Enhancing Postharvest Quality and Shelf Life of Strawberries through Advanced Coating Technologies: A Comprehensive Investigation of Chitosan and Glycine Betaine Nanoparticle Treatments
Abstract
:1. Introduction
2. Results and Discussion
2.1. Decay Percentage
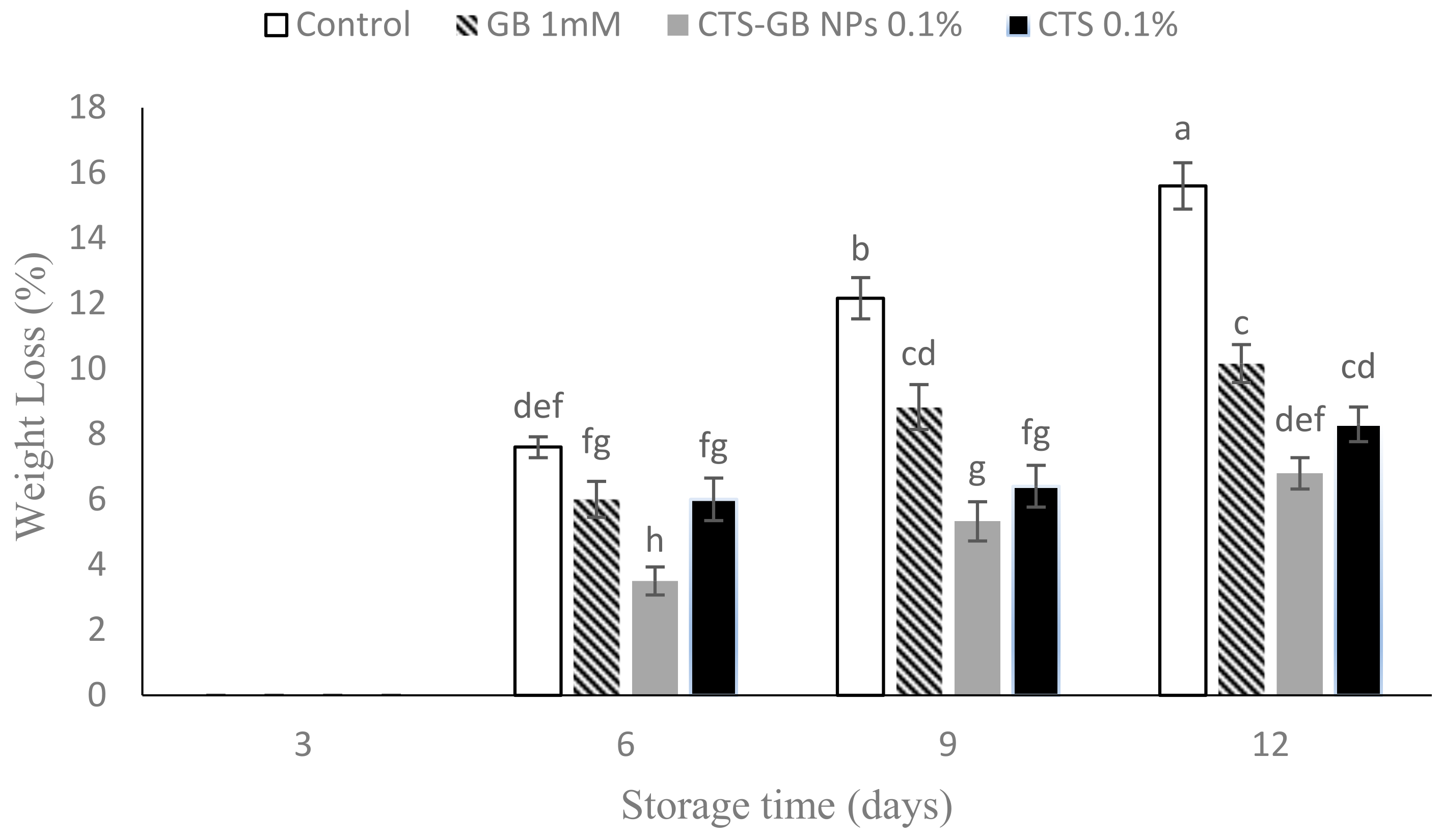
2.2. Weight Loss
2.3. Fruit Tissue Firmness
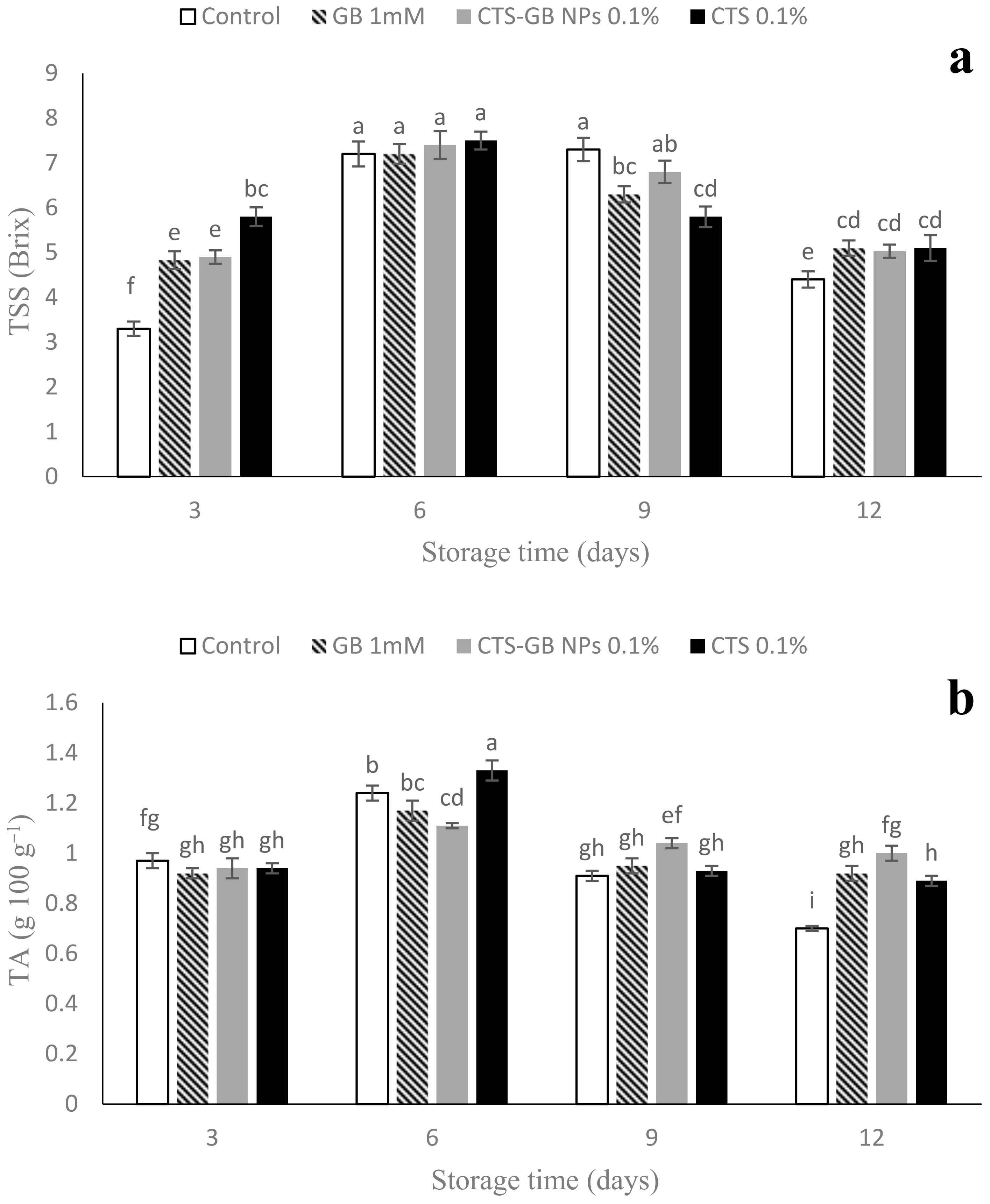
2.4. Total Soluble Solids (TSSs) and Titratable Acidity (TA)
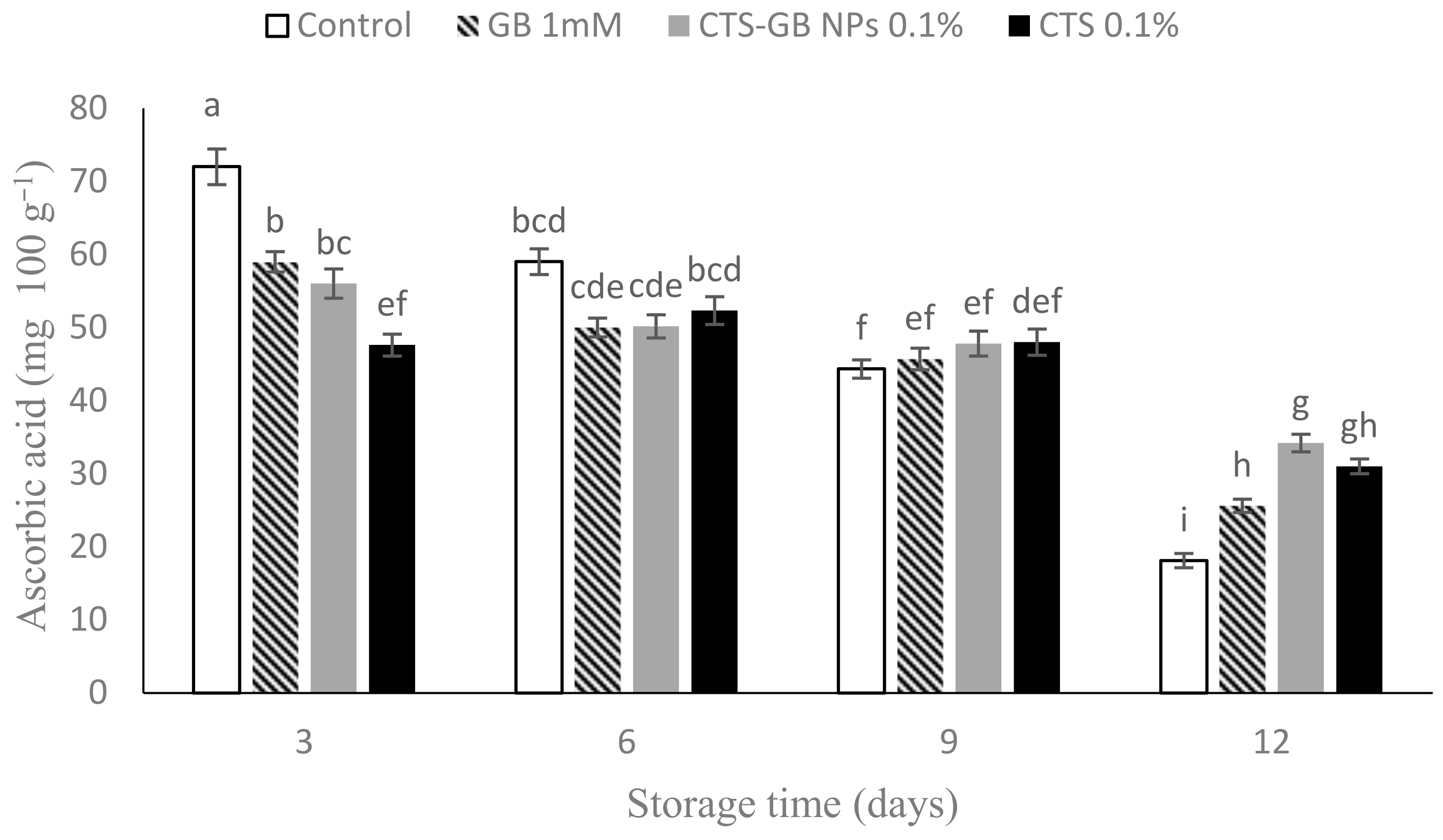
2.5. Ascorbic Acid
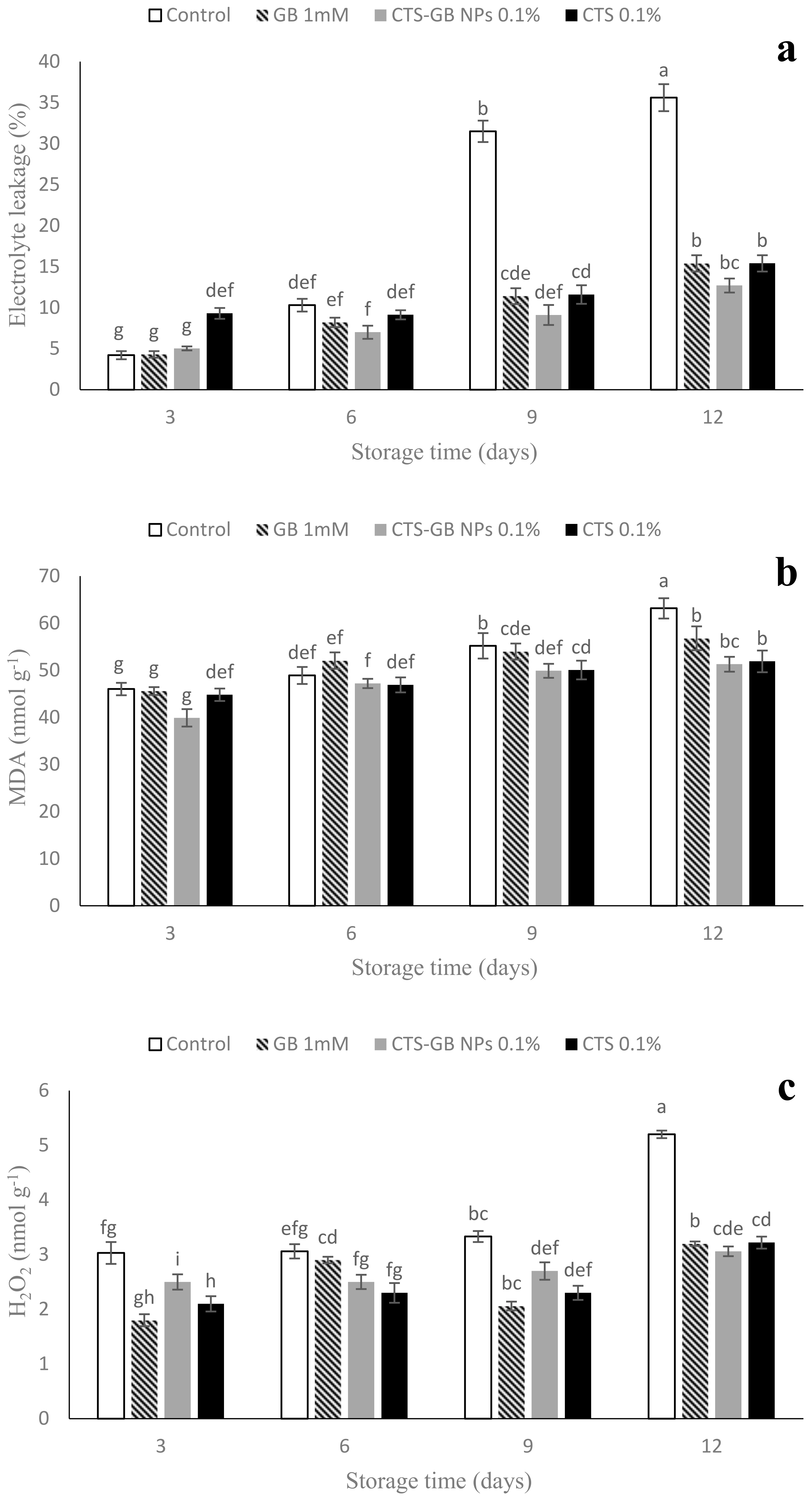
2.6. Electrolyte Leakage (EL), Malondialdehyde (MDA), and Hydrogen Peroxide (H2O2)
2.7. Total Anthocyanin Content
2.8. Total Phenol, Flavonoid, and Antioxidant Capacity
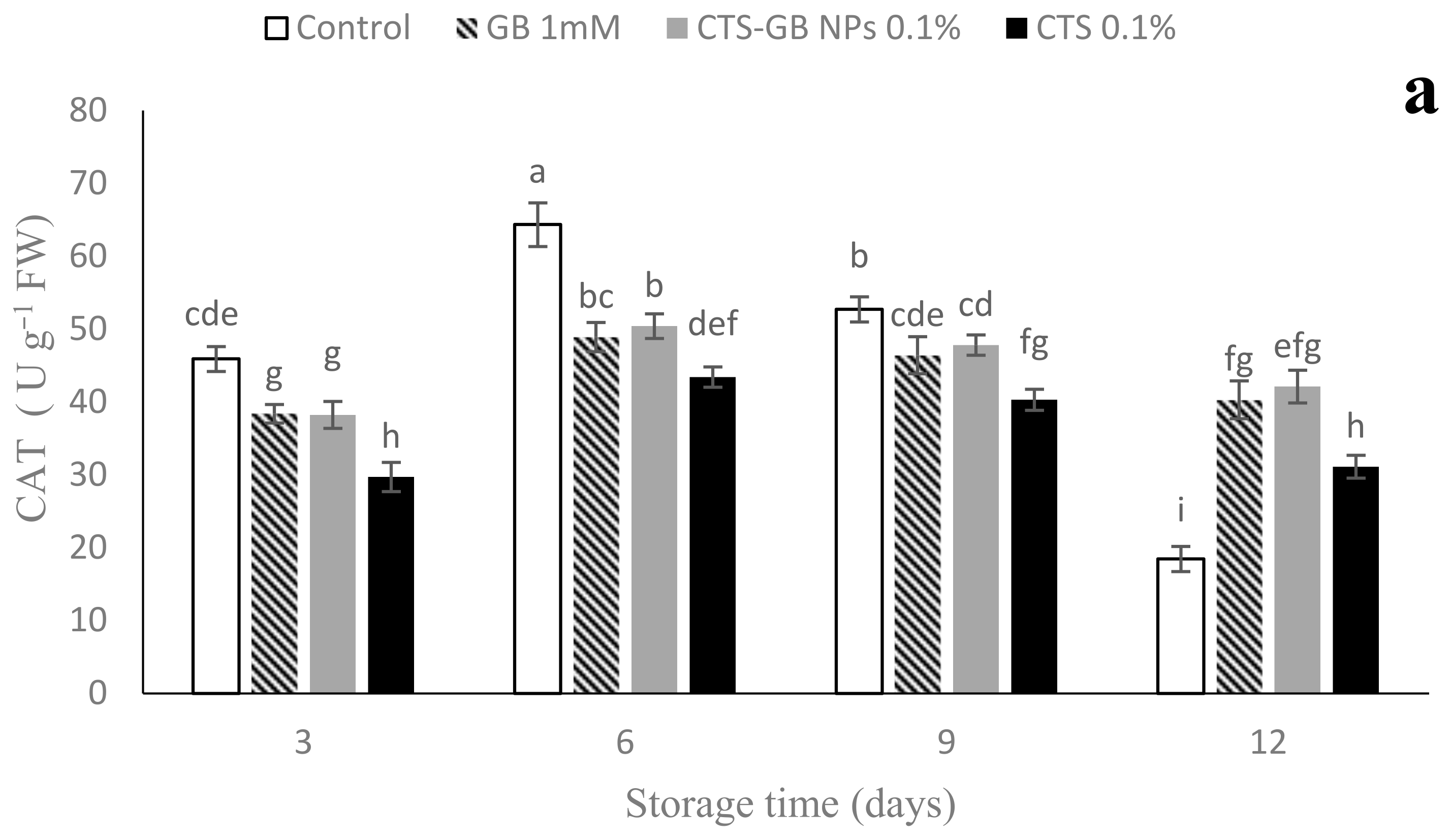
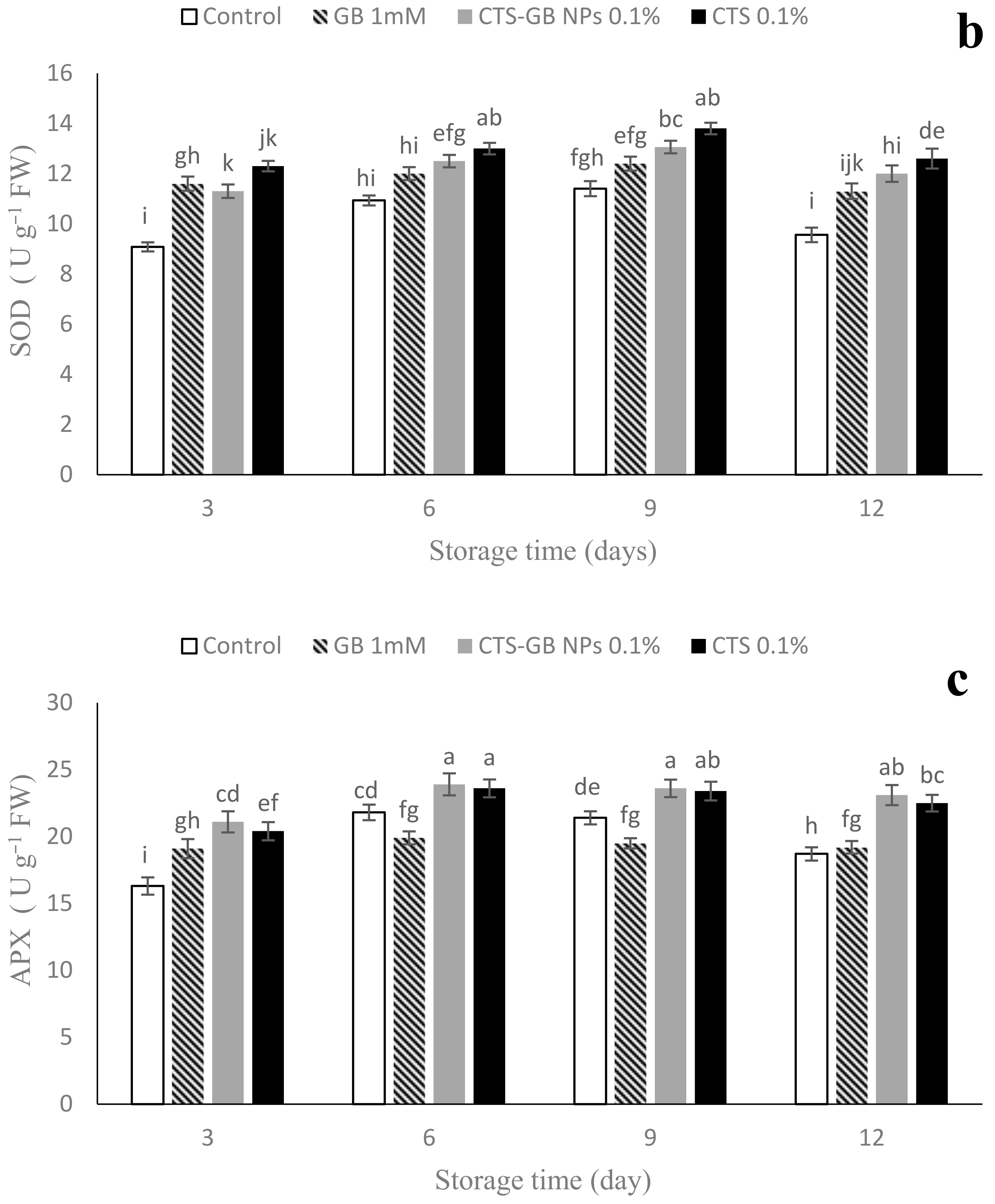
2.9. Antioxidant Enzymes Activities
3. Materials and Methods
3.1. Plant Material, Trial Location, and Time
3.2. Nanocomposite Preparation
3.3. Application of Treatments
3.4. Decay Percentage
3.5. Weight Loss Percentage
3.6. Fruit Tissue Firmness
3.7. Total Soluble Solids (TSSs) and Titratable Acidity (TA)
3.8. Ascorbic Acid Content
3.9. Anthocyanin Content
3.10. Total Phenol and Flavonoid Content, and Antioxidant Capacity
3.11. Malondialdehyde (MDA) and Hydrogen Peroxide (H2O2) Content
3.12. Antioxidant Enzymes Activity
3.12.1. Catalase (CAT) Activity
3.12.2. Superoxide Dismutase (SOD) Activity
3.12.3. Ascorbate Peroxidase (APX) Activity
3.13. Experimental Design and Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buendia, B.; Gil, M.; Tudela, J.A.; Gady, A.L. HPLC-MS Analysis of Pro anthocyanidin Oligomers and Other Phenolics in 15 Strawberry Cultivars. J. Agric. Food Chem. 2010, 58, 3916–3926. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Bautista-Banos, S.; Garcia-Dominguez, E.; BarreraNecha, L.L.; Reyes-Chilpa, R.; Wilson, C.L. Seasonal evaluation of the postharvest fungicidal activity of powders and extracts of huamuchil (Pithecellobiom dulce): Action against Botrytis cinerea, Penicillium digitatum and Rhizopus stolonifer of strawberry fruit. Postharvest Biol. Technol. 2003, 29, 81–92. [Google Scholar] [CrossRef]
- Riberio, C.; Vicente, A.A.; Teixeira, J.A.; Miranda, C. Optimization of edible coating composition to retard strawberry fruit senescence. Postharvest Biol. Technol. 2007, 44, 63–70. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Xia, W. Hypocholesterolaemic effects of different chitosan samples in vitro and in vivo. Food Chem. 2008, 107, 419–425. [Google Scholar] [CrossRef]
- Chien, P.; Sheu, F.; Yang, F. Effects of Edible Chitosan Coating on Quality and Shelf Life of Sliced Mango Fruit. J. Food Eng. 2007, 78, 225–229. [Google Scholar] [CrossRef]
- Wang, S.Y.; Gao, H.Y. Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria x aranassa Duch.). LWT-Food Sci. Technol. 2013, 52, 71–79. [Google Scholar] [CrossRef]
- Adiletta, G.; Zampella, L.; Coletta, C.; Petriccione, M. Chitosan coating to preserve the qualitative traits and improve antioxidant system in fresh figs (Ficus carica L.). Agriculture 2019, 9, 84. [Google Scholar] [CrossRef]
- Malekzadeh, P.; Hatamnia, A.A. Effect of glycine betaine treatment on reducing cold damage to pomegranate fruit during storage. J. Plant Environ. Physiol. 2015, 10, 39–47. [Google Scholar]
- Rabbani, G.; Choi, I. Roles of osmolytes in protein folding and aggregation in cells and their biotechnological applications. Int. J. Biol. Macromol. 2018, 109, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Seaidpor, F.; Sayyari, M.; Ghanbari, F. Effect of glycin betaine on frost resistance of cucumber seedlings. J. Agric. 2015, 17, 53–67. [Google Scholar]
- Fugui, W.; Tao, H.; Hai-ying, Z.; Li, X. Effect of exogenous glycine betaine on oxidative metabolism in cucumber during low-temperature storage. Food Sci. 2013, 34, 313–316. [Google Scholar]
- Awad, M.A.; Al-Qurashi, A.D.; Mohamed, S.A. Postharvest trans-resveratrol and glycine betaine treatments affect quality, antioxidant capacity, antioxidant compounds and enzymes activities of ‘El-Bayadi’ table grapes after storage and shelf life. Sci. Hortic. 2015, 197, 350–356. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, S.; Yuan, M.; Song, H.; Wang, T.; Zhang, W.; Zhang, Z. Effect of glycine betaine on chilling injury in relation to energy metabolism in papaya fruit during cold storage. Food Sci. Nutr. 2019, 7, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shan, T.; Xie, B.; Ling, C.; Shao, S.; Jin, P.; Zheng, Y. Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chem. 2019, 272, 530–538. [Google Scholar] [CrossRef]
- Wang, J.; Lv, M.; He, H.; Jiang, Y.; Yang, J.; Ji, S. Glycine betaine alleviated peel browning in cold-stored ‘Nanguo’ pears during shelf life by regulating phenylpropanoid and soluble sugar metabolisms. Sci. Hortic. 2020, 262, 109100. [Google Scholar] [CrossRef]
- Molaei, S.; Rabiei, V.; Soleimani, A.; Razavi, F. Exogenous application of glycine betaine increases the chilling tolerance of pomegranate fruits cv. Malase Saveh during cold storage. J. Food Process. Preserv. 2021, 45, e15315. [Google Scholar] [CrossRef]
- Shi, S.; Wang, W.; Liu, L.; Wu, S.; Wei, Y.; Li, W. Effect of chitosan/nano-silica coating on the physicochemical characteristics of longan fruit under ambient temperature. J. Food Eng. 2013, 118, 125–131. [Google Scholar] [CrossRef]
- Adeel, M.; Shakoor, N.; Shafiq, M.; Pavlicek, A.; Part, F.; Zafiu, C.; Raza, A.; Ahmad, M.A.; Jilani, G.; White, J.C.; et al. A critical review of the environmental impacts of manufactured nano-objects on earthworm species. Environ. Pollut. 2021, 290, 118041. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Senjen, R. Nanotechnology used for food packaging and food contact materials. Nanotechnol. Food Agric. 2008, 2, 14–68. [Google Scholar]
- Xing, Y.; Li, W.; Wang, Q.; Li, X.; Xu, Q.; Guo, X. Antimicrobial nanoparticles incorporated in edible coatings and films for the preservation of fruits and vegetables. Molecules 2019, 24, 1695. [Google Scholar] [CrossRef] [PubMed]
- Van, T.B.; Duyen, H.H.; Ha, V.H. Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria x ananassa Duch.). Postharvest Biol. Technol. 2020, 162, 103–111. [Google Scholar]
- Bahmani, R.; Razavi, F.; Mortazavi, S.N.; Gohari, G.; Juarez-Maldonado, A. Evaluation of Proline-Coated Chitosan Nanoparticles on Decay Control and Quality Preservation of Strawberry Fruit (cv. Camarosa) during Cold Storage. Horticulturae 2022, 8, 648. [Google Scholar] [CrossRef]
- Nasr, F.; Pateiro, M.; Rabiei, V.; Razavi, F.; Formaneck, S.; Gohari, G.; Lorenzo, J.M. Chitosan-Phenylalanine Nanoparticles (Cs-Phe Nps) Extend the Postharvest Life of Persimmon (Diospyros kaki) Fruits under Chilling Stress. Coatings 2021, 11, 819. [Google Scholar] [CrossRef]
- Hernandez-Lopez, G.; Ventura-Aguilar, R.I.; Correa-Pacheco, Z.N.; Bautista-Banos, S.; Barrera-Necha, L.L. Nanostructured chitosan edible coating loaded with α-pinene for the preservation of the postharvest quality of Capsicum annum L. and Alternaria alternate control. Int. J. Biol. Macromol. 2020, 165, 1881–1888. [Google Scholar]
- Abdel-Rahman, F.A.; Monir, G.A.; Hassan, M.S.S.; Ahmed, Y.; Refaat, M.H.; Ismail, I.A.; El-Garhy, H.A.S. Exogenously Applied Chitosan and Chitosan Nanoparticles Improved Apple Fruit Resistance to Blue Mold, Upregulated Defense-Related Genes Expression, and Maintained Fruit Quality. Horticulturae 2021, 7, 224. [Google Scholar] [CrossRef]
- Wantat, A.; Seraypheap, K.; Rojsitthisak, P. Effect of chitosan coatings supplemented with chitosan-montmorillonite nanocomposites on postharvest quality of ‘Hom Thong’ banana fruit. Food Chem. 2022, 374, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, D.; Belwal, T.; Li, L.; Chen, H.; Xu, T.; Luo, Z. Effect of Nano-SiOx/Chitosan Complex Coating on the Physicochemical Characteristics and Preservation Performance of Green Tomato. Molecules 2019, 24, 4552. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, S.; Ren, Y.; Li, H.; Zhang, X.; Di, J. Jujube preservation using chitosan film with nano-silicon dioxide. J. Food Eng. 2012, 113, 408–414. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Razavi, F.; Rabiei, V.; Gohari, G.; Palou, L. Application of Glycine betaine coated chitosan nanoparticles alleviate chilling injury and maintain quality of plum (Prunus domestica L.) fruit. Int. J. Biol. Macromol. 2022, 207, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Harker, F.R.; Elgar, H.J.; Watkins, C.B.; Jackson, P.J.; Hallett, I.C. Physical and mechanical changes in strawberry fruit after high carbon dioxide treatments. Postharvest Biol. Technol. 2000, 19, 139–146. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Qiy, L.; Keping, K.; Lin, C.; Apaliya, T.; Gu, X.; Zhang, H. Mechanisms of glycine betaine enhancing oxidative stress tolerance and biocontrol efficacy of Pichia caribbica against blue mold on apples. Biol. Control. 2017, 108, 55–63. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, T.; Zuo, J.; Gao, L.; Fan, L. Amelioration of postharvest chilling injury in sweet pepper by glycine betaine. Postharvest Biol. Technol. 2016, 112, 114–120. [Google Scholar] [CrossRef]
- Abdelhai, M.; Awad, F.; Komla, Q.; Godana, M.; Zhang, H. Enhancement the biocontrol efficacy of Sporidiobolus pararoseus Y16 against apple blue mold decay by glycine betaine and its mechanism. Biol. Control. 2019, 139, 142–165. [Google Scholar] [CrossRef]
- Eshghi, S.; Mohammadi, A.; Badii, F.; Hoseini, M.Z.; Ahmadi, K. Effect of nanochitosan-based coating with and without copper loaded on physicochemical and bioactive components of fresh strawberry fruit (Fragaria × ananassa Duchesne) during storage. Food Bioprocess Technol. 2014, 7, 2397–2409. [Google Scholar] [CrossRef]
- Romanazzi, G. Chitosan treatment for the control of postharvest decay of table grapes, strawberries and sweet cherries. Fresh Prod. 2010, 4, 111–115. [Google Scholar]
- Saki, M.; Kaji, B.; Abbasifar, A.; Shahrjerdi, I. Effect of chitosan coating combined with thymol essential oil on physicochemical and qualitative properties of fresh fig (Ficus carica L.) fruit during cold storage. J. Food Meas. Charact. 2019, 13, 1147–1158. [Google Scholar] [CrossRef]
- Resende, N.S.; Gonçalves, G.A.S.; Reis, K.C.; Tonoli, G.H.D.; Boas, E. Chitosan/cellulose nanofibril nanocomposite and its effect on quality of coated strawberries. J. Food Qual. 2018, 2018, 1727426. [Google Scholar] [CrossRef]
- Xing, Y.; Yang, H.; Guo, X.; Bia, X.; Liu, X.; Xua, Q.; Wang, Q.; Lia, W.; Lia, X.; Shui, Y.; et al. Effect of chitosan/nano-tiO2 composite coatings on the postharvest quality and physicochemical characteristics of mango fruits. Sci. Hortic. 2020, 263, 109–135. [Google Scholar] [CrossRef]
- Devlieghere, F.; Vermeulen, A.; Debevere, J. Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004, 21, 703–714. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinauer Associates: Sunderland, UK, 2006. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Chi, H.; Song, S.; Luo, M.; Zhang, C.; Li, W.; Li, L.; Qin, Y. Effect of PLA nanocomposite films containing bergamot essential oil, TiO2 nanoparticles, and Ag nanoparticles on shelf life of mangoes. Sci. Hortic. 2019, 249, 192–198. [Google Scholar] [CrossRef]
- Kaewklin, Y.; Siripatrawan, U.; Suwanagul, A.; Sukleed, Y. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 12, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Fang, Y.; Yang, Y.; Ma, N.; Zhao, L. Effect of nanocomposite-based packaging on postharvest quality of ethylene-treated kiwifruit (Actinidia deliciosa) during cold storage. Food Res. Int. 2011, 44, 1589–1596. [Google Scholar] [CrossRef]
- Kashappa, D.G.; Hyun, P.J. Study of gamma irradiation effects on chitosan micro particles. Drug Deliv. 2006, 13, 39–50. [Google Scholar]
- Yang, W.J.; Rich, P.J.; Axtell, J.D.; Wood, K.V.; Bonhm, C.C.; Ejeta, G.; Mickelbart, M.V.; Rhodes, D. Genotypic Variation for Glycinebetaine in Sorghum. Crop Sci. 2003, 43, 162–169. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Xu, Q.; Yun, J.; Lu, Y.; Tang, Y. Effects of chitosan coating enriched with cinnamon oil on qualitative properties of sweet pepper (Capsicum annuum L.). Food Chem. 2011, 124, 1443–1450. [Google Scholar] [CrossRef]
- Duan, X.W.; Liu, T.; Zhang, D.; Su, X.G.; Lin, H.T.; Jiang, Y.M. Effect of pureoxygen atmosphere on antioxidant enzyme and antioxidant activity of harvested litchi fruit during storage. Food Res. Int. 2011, 44, 1905–1911. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Shiri, M.A.; Sanavi, M. Effect of chitosan coating on some quality indices of apricot (Prunus armeniaca L.) during cold storage. Casp. J. Environ. Sci. 2010, 8, 25–33. [Google Scholar]
- Liu, J.; Tian, S.; Menga, X.; Xu, Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Srinivasa, P.C.; Baskaran, R.; Ramesh, M.N.; Prashanth, H. Storage studies of mango packed using biodegradable chitosan film. Eur. Food Res. Technol. 2002, 215, 504–508. [Google Scholar]
- Abou-Aly, H.E.; Mady, M.A. Complemented effect of glycine betainea and biofertilizers on growth and produtivity of sweet pepper (Capsicum annuum L.) plant under high temperature condition. J. Plant Prod. 2014, 5, 505–526. [Google Scholar] [CrossRef]
- Li, M.; Zhi, H.; Dong, Y. Influence of Preharvest and Postharvest Applications of Glycine Betaine on Fruit Quality Attributes and Storage Disorders of ‘Lapins’ and ‘Regina’ Cherries. Hortic. Sci. 2019, 54, 1540–1545. [Google Scholar] [CrossRef]
- Dong, H.; Cheng, L.; Tan, J.; Zheng, K.; Jiang, Y. Effects Of chitosan coating on quality and shelf life of peeled litchi fruit. J. Food Eng. 2004, 64, 355–358. [Google Scholar] [CrossRef]
- Eissa, H.A. Effect of chitosan coating on shelf life and quality of fresh-cut mushroom. J. Food Qual. 2007, 30, 623–645. [Google Scholar] [CrossRef]
- Gholamipourfard, K.; Kamari, S.; Ghasemnejad, M.; Fotouhi Ghazvini, R. Effect of chitosan coating on weight loss and postharvest quality of green pepper (Capsicum annum L.) fruits. Acta Hortic. 2010, 877, 821–826. [Google Scholar] [CrossRef]
- Smirnoff, N.; Stewart, G.R. Stress metabolites and their role in coastal plants. Vegetatio 1985, 62, 273–278. [Google Scholar] [CrossRef]
- Einset, J.; Connolly, E.L. Glycine betaine enhances extracellular processes blocking ROS signaling during stress. Plant Signal Behav. 2009, 4, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Shan, W.; Ling Cai, D.; Chen, J.Y.; Lu, W.J.; Su, X.G.; Kuang, J.F. Postharvest application of glycine betaine ameliorates chilling injury in cold-stored banana fruit by enhancing antioxidant system. Sci. Hortic. 2021, 287, 110264. [Google Scholar] [CrossRef]
- Goncalves, B.; Morais, M.C.; Sequeira, A.; Ribeiro, C.; Guedes, F.; Silva, A.P.; Aires, A. Quality preservation of sweet cherry cv. ‘staccato’ by using glycine-betaine or Ascophyllum nodosum. Food Chem. 2020, 322, 126713. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, F.; Rao, J. Effect of putrescine treatment on chilling injury, fatty acid composition and antioxidant system in kiwifruit. Soc. Psychiatry 2016, 45, 11–25. [Google Scholar] [CrossRef]
- Borsani, J.; Budde, C.O.; Porrini, L.; Lauxmann, M.A.; Lombardo, V.A.; Murray, R.; Andreo, C.S.; Drincovich, M.F.; Lara, M.V. Carbon metabolism of peach fruit after harvest: Changes in enzymes involved in organic acid and sugar level modifications. J. Exp. Bot. 2009, 60, 1823–1837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, P.; Huang, Y.; Shan, T.; Wang, L.; Li, Y.; Zheng, Z. Effect of hot water combined with glycine betaine alleviates chilling injury in cold-stored loquat fruit. Postharvest Biol. Technol. 2019, 118, 141–147. [Google Scholar] [CrossRef]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the postharvest quality and anti oxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, X.; Dong, C.; Chen, W. Effects of wax treatment on quality and postharvest physiology of pineapple fruit in cold storage. Afr. J. Biotechnol. 2011, 10, 7592–7603. [Google Scholar]
- Ghasemnezhad, M.; Zareh, S.; Rassa, M.; Sajedi, R.H. Effect of chitosan coating on maintenance of aril quality, microbial population and PPO activity of pomegranate (Punica granatum L. cv. Tarom) at cold storage temperature. J. Sci. Food Agric. 2013, 93, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, J.; Jiang, W. Effects of chitosan on shelf life of cold-stored litchi fruit at ambient temperature. Food Sci. Technol. 2005, 38, 757–761. [Google Scholar] [CrossRef]
- Han, C.; Zhao, Y.; Leonard, S.W.; Traber, M.G. Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria x ananassa) and raspberries (Rubus ideaus). Postharvest Biol. Technol. 2004, 33, 67–78. [Google Scholar] [CrossRef]
- Aaby, K.; Remberg, S.F. Strawberry phenolics and impact of ripening. In Processing and Impact on Active Components in Food; Academic Press: Cambridge, MA, USA, 2015; pp. 157–164. [Google Scholar]
- Gohari, G.; Molaei, S.; Kheiry, A.; Ghafouri, M.; Razavi, F.; Lorenzo, J.; Juárez-Maldonado, A. Exogenous Application of Proline and L-Cysteine Alleviates Internal Browning and Maintains Eating Quality of Cold Stored Flat ‘Maleki’ Peach Fruits. Horticulturae 2021, 27, 469. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Yang, H. Postharvest chitosan-g-salicylic acid application alleviates chilling injury and preserves cucumber fruit quality during cold storage. Food Chem. 2015, 174, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Bodbodak, S. Physiological and biochemical mechanisms regulating chilling tolerance in fruits and vegetables under postharvest salicylates and jasmonates treatments. Sci. Hortic. 2013, 156, 73–85. [Google Scholar] [CrossRef]
- Hernández-Muñoza, P.; Almenara, E.; José, J.; Gavara, R. Effect of calcium dips and chitosan coatings on postharvest life of strawberries (Fragaria x ananassa). Postharvest Biol. Technol. 2006, 39, 247–253. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, T.; Zhang, P.; Wang, Z.Y. Melatonin attenuates postharvest physiological deterioration of cassava storage roots. J. Pineal Res. 2016, 60, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, A.S.; Malik, A.U.; Shahid, M. Effect of controlled atmosphere storage on pericarp browning, bioactive compounds and antioxidant enzymes of litchi fruit. Food Chem. 2016, 206, 18–29. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahmani, R.; Razavi, F.; Mortazavi, S.N.; Gohari, G.; Juárez-Maldonado, A. Enhancing Postharvest Quality and Shelf Life of Strawberries through Advanced Coating Technologies: A Comprehensive Investigation of Chitosan and Glycine Betaine Nanoparticle Treatments. Plants 2024, 13, 1136. https://doi.org/10.3390/plants13081136
Bahmani R, Razavi F, Mortazavi SN, Gohari G, Juárez-Maldonado A. Enhancing Postharvest Quality and Shelf Life of Strawberries through Advanced Coating Technologies: A Comprehensive Investigation of Chitosan and Glycine Betaine Nanoparticle Treatments. Plants. 2024; 13(8):1136. https://doi.org/10.3390/plants13081136
Chicago/Turabian StyleBahmani, Reza, Farhang Razavi, Seyed Najmmaddin Mortazavi, Gholamreza Gohari, and Antonio Juárez-Maldonado. 2024. "Enhancing Postharvest Quality and Shelf Life of Strawberries through Advanced Coating Technologies: A Comprehensive Investigation of Chitosan and Glycine Betaine Nanoparticle Treatments" Plants 13, no. 8: 1136. https://doi.org/10.3390/plants13081136




