume Intercropping for Enhanced Resource Use Efficiency and Crop Productivity in the Guinea Savanna of Northern Ghana. Field Crops Res. 2017, 213, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Yin, X.; Ren, J.; Zhang, M.; Tang, L.; Zheng, Y. Complementation Drives Higher Growth Rate and Yield of Wheat and Saves Nitrogen Fertilizer in Wheat and Faba Bean Intercropping. Field Crop Res. 2018, 221, 119–129. [Google Scholar] [CrossRef]
- Izaurralde, R.C.; McGill, W.B.; Juma, N.G. Nitrogen Fixation Efficiency, Interspecies N Transfer, and Root Growth in Barley-Field Pea Intercrop on a Black Chernozemic Soil. Biol. Fertil. Soils 1992, 13, 11–16. [Google Scholar] [CrossRef]
- Jensen, E.S. Grain Yield, Symbiotic N2 Fixation and Interspecific Competition for Inorganic N in Pea-Barley Intercrops. Plant Soil 1996, 182, 25–38. [Google Scholar] [CrossRef]
- Danso, S.K.A.; Zapata, F.; Hardarson, G.; Fried, M. Nitrogen Fixation in Fababeans as Affected by Plant Population Density in Sole or Intercropped Systems with Barley. Soil Biol. Biochem. 1987, 19, 411–415. [Google Scholar] [CrossRef]
- Rodriguez, C.; Carlsson, G.; Englund, J.-E.; Flöhr, A.; Pelzer, E.; Jeuffroy, M.-H.; Makowski, D.; Jensen, E.S. Grain Legume-Cereal Intercropping Enhances the Use of Soil-Derived and Biologically Fixed Nitrogen in Temperate Agroecosystems. A Meta-Analysis. Eur. J. Agron. 2020, 118, 126077. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Jensen, E.S. Evaluating Pea and Barley Cultivars for Complementarity in Intercropping at Different Levels of Soil N Availability. Field Crops Res. 2001, 72, 185–196. [Google Scholar] [CrossRef]
- Andersen, M.K.; Hauggaard-Nielsen, H.; Ambus, P.; Jensen, E.S. Biomass Production, Symbiotic Nitrogen Fixation and Inorganic N Use in Dual and Tri-Component Annual Intercrops. Plant Soil 2005, 266, 273–287. [Google Scholar] [CrossRef]
- Nguyen, C. Rhizodeposition of Organic C by Plant: Mechanisms and Controls. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 97–123. ISBN 978-90-481-2666-8. [Google Scholar]
- Moyer-Henry, K.A.; Burton, J.W.; Israel, D.W.; Rufty, T.W. Nitrogen Transfer between Plants: A 15N Natural Abundance Study with Crop and Weed Species. Plant Soil 2006, 282, 7–20. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, L.; Shao, Y.; Wang, J. Root and Hyphal Interactions Influence N Transfer by Arbuscular Mycorrhizal Fungi in Soybean/Maize Intercropping Systems. Fungal Ecol. 2023, 64, 101240. [Google Scholar] [CrossRef]
- Fujita, K.; Ogata, S.; Matsumoto, K.; Masuda, T.; Ofosu-Budu, G.K.; Kuwata, K. Nitrogen Transfer and Dry Matter Production in Soybean and Sorghum Mixed Cropping System at Different Population Densities. Soil Sci. Plant Nutr. 1990, 36, 233–241. [Google Scholar] [CrossRef]
- Chu, G.X.; Shen, Q.R.; Cao, J.L. Nitrogen Fixation and N Transfer from Peanut to Rice Cultivated in Aerobic Soil in an Intercropping System and Its Effect on Soil N Fertility. Plant Soil 2004, 263, 17–27. [Google Scholar] [CrossRef]
- Gungaabayar, A.; Jha, A.; Warkentin, T.; Knight, D.; Penner, G.; Biligetu, B. Forage Yield and Biological Nitrogen Fixation of Pea–Cereal Intercrops for Hay Production. Agron. J. 2023, 115, 607–619. [Google Scholar] [CrossRef]
- Corre-Hellou, G.; Fustec, J.; Crozat, Y. Interspecific Competition for Soil N and Its Interaction with N2 Fixation, Leaf Expansion and Crop Growth in Pea–Barley Intercrops. Plant Soil 2006, 282, 195–208. [Google Scholar] [CrossRef]
- Neumann, A.; Schmidtke, K.; Rauber, R. Effects of Crop Density and Tillage System on Grain Yield and N Uptake from Soil and Atmosphere of Sole and Intercropped Pea and Oat. Field Crops Res. 2007, 100, 285–293. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Gooding, M.; Ambus, P.; Corre-Hellou, G.; Crozat, Y.; Dahlmann, C.; Dibet, A.; von Fragstein, P.; Pristeri, A.; Monti, M.; et al. Pea–Barley Intercropping for Efficient Symbiotic N2-Fixation, Soil N Acquisition and Use of Other Nutrients in European Organic Cropping Systems. Field Crop Res. 2009, 113, 64–71. [Google Scholar] [CrossRef]
- Bedoussac, L.; Journet, E.-P.; Hauggaard-Nielsen, H.; Naudin, C.; Corre-Hellou, G.; Prieur, L.; Jensen, E.S.; Justes, E. Eco-Functional Intensification by Cereal-Grain Legume Intercropping in Organic Farming Systems for Increased Yields, Reduced Weeds and Improved Grain Protein Concentration. In Organic Farming, Prototype for Sustainable Agricultures; Springer: Dordrecht, The Netherlands, 2014; pp. 47–63. ISBN 978-94-007-7927-3. [Google Scholar]
- Voisin, A.-S.; Salon, C.; Munier-Jolain, N.G.; Ney, B. Quantitative Effects of Soil Nitrate, Growth Potential and Phenology on Symbiotic Nitrogen Fixation of Pea (Pisum sativum L.). Plant Soil 2002, 243, 31–42. [Google Scholar] [CrossRef]
- Naudin, C.; Corre-Hellou, G.; Voisin, A.-S.; Oury, V.; Salon, C.; Crozat, Y.; Jeuffroy, M.-H. Inhibition and Recovery of Symbiotic N2 Fixation by Peas (Pisum sativum L.) in Response to Short-Term Nitrate Exposure. Plant Soil 2011, 346, 275–287. [Google Scholar] [CrossRef]
- Zhao, F.; Sun, Z.; Feng, L.; Zhang, Y.; Feng, C.; Bai, W.; Zheng, J.; Zhang, Z.; Yang, N.; Cai, Q.; et al. Biological N Fixation but Not Mineral N Fertilization Enhances the Accumulation of N in Peanut Soil in Maize/Peanut Intercropping System. J. Agric. Food Res. 2022, 10, 100365. [Google Scholar] [CrossRef]
- Mead, R.; Willey, R.W. The Concept of a ‘Land Equivalent Ratio’ and Advantages in Yields from Intercropping. Exp. Agric. 1980, 16, 217–228. [Google Scholar] [CrossRef]
- Vandermeer, J.H. The Ecology of Intercropping; Cambridge University Press: Cambridge, UK, 1989; ISBN 978-0-521-34689-4. [Google Scholar]
- Fetene, M.; Feten, M. Intra- and Inter-Specific Competition between Seedlings of Acacia Etbaica and a Perennial Grass (Hyparrhenia hirta). J. Arid. Environ. 2003, 55, 441–451. [Google Scholar] [CrossRef]
- Kaci, G.; Ouaret, W.; Rahmoune, B. Wheat-Faba Bean Intercrops Improve Plant Nutrition, Yield, and Availability of Nitrogen (N) and Phosphorus (P) in Soil. Agron. Res. 2022, 20, 603–616. [Google Scholar] [CrossRef]
- Tavoletti, S.; Merletti, A. A Comprehensive Approach to Evaluate Durum Wheat–Faba Bean Mixed Crop Performance. Front. Plant Sci. 2022, 13, 733116. [Google Scholar] [CrossRef] [PubMed]
- Polthanee, A.; Trelo-ges, V. Growth, Yield and Land Use Efficiency of Corn and Legumes Grown under Intercropping Systems. Plant Prod. Sci. 2003, 6, 139–146. [Google Scholar] [CrossRef]
- Cowden, R.J.; Shah, A.N.; Lehmann, L.M.; Kiær, L.P.; Henriksen, C.B.; Ghaley, B.B. Nitrogen Fertilizer Effects on Pea–Barley Intercrop Productivity Compared to Sole Crops in Denmark. Sustainability 2020, 12, 9335. [Google Scholar] [CrossRef]
- Del Pozo, A.; Garnier, E.; Aronson, J. Contrasted Nitrogen Utilization in Annual C3 Grass and Legume Crops: Physiological Explorations and Ecological Considerations. Acta Oecol. 2000, 21, 79–89. [Google Scholar] [CrossRef]
- Del Pozo, A.; Pérez, P.; Gutiérrez, D.; Alonso, A.; Morcuende, R.; Martínez-Carrasco, R. Gas Exchange Acclimation to Elevated CO2 in Upper-Sunlit and Lower-Shaded Canopy Leaves in Relation to Nitrogen Acquisition and Partitioning in Wheat Grown in Field Chambers. Environ. Exp. Bot. 2007, 59, 371–380. [Google Scholar] [CrossRef]
- Yu-zheng, Z.; Han-qing, Z.; Ping, L.; Dong-sheng, Z.; Xing-yu, H.; Zhi-qiang, G. Leaf Nitrogen Have a Better Relationship with Photosynthesis Performance across Wheat Species under Elevated CO2 and Drought. Plant Physiol. Biochem. 2021, 166, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Dordas, C.A.; Lithourgidis, A.S.; Matsi, T.; Barbayiannis, N. Application of Liquid Cattle Manure and Inorganic Fertilizers Affect Dry Matter, Nitrogen Accumulation, and Partitioning in Maize. Nutr. Cycl. Agroecosyst. 2008, 80, 283–296. [Google Scholar] [CrossRef]
- Lithourgidis, A.S.; Dordas, C.A. Forage Yield, Growth Rate, and Nitrogen Uptake of Faba Bean Intercrops with Wheat, Barley, and Rye in Three Seeding Ratios. Crop Sci. 2010, 50, 2148–2158. [Google Scholar] [CrossRef]
- Tosti, G.; Guiducci, M. Durum Wheat–Faba Bean Temporary Intercropping: Effects on Nitrogen Supply and Wheat Quality. Eur. J. Agron. 2010, 33, 157–165. [Google Scholar] [CrossRef]
- Suryapani, S.; Umar, S.; Malik, A.A.; Ahmad, A. Symbiotic Nitrogen Fixation by Lentil Improves Biochemical Characteristics and Yield of Intercropped Wheat Under Low Fertilizer Input. J. Crop Improv. 2013, 27, 53–66. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Morshedloo, M.R.; Maggi, F. Evaluation of Competition, Essential Oil Quality and Quantity of Peppermint Intercropped with Soybean. Ind. Crops Prod. 2018, 111, 743–754. [Google Scholar] [CrossRef]
- Duan, Y.; Shen, J.; Zhang, X.; Wen, B.; Ma, Y.; Wang, Y.; Fang, W.; Zhu, X. Effects of Soybean–Tea Intercropping on Soil-Available Nutrients and Tea Quality. Acta Physiol. Plant 2019, 41, 140. [Google Scholar] [CrossRef]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.-L.; Barbottin, A.; Jeuffroy, M.-H.; Gate, P.; Agati, G.; et al. Optically Assessed Contents of Leaf Polyphenolics and Chlorophyll as Indicators of Nitrogen Deficiency in Wheat (Triticum aestivum L.). Field Crops Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Wang, R.; Sun, Z.; Bai, W.; Wang, E.; Wang, Q.; Zhang, D.; Zhang, Y.; Yang, N.; Liu, Y.; Nie, J.; et al. Canopy Heterogeneity with Border-Row Proportion Affects Light Interception and Use Efficiency in Maize/Peanut Strip Intercropping. Field Crops Res. 2021, 271, 108239. [Google Scholar] [CrossRef]
- Pellicanò, A.; Romeo, M.; Pristeri, A.; Preiti, G.; Monti, M. Cereal-Pea Intercrops to Improve Sustainability in Bioethanol Production. Agron. Sustain. Dev. 2015, 35, 827–835. [Google Scholar] [CrossRef]
- Szumigalski, A.R.; Van Acker, R.C. Land Equivalent Ratios, Light Interception, and Water Use in Annual Intercrops in the Presence or Absence of In-Crop Herbicides. Agron. J. 2008, 100, 1145–1154. [Google Scholar] [CrossRef]
- Du, X.; Chen, B.; Shen, T.; Zhang, Y.; Zhou, Z. Effect of Cropping System on Radiation Use Efficiency in Double-Cropped Wheat–Cotton. Field Crops Res. 2015, 170, 21–31. [Google Scholar] [CrossRef]
- Liu, X.; Rahman, T.; Song, C.; Yang, F.; Su, B.; Cui, L.; Bu, W.; Yang, W. Relationships among Light Distribution, Radiation Use Efficiency and Land Equivalent Ratio in Maize-Soybean Strip Intercropping. Field Crops Res. 2018, 224, 91–101. [Google Scholar] [CrossRef]
- Jensen, E.S.; Carlsson, G.; Hauggaard-Nielsen, H. Intercropping of Grain Legumes and Cereals Improves the Use of Soil N Resources and Reduces the Requirement for Synthetic Fertilizer N: A Global-Scale Analysis. Agron. Sustain. Dev. 2020, 40, 5. [Google Scholar] [CrossRef]
- Boutagayout, A.; Belmalha, S.; Nassiri, L.; El Alami, N.; Jiang, Y.; Lahlali, R.; Bouiamrine, E.H. Weed Competition, Land Equivalent Ratio and Yield Potential of Faba Bean (Vicia faba L.)-Cereals (Triticum aestivum L. and/or Avena sativa L.) Intercropping under Low-Input Conditions in Meknes Region, Morocco. Vegetos 2023, 1–14. [Google Scholar] [CrossRef]
- Nurgi, N.; Tana, T.; Dechassa, N.; Tesso, B.; Alemayehu, Y. Effect of Spatial Arrangement of Faba Bean Variety Intercropping with Maize on Yield and Yield Components of the Crops. Heliyon 2023, 9, e16751. [Google Scholar] [CrossRef]
- Bedoussac, L.; Justes, E. A Comparison of Commonly Used Indices for Evaluating Species Interactions and Intercrop Efficiency: Application to Durum Wheat–Winter Pea Intercrops. Field Crops Res. 2011, 124, 25–36. [Google Scholar] [CrossRef]
- Sahota, T.S.; Malhi, S.S. Intercropping Barley with Pea for Agronomic and Economic Considerations in Northern Ontario. Agric. Sci. 2012, 3, 889–895. [Google Scholar] [CrossRef]
- Monti, M.; Pellicanò, A.; Santonoceto, C.; Preiti, G.; Pristeri, A. Yield Components and Nitrogen Use in Cereal-Pea Intercrops in Mediterranean Environment. Field Crop Res. 2016, 196, 379–388. [Google Scholar] [CrossRef]
- Dhima, K.V.; Vasilakoglou, I.B.; Keco, R.X.; Dima, A.K.; Paschalidis, K.A.; Gatsis, T.D. Forage Yield and Competition Indices of Faba Bean Intercropped with Oat. Grass Forage Sci. 2014, 69, 376–383. [Google Scholar] [CrossRef]
- Ghaley, B.B.; Hauggaard-Nielsen, H.; Høgh-Jensen, H.; Jensen, E.S. Intercropping of Wheat and Pea as Influenced by Nitrogen Fertilization. Nutr. Cycl. Agroecosyst. 2005, 73, 201–212. [Google Scholar] [CrossRef]
- Brooker, R.W.; Bennett, A.E.; Cong, W.-F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.M.; Jones, H.G.; Karley, A.J.; et al. Improving Intercropping: A Synthesis of Research in Agronomy, Plant Physiology and Ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef]
- Jensen, E.S.; Bedoussac, L.; Carlsson, G.; Journet, E.-P.; Justes, E.; Hauggaard-Nielsen, H. Enhancing Yields in Organic Crop Production by Eco-Functional Intensification. Sustain. Agric. Res. 2015, 4, 42–50. [Google Scholar] [CrossRef]
- Saia, S.; Urso, V.; Amato, G.; Frenda, A.S.; Giambalvo, D.; Ruisi, P.; Di Miceli, G. Mediterranean Forage Legumes Grown Alone or in Mixture with Annual Ryegrass: Biomass Production, N2 Fixation, and Indices of Intercrop Efficiency. Plant Soil 2016, 402, 395–407. [Google Scholar] [CrossRef]
- Bacchi, M.; Monti, M.; Calvi, A.; Lo Presti, E.; Pellicanò, A.; Preiti, G. Forage Potential of Cereal/Legume Intercrops: Agronomic Performances, Yield, Quality Forage and LER in Two Harvesting Times in a Mediterranean Environment. Agronomy 2021, 11, 121. [Google Scholar] [CrossRef]
- Guinet, M.; Nicolardot, B.; Revellin, C.; Durey, V.; Carlsson, G.; Voisin, A.-S. Comparative Effect of Inorganic N on Plant Growth and N2 Fixation of Ten Legume Crops: Towards a Better Understanding of the Differential Response among Species. Plant Soil 2018, 432, 207–227. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.; Xiao, J.; Tang, L.; Zheng, Y. Interactive Influences of Intercropping by Nitrogen on Flavonoid Exudation and Nodulation in Faba Bean. Sci. Rep. 2019, 9, 4818. [Google Scholar] [CrossRef] [PubMed]
- Banik, P.; Sharma, R.C. Yield and Resource Utilization Efficiency in Baby Corn—Legume-Intercropping System in the Eastern Plateau of India. J. Sustain. Agric. 2009, 33, 379–395. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Yu, C.-B.; Cheng, X.; Li, C.-J.; Sun, J.-H.; Zhang, F.-S.; Lambers, H.; Li, L. Intercropping Alleviates the Inhibitory Effect of N Fertilization on Nodulation and Symbiotic N2 Fixation of Faba Bean. Plant Soil 2009, 323, 295–308. [Google Scholar] [CrossRef]
- Hu, F.; Zhao, C.; Feng, F.; Chai, Q.; Mu, Y.; Zhang, Y. Improving N Management through Intercropping Alleviates the Inhibitory Effect of Mineral N on Nodulation in Pea. Plant Soil 2017, 412, 235–251. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Andersen, M.K.; Jørnsgaard, B.; Jensen, E.S. Density and Relative Frequency Effects on Competitive Interactions and Resource Use in Pea–Barley Intercrops. Field Crop Res. 2006, 95, 256–267. [Google Scholar] [CrossRef]
- Zhao, C.; Fan, Z.; Coulter, J.A.; Yin, W.; Hu, F.; Yu, A.; Fan, H.; Chai, Q. High Maize Density Alleviates the Inhibitory Effect of Soil Nitrogen on Intercropped Pea. Agronomy 2020, 10, 248. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; McElroy, M.S.; Chapagain, T.; Papadopoulos, Y.A.; Raizada, M.N. Belowground Nitrogen Transfer from Legumes to Non-Legumes under Managed Herbaceous Cropping Systems. A Review. Agron. Sustain. Dev. 2016, 36, 58. [Google Scholar] [CrossRef]
- Naudin, C.; Corre-Hellou, G.; Pineau, S.; Crozat, Y.; Jeuffroy, M.-H. The Effect of Various Dynamics of N Availability on Winter Pea–Wheat Intercrops: Crop Growth, N Partitioning and Symbiotic N2 Fixation. Field Crop Res. 2010, 119, 2–11. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Zhang, H.; Shao, Z.; Sun, B.; Gao, Q. Enhancement of Rhizosphere Citric Acid and Decrease of NO3−/NH4+ Ratio by Root Interactions Facilitate N Fixation and Transfer. Plant Soil 2020, 447, 169–182. [Google Scholar] [CrossRef]
- Bedoussac, L.; Justes, E. The Efficiency of a Durum Wheat-Winter Pea Intercrop to Improve Yield and Wheat Grain Protein Concentration Depends on N Availability during Early Growth. Plant Soil 2010, 330, 19–35. [Google Scholar] [CrossRef]
- Jiao, N.; Wang, F.; Ma, C.; Zhang, F.; Jensen, E.S. Interspecific Interactions of Iron and Nitrogen Use in Peanut (Arachis hypogaea L.)-Maize (Zea mays L.) Intercropping on a Calcareous Soil. Eur. J. Agron. 2021, 128, 126303. [Google Scholar] [CrossRef]
- Zhu, S.-G.; Zhu, H.; Zhou, R.; Zhang, W.; Wang, W.; Zhou, Y.-N.; Wang, B.-Z.; Yang, Y.-M.; Wang, J.; Tao, H.-Y.; et al. Intercrop Overyielding Weakened by High Inputs: Global Meta-Analysis with Experimental Validation. Agric. Ecosyst. Environ. 2023, 342, 108239. [Google Scholar] [CrossRef]
- Li, C.; Hoffland, E.; Kuyper, T.W.; Yu, Y.; Zhang, C.; Li, H.; Zhang, F.; van der Werf, W. Syndromes of Production in Intercropping Impact Yield Gains. Nat. Plants 2020, 6, 653–660. [Google Scholar] [CrossRef]
- Unkovich, M.; Herridge, D.; Peoples, M.; Cadisch, G.; Boddey, B.; Giller, K.; Alves, B.; Chalk, P.M. Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems; ACIAR Monograph; Australian Centre for International Agricultural Research: Canberra, ACT, Australia, 2008. [Google Scholar]
- Kohl, D.H.; Shearer, G. Isotopic Fractionation Associated with Symbiotic N2 Fixation and Uptake of NO3− by Plants. Plant Physiol. 1980, 66, 51–56. [Google Scholar] [CrossRef]
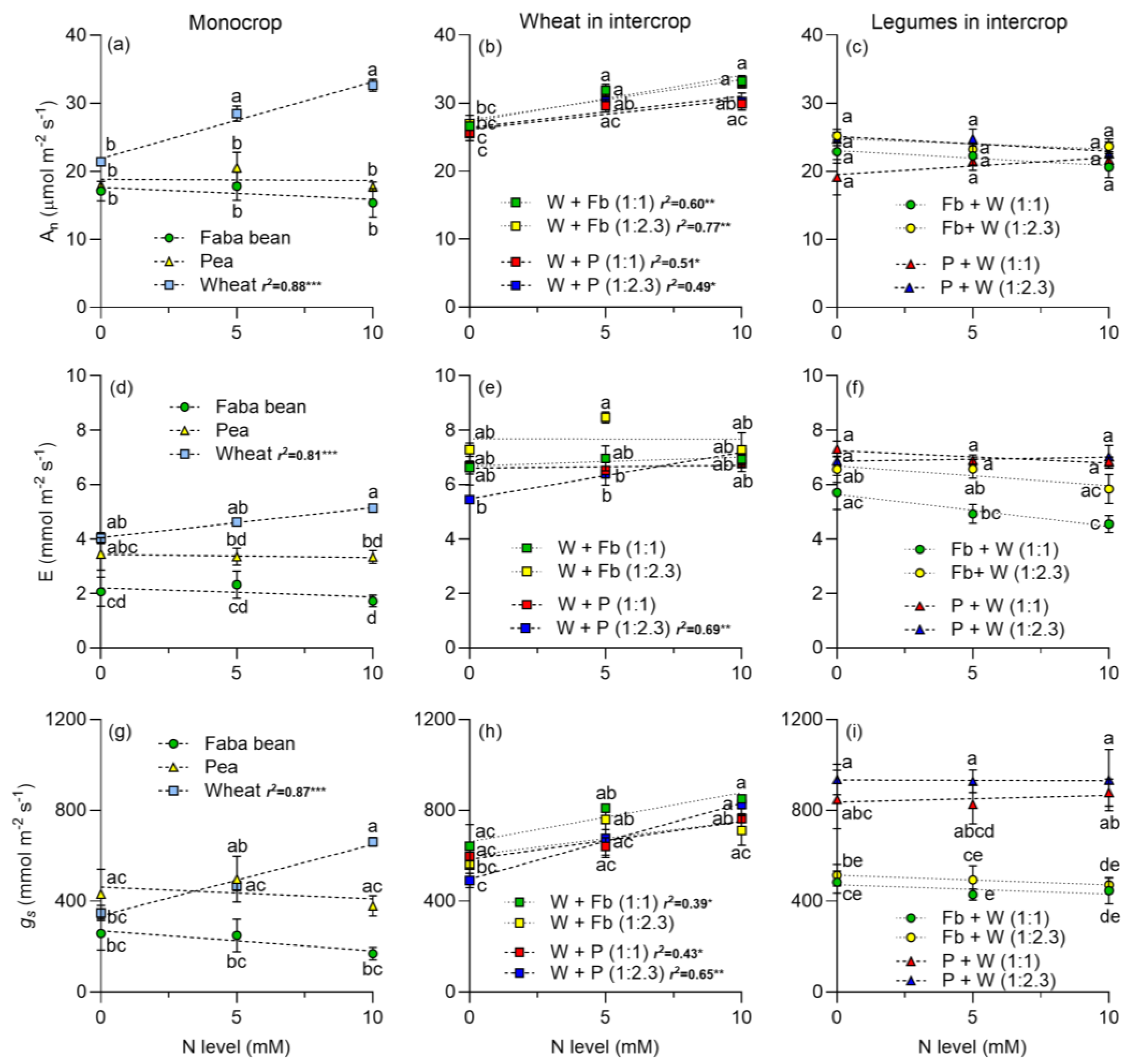
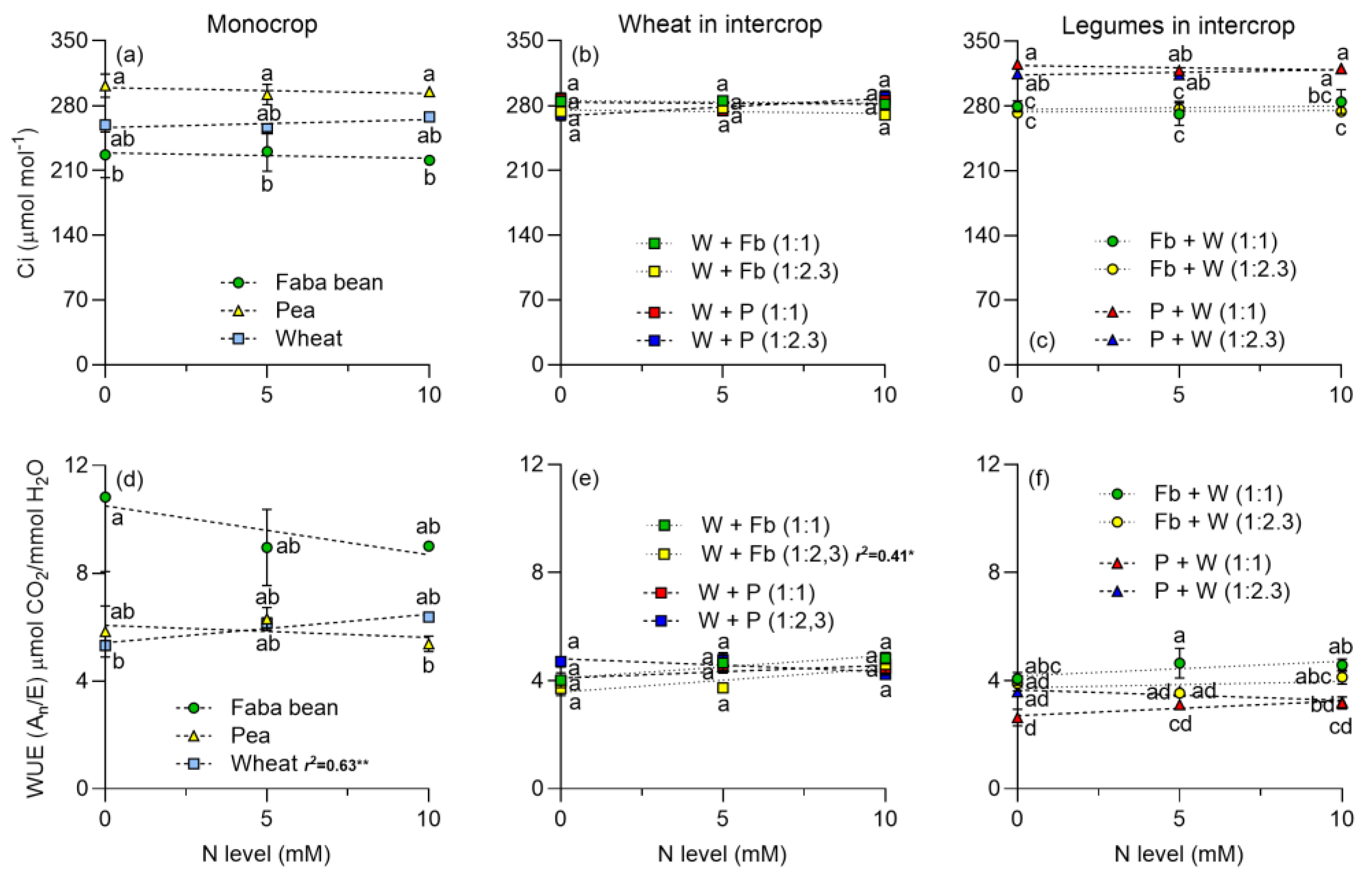
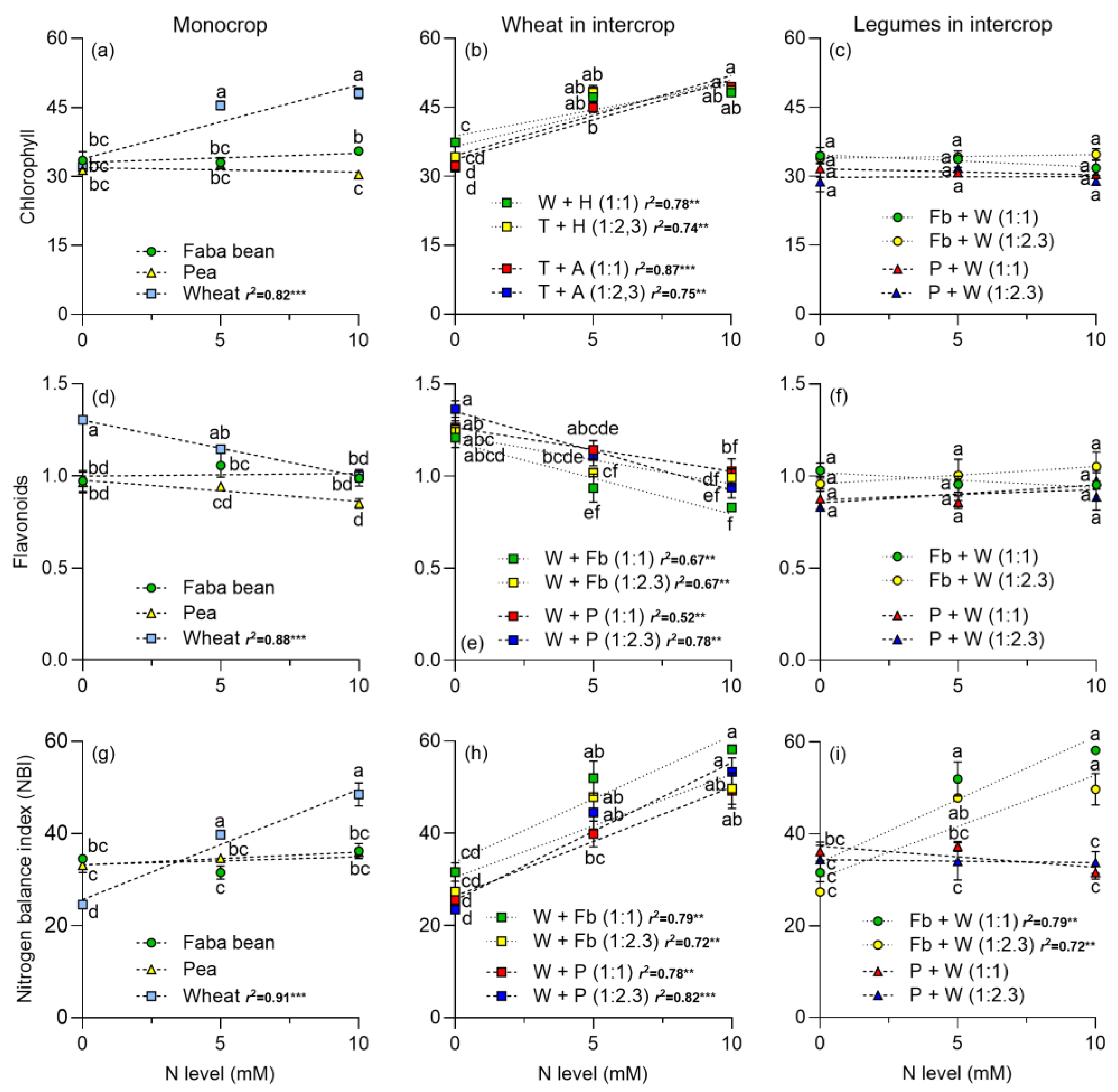
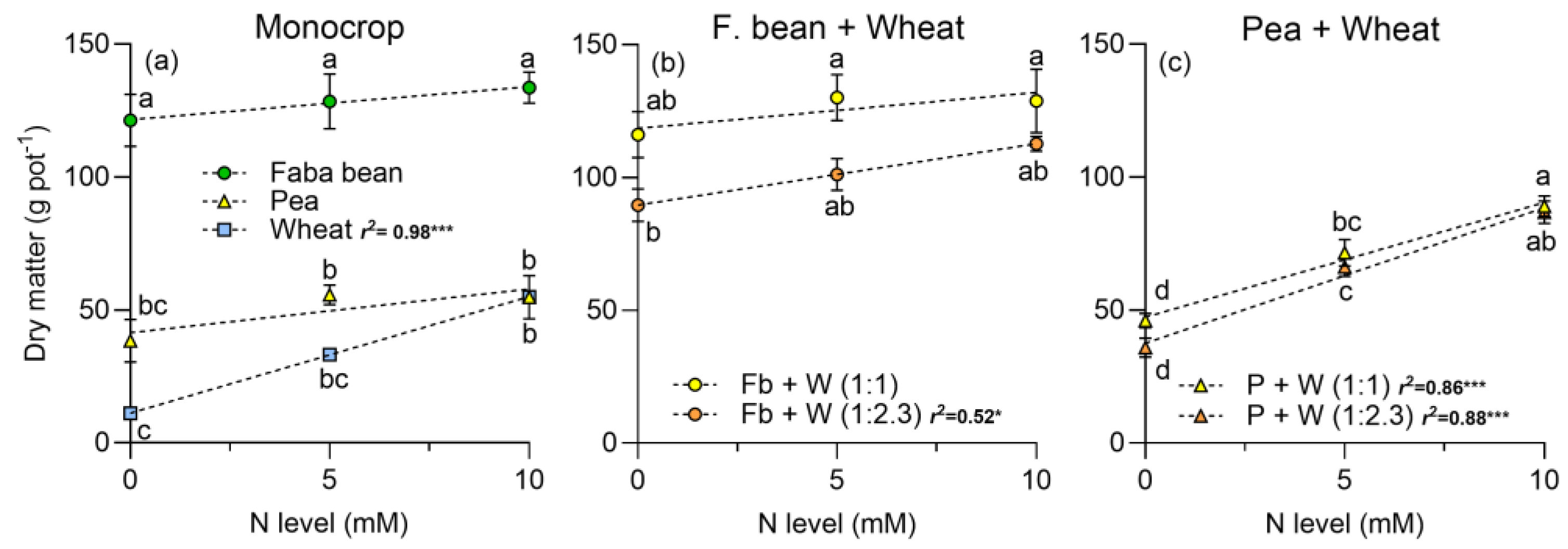

| Factors | Variable | df | F-Value | p-Value |
|---|---|---|---|---|
| A: Plant density | V. faba shoot DM | 2 | 10.20 | <0.001 *** |
| B: N dose | 2 | 3.11 | 0.06 | |
| A × B | 4 | 0.23 | 0.92 | |
| A: Plant density | P. sativum shoot DM | 2 | 11.17 | <0.001 *** |
| B: N dose | 2 | 40.36 | <0.001 *** | |
| A × B | 4 | 3.23 | 0.03 * | |
| A: Plant density | V. faba nodule DM | 2 | 6.19 | 0.006 ** |
| B: N dose | 2 | 1.44 | 0.25 | |
| A × B | 4 | 0.39 | 0.81 | |
| A: Plant density | P. sativum nodule DM | 2 | 3.08 | 0.12 |
| B: N dose | 2 | 0.95 | 0.40 | |
| A × B | 4 | 2.03 | 0.12 | |
| A: Plant density | LER for V. faba | 1 | 0.94 | 0.35 |
| B: N dose | 2 | 38.3 | <0.001 *** | |
| A × B | 2 | 0.09 | 0.91 | |
| A: Plant density | LER for P. sativum | 1 | 0.93 | 0.35 |
| B: N dose | 2 | 2.16 | 0.14 | |
| A × B | 2 | 0.12 | 0.88 |
| Factors | Variable | df | F-Value | p-Value |
|---|---|---|---|---|
| A: Plant density | %Ndfa V. faba | 2 | 9.96 | <0.001 *** |
| B: N level | 2 | 57.5 | <0.001 *** | |
| A × B | 4 | 0.32 | 0.86 | |
| A: Plant density | %Ndfa P. sativum | 2 | 72.3 | <0.00 *** |
| B: N level | 2 | 56.3 | <0.001 *** | |
| A × B | 4 | 7.07 | <0.001 *** | |
| A: Plant density | %N transferred V. faba | 1 | 0.87 | 0.36 |
| B: N level | 2 | 3.74 | 0.04 * | |
| A × B | 4 | 0.38 | 0.68 | |
| A: Plant density | %N transferred P. sativum | 1 | 0.06 | 0.80 |
| B: N level | 2 | 15.8 | <0.001 *** | |
| A × B | 4 | 1.05 | 0.37 | |
| A: Plant density | LERN V. faba | 1 | 2.22 | 0.15 |
| B: N level | 2 | 8.02 | 0.004 ** | |
| A × B | 2 | 2.60 | 0.10 | |
| A: Plant density | LERN P. sativum | 1 | 0.008 | 0.93 |
| B: N level | 2 | 0.93 | 0.41 | |
| A × B | 2 | 0.03 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salinas-Roco, S.; Morales-González, A.; Espinoza, S.; Pérez-Díaz, R.; Carrasco, B.; del Pozo, A.; Cabeza, R.A. N2 Fixation, N Transfer, and Land Equivalent Ratio (LER) in Grain Legume–Wheat Intercropping: Impact of N Supply and Plant Density. Plants 2024, 13, 991. https://doi.org/10.3390/plants13070991
Salinas-Roco S, Morales-González A, Espinoza S, Pérez-Díaz R, Carrasco B, del Pozo A, Cabeza RA. N2 Fixation, N Transfer, and Land Equivalent Ratio (LER) in Grain Legume–Wheat Intercropping: Impact of N Supply and Plant Density. Plants. 2024; 13(7):991. https://doi.org/10.3390/plants13070991
Chicago/Turabian StyleSalinas-Roco, Sebastian, Amanda Morales-González, Soledad Espinoza, Ricardo Pérez-Díaz, Basilio Carrasco, Alejandro del Pozo, and Ricardo A. Cabeza. 2024. "N2 Fixation, N Transfer, and Land Equivalent Ratio (LER) in Grain Legume–Wheat Intercropping: Impact of N Supply and Plant Density" Plants 13, no. 7: 991. https://doi.org/10.3390/plants13070991





