Polar Expansion Dynamics in the Plant Kingdom: A Diverse and Multifunctional Journey on the Path to Pollen Tubes
Abstract
:1. Introduction
2. Common Components and Processes Associated with Polar Expansion in Plants
2.1. Cell Wall
2.1.1. Pectin and Pectin Methylesterase (PME)
2.1.2. Cellulose, Callose and Xyloglucans
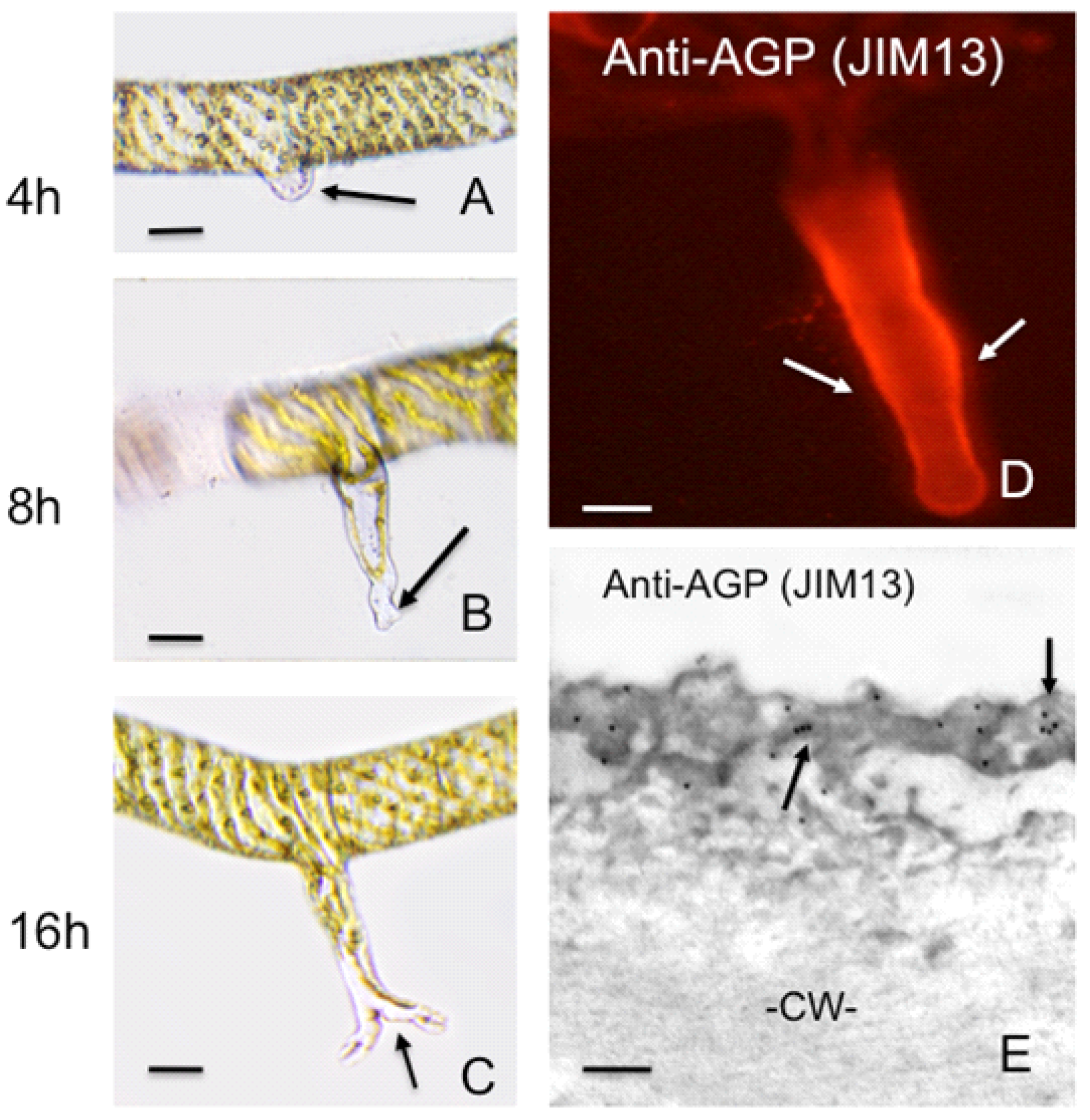
2.1.3. Arabinogalactan Proteins (AGPs)
2.1.4. Expansins
2.2. Exo- and Endocytosis: Actin and Actin-Associated Proteins
2.2.1. Actin
2.2.2. Actin Binding Proteins
2.3. Microtubules
2.4. Phosphoinositides
2.5. ROP/Rac GTPases
2.6. Ca2+ Dynamics
2.7. Reactive Oxygen Species
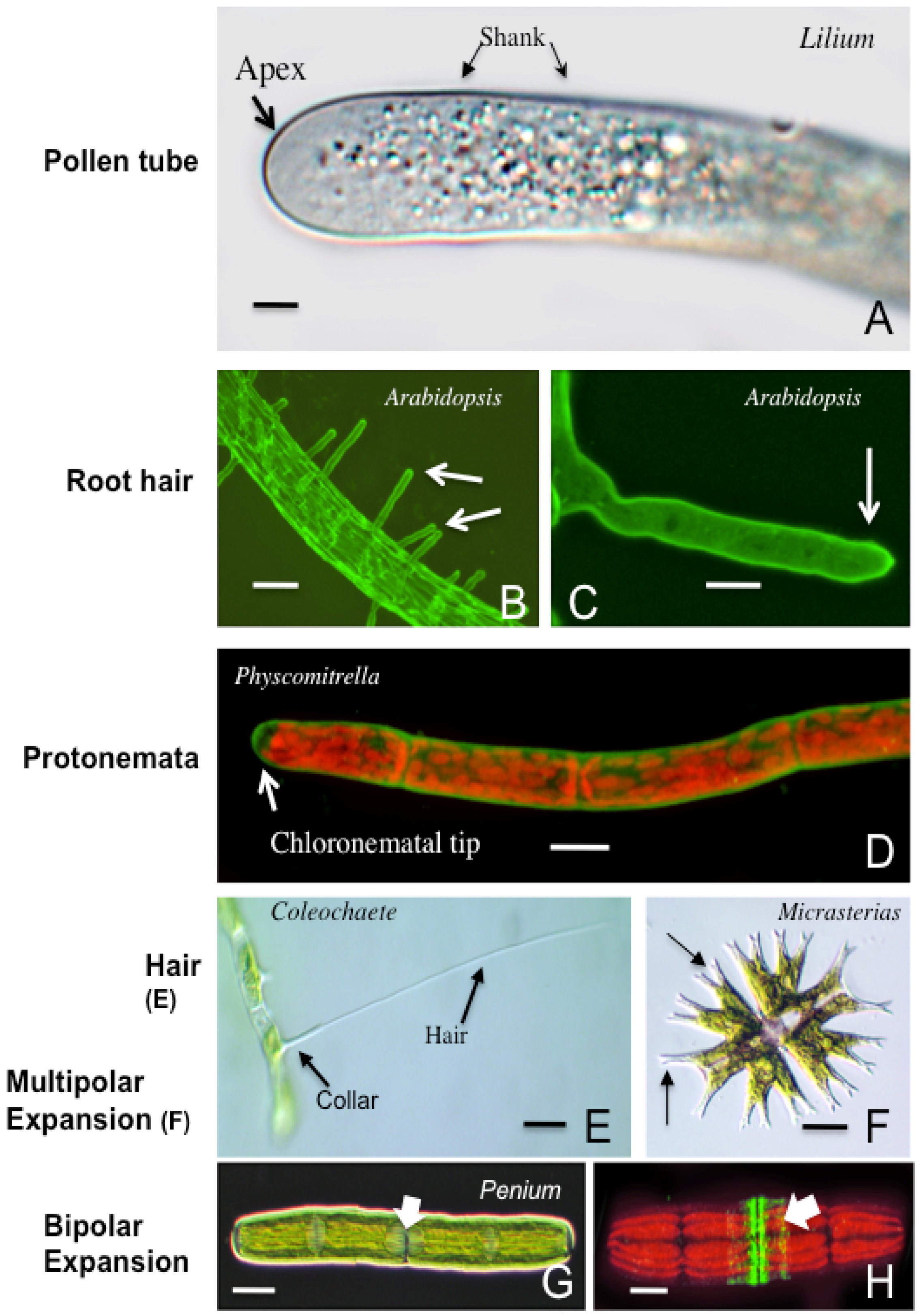
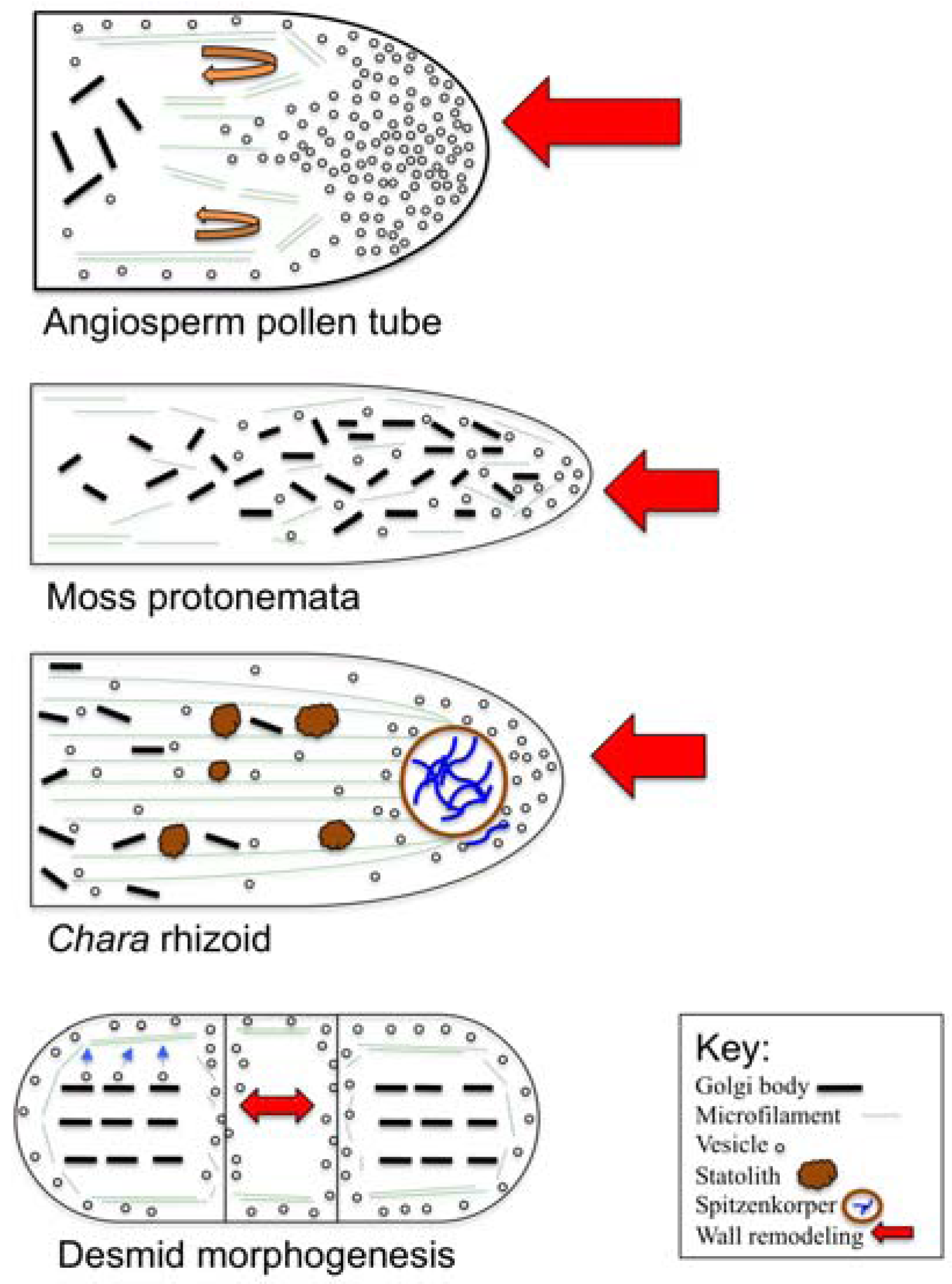
3. Polar Growth Mechanisms in Early Divergent Plants
3.1. The Charophycean Green Algae (CGA; Streptophyta)
3.2. Physcomitrella Patens and Mosses
3.3. Polar Expansion, Early Divergent Plants and Possible Roles
4. Polar Expansion in Primitive Vascular Plants: Fern Rhizoids
5. The Pollen Tube
6. Root Hairs
7. Conclusions
- (1)
- Localized alterations (i.e., remodeling) to cell wall chemistry and structure are focal points for polar growth. These zones are produced via targeted cell wall secretion and/or alterations to pre-existing wall zones and results polymer composites that can resist internal turgor but allow for controlled expansion on a narrow front.
- (2)
- Polar expansion requires new plasma membrane and cell wall material. This entails targeted secretion of endomembrane (i.e., primarily Golgi-based) components that are transported/directed to the expansion tip via the actin cytoskeleton. This entails complex and integrated interactions of actin with actin-binding proteins that are in turn, regulated by various signal transduction molecules. The microtubule cytoskeleton may also play a significant role in the polar expansion process.
- (3)
- Polar expansion directed by targeted secretion requires high levels and/or gradients of Ca2+.
- (4)
- Many regulatory molecules and mechanisms are involved with polar growth. These include, phospholipids, Rho-GTPases and ROS, to name just a few or what is currently known.
| Structures | Taxonomic groups | Purpose | References |
|---|---|---|---|
| Pollen tubes | Angiosperms, Gymnosperms | Delivery of male gamete to female gametangium | [3,132,133,134,135,136,137] |
| Root hairs | Vascular plants | Absorption, anchoring, symbiosis | [138,139,140] |
| Protonemata | Bryophytes | Substrate “exploration”, anchoring | [117,118,119,120,121,122] |
| Rhizoids | Ferns, bryophytes, Charales | Absorption, anchoring | [108,109,110,111,112,113,114,115,127,128,129,130] |
| Hairs or setae | Coleochaetales | Unknown | [105,106,107] |
| Wound induced rhizoids | Spirogyra (Zygnematales) | Anchoring | [101,102,103,104] |
| Lobed cells | Desmids (Zygnematales) | Maximizing chloroplast surface area | [87,88,91,92,93,94,95,96,97,98,99,100] |
Acknowledgements
References
- Geitmann, A.; Ortega, J.K.E. Mechanics and modeling of plant cell growth. Trends Plant Sci. 2009, 14, 467–478. [Google Scholar] [CrossRef]
- Palin, R.; Geitmann, A. The role of pectin in plant morphogenesis. Biosystems 2012, 109, 397–402. [Google Scholar] [CrossRef]
- Chebli, Y.; Geitmann, A. Mechanical properties governing pollen tube growth. Funct. Plant Sci. Biotech. 2007, 1, 232–245. [Google Scholar]
- Wolf, S.; Hematy, K.; Hofte, H. Growth control and cell wall signaling in plants. Ann. Rev. Plant Biol. 2012, 63, 381–407. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Wolf, S.; Greiner, S. Growth control by cell wall pectins. Protoplasma 2012, 249, S169–S175. [Google Scholar] [CrossRef]
- Wolf, S.; Mouille, G.; Pelloux, J. Homogalacturonan methyl-esterification and plant development. Mol. Plant 2009, 2, 851–860. [Google Scholar] [CrossRef]
- Braybrook, S.A.; Hofte, H.; Peaucelle, A. Probing the mechanical contributions of the pectin matrix. Insights for cell growth. Plant Signal. Behav. 2012, 7, 1037–1041. [Google Scholar] [CrossRef]
- Dardelle, F.; Lehner, A.; Ramdani, Y.; Bardor, M.; Lerouge, P.; Driouich, A.; Mollet, J.-C. Biochemical and immunocytochemical characterizations of Arabidopsis pollen tube cell wall. Plant Physiol. 2010, 153, 1563–1576. [Google Scholar] [CrossRef]
- Bosch, M.; Hepler, P.K. Pectin methylesterases and pectin dynamics in pollen tubes. Plant Cell 2006, 17, 3219–3226. [Google Scholar]
- Bosch, M.; Cheung, A.Y.; Hepler, P.K. Pectin methylesterase, a regulator of pollen tube growth. Plant Physiol. 2005, 138, 1334–346. [Google Scholar] [CrossRef]
- Hothorn, M. Structural insights into the target specificity of plant invertase and pectin methylesterase inhibitory proteins. Plant Cell 2004, 16, 3437–447. [Google Scholar] [CrossRef]
- Jolie, R.P.; Duvetter, T.; van Loey, A.M.; Hendrickx, M.E. Pectinmethylesterase and its proteinaceious inhibitor: A review. Carb. Res. 2010, 345, 2583–2595. [Google Scholar] [CrossRef]
- Aouar, L.; Chebli, Y.; Getimann, A. Morphogenesis of complex plant cell shapes: The mechanical role of crystalline cellulose in growing pollen tubes. Sex. Plant Reprod. 2010, 23, 15–27. [Google Scholar] [CrossRef]
- Suslov, D.; Verbelen, J.-P.; Vissenberg, K. Onion epidermis as a new model to study the control of growth anisotropy in higher plants. J. Expt. Bot. 2009, 60, 4175–4187. [Google Scholar] [CrossRef]
- Park, S.; Szumlanski, A.L.; Gu, F.; Guo, F.; Nielsen, E. A role for CLSD3 during cell-wall synthesis in apical plasma membranes of tip-growing root-hair cells. Nature Cell Biol. 2011, 13, 973–981. [Google Scholar] [CrossRef]
- Cai, G.; Faleri, C.; del Casino, C.; Emons, A.M.E.; Cresti, M. Ditribution of callose synthase, cellulose synthase and sucrose synthase in tobacco pollen tube is controlled in dissimilar ways by actin filaments and microtubules. Plant Physiol. 2011, 155, 1169–1190. [Google Scholar] [CrossRef]
- Vissenberg, K.; Fry, S.C.; Pauly, M.; Hofte, H.; Verbelen, J.-P. XTH acts at the microfibril-matrix interface during cell elongation. J. Exp. Bot. 2005, 56, 673–683. [Google Scholar] [CrossRef]
- Van Sandt, V.S.T.; Stieperaere, H.; Guisez, Y.; Verbelen, J.-P.; Vissengerg, K. XET activity is found near sites of growth and cell elongation in bryophytes and some green algae: New insights into the evolution of primary cell wall elongation. Ann. Bot. 2007, 99, 39–51. [Google Scholar] [CrossRef]
- Benatti, M.; Penning, B.W.; Carpita, N.C.; McCann, M.C. We are good to grow: Dynamic integration of cell wall architecture with the machinery of growth. Front. Plant Sci. 2012. [Google Scholar] [CrossRef]
- Nguema-Ona, E.; Cimbra, S.; Vicre-Gilbouin, A.; Mollet, J.C.; Driouich, A. Arabinogalactan proteins in root and pollen-tube cells: Distribution and functional aspects. Ann. Bot. 2012, 110, 383–404. [Google Scholar] [CrossRef]
- Velasquez, S.M.; Martiniano, M.; Ricardi, M.M.; Dorosz, J.G.; Fernandez, P.V.; Nadra, A.D.; Pol-Fachin, L.; Egelund, J.; Gille, S.; Harholt, J.; et al. O-glycosylated cell wall proteins are essential in root hair growth. Science 2011, 332, 1401–1403. [Google Scholar] [CrossRef]
- Lee, K.J.D.; Sakata, Y.; Mau, S.-L.; Pettolino, F.; Bacic, A.; Quatrano, R.S.; Knight, C.D.; Knox, J.P. Arabinogalactan proteins are required for apical cell extension in the moss Physcomitrella patens. Plant Cell 2005, 17, 3051–3065. [Google Scholar] [CrossRef]
- Boisson-Dernier, A.; Kessler, S.A.; Grossniklaus, U. The walls have ears: The role of CrRLK1Ls in sensing and transducing extracellular signals. J. Exp. Bot. 2011, 62, 1581–1591. [Google Scholar] [CrossRef]
- Zang, Y.; Yang, J.; Showalter, A. AtAGP18, a lysine-rich arabinogalactan protein in Arabidopsis thaliana, functions in plant growth and development as a putative co-receptor for signal transduction. Plant Signal. Behav. 2011, 6, 855–857. [Google Scholar] [CrossRef]
- Domozych, D.S.; Elliott, L.; Kiemle, S.N.; Gretz, M.R. Pleurotaenium trabecula, a dsmid of wetland biofilms: The extracellular matrix and adhesion mechanisms. J. Phycol. 2007, 43, 1022–1038. [Google Scholar] [CrossRef]
- Cosgrove, D.J.; Li, L.C.; Cho, H.; Hoffmann-Benning, S.; Moore, R.C.; Blecker, D. The growing world of expansins. Plant Cell Physiol. 2002, 43, 1436–1444. [Google Scholar] [CrossRef]
- Li, Y.; Darley, C.P.; Ongaro, V.; Fleming, A.; Schipper, O.; Baldauf, S.L.; McQueen-Mason, S.J. Plant expansins are a complex multigene family with an ancient evolutionary origin. Plant Physiol. 2002, 128, 854–864. [Google Scholar] [CrossRef]
- McQueen-Mason, S.; Durachko, D.M.; Cosgrove, D.J. Two endogenous proteins that induce cell wall extension in plants. Plant Cell 1992, 4, 1425–1433. [Google Scholar]
- Sampedro, J.; Cosgrove, D.J. The expansin superfamily. Genome Biol. 2005, 6. [Google Scholar] [CrossRef]
- Ketelaar, T.; Galway, M.E.; Mulder, B.M.; Emons, A.M.C. Rates of exocytosis and endocytosis in Arabidopsis root hais and pollen tubes. J. Microsc. 2008, 231, 265–273. [Google Scholar] [CrossRef]
- Moscatelli, A.; Ciampolini, F.; Rodighiero, S.; Oneill, E.; Cresti, M.; Santo, N.; Idilli, A. Distinct endocytic pathways identified in tobacco pollen tubes using charged nanogold. J. Cell Sci. 2007, 120, 3804–3819. [Google Scholar] [CrossRef]
- Moscatelli, A.; Idilli, A.I. Pollen tube growth: A delicate equilibrium between secretory and endocytic pathways. J. Integr. Plant Biol. 2009, 51, 727–739. [Google Scholar] [CrossRef]
- Kato, N.; He, H.; Steger, A. A systems model of vesicle trafficking in Arabidopsis pollen tubes. Plant Physiol. 2010, 152, 590–601. [Google Scholar]
- Bove, J.; Vaillancourt, B.; Kroeger, J.; Hepler, P.K.; Wiseman, P.W.; Geitmann, A. Magnitude and direction of vesicle dynamics in growing pollen tubes using spatiotemporal image correlation spectroscopy and fluorescence recovery after bleaching. Plant Physiol. 2008, 147, 1646–1658. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; Lee, D.; McCormick, S. Interdependence of endomembrane trafficking and actin dynamics during polarized growth of Arabidopsis pollen tubes. Plant Physiol. 2010, 152, 2200–2210. [Google Scholar] [CrossRef]
- Zonia, L.; Munnik, T. Vesicle trafficking dynamics and visualization of zones of exocytosis and endocytosis in tobacco pollen tubes. J. Exp. Bot. 2010, 59, 861–873. [Google Scholar] [CrossRef]
- Campanoni, P.; Blatt, M.R. Membrane trafficking and polar growth in root hairs and pollen tubes. J. Exp. Bot. 2006, 58, 65–74. [Google Scholar] [CrossRef]
- Samaj, J.; Muller, J.; Beck, M.; Bohm, N.; Menzel, D. Vesicular trafficking, cytoskeleton and signaling in root hairs and pollen tubes. Trends Plant Sci. 2006, 11, 594–600. [Google Scholar] [CrossRef]
- Daher, F.B.; Geitmann, A. Actin is involved in pollen tube tropism through redefining the spatial targeting of secretory vesicles. Traffic 2011, 12, 1537–1551. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, Z. Rapid tip growth: Insights from pollen tubes. Sem. Cell Devel. Biol. 2010, 22, 816–824. [Google Scholar]
- Kroeger, J.; Geitmann, A. The pollen tube paradigm revisited. Curr. Opin. Plant Biol. 2012, 15, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, X.; Bao, C.; Khurana, P.; Wang, Q.; Xie, Y.; Zheng, Y.; Chen, N.; Blanchoin, L.; Staiger, C.J.; Huang, S. Arabidopsis VILLIN5 an actin filament bundling and severing protein, is necessary for normal pollen tube growth. Plant Cell 2010, 22, 2749–2767. [Google Scholar] [CrossRef]
- Blanchoin, L.; Boujemaa-Paterski, R.; Henty, J.L.; Khurana, P.; Staiger, C.J. Actin dynamics in plant cells: A team effort from multiple proteins orchestrates this very fast-paced game. Curr. Opin. Plant Sci. 2010, 13, 714–723. [Google Scholar] [CrossRef]
- Staiger, C.J.; Poulter, N.S.; Henty, J.L.; Franlin-Tong, V.E.; Blanchoin, L. Regulation of actin dynamics by-actin-binding proteins in pollen. J. Exp. Bot. 2010, 61, 1969–1986. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Niroomand, S.; Zou, Y.; Wu, H.-M. A transmembrane formin nucleates subapical actin assembly and controls tip-focused growth in pollen tubes. Proc. Natl. Acad. Sci. USA 2010, 107, 16390–16395. [Google Scholar]
- Lee, Y.J.; Yang, Z. Tip growth: signaling in the apical dome. Curr. Opin. Plant Biol. 2008, 11, 662–671. [Google Scholar] [CrossRef]
- Lee, Y.J.; Szumlanski, A.; Nielsen, E.; Yang, Z. Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J. Cell Biol. 2008, 181, 1155–1168. [Google Scholar] [CrossRef]
- Pei, W.; Du, F.; Zhang, Y.; He, T.; Ren, H. Control of the actin cytoskeleton in root hair development. Plant Sci. 2012. [Google Scholar] [CrossRef]
- Vidali, L.; Burkhart, G.M.; Augustine, R.C.; Kerdavid, E.; Tuzel, E.; Bezanilla, M. Myosin XI is essential for tip growth in Physcomitrella patens. Plant Cell 2010, 22, 1868–1882. [Google Scholar] [CrossRef]
- Vidali, L.; Rounds, C.M.; Hepler, P.K.; Bezanilla, M. Lifeact-mEGFP reveals a dynamic apical F-actin network in tip growing plant cells. PLoS One 2009, 4, e5744. [Google Scholar]
- Braun, M.; Limbach, C. Rhizoids and protonemata of characean algae: Model cells for research on polarized growth and plant gravity sensing. Protoplasma 2006, 229, 133–142. [Google Scholar] [CrossRef]
- Meindl, U. Micrasterias cells as a model system for research on morphogenesis. Microbiol. Rev. 1993, 50, 415–33. [Google Scholar]
- Yoshida, K.; Shimmen, T. Involvement of actin filaments in rhizoid morphogenesis of Spirogyra. Physiol. Plant 2009, 135, 98–107. [Google Scholar] [CrossRef]
- Vidali, L.; van Gisbergen, P.A.C.; Guerin, C.; Franco, P.; Li, M.; Burkart, G.M.; Augustine, R.C.; Blanchoin, L.; Bezanilla, M. Rapid formin-mediated actin-filament elongation is essential for polarized plant cell growth. Proc. Natl. Acad. Sci. USA 2009, 106, 13341–13346. [Google Scholar]
- Yarmola, E.G.; Bubb, M.R. Profilin: Emerging concepts and lingering misconceptions. Trends Bioch. Sci. 2006, 31, 197–205. [Google Scholar] [CrossRef]
- Thomas, C. Bundling actin filaments from membranes: Some novel players. Front. Plant Sci. 2012. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Y.; Du, F.; Cao, L.; Dong, H.; Ren, H. Arabidopsis VILLIN4 is involved in root hair growth through regulating actin organization in a Ca2+-dependent manner. New Phytol. 2011, 190, 667–682. [Google Scholar] [CrossRef]
- Tominaga, M.; Yokota, E.; Vidali, L.; Sonobe, S.; Hepler, P.K.; Shimmen, T. The role of plant vilin in the organization of the actin cytoskeleton, cytoplasmic streaming and the architecture of the transvacuolar strand in root hair cells of Hydrocharis. Planta 2000, 210, 836–843. [Google Scholar] [CrossRef]
- Perroud, P.F.; Quatrano, R.S. The role of ARPC4 in tip growth and alignment of the polar axis in filaments of Physcomitrella patens. Cell Motil. Cytoskel. 2006, 63, 162–171. [Google Scholar] [CrossRef]
- Mathur, J. Local interactions shape plant cells. Curr. Opin. Plant Biol. 2006, 18, 40–46. [Google Scholar] [CrossRef]
- Petrasek, J.; Schwarzerova, K. Actin and microtubule cytoskeleton interactions. Curr. Opin. Plant Biol. 2008, 12, 1–7. [Google Scholar] [CrossRef]
- Bibikova, T.N.; Blancfluor, E.B.; Gilroy, S. Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 1999, 17, 657–665. [Google Scholar] [CrossRef]
- Ischebeck, T.; Vu, L.H.; Jin, X.; Stenzel, I.; Lofke, C.; Heilmann, I. Functional cooperativity of enzymes of phosphoinositide conversion according to synergistic effects on pectin secretion in tobacco pollen tubes. Mol. Plant 2010, 3, 870–881. [Google Scholar] [CrossRef]
- Saavedra, L.; Balbi, V.; Lerche, J.; Mikami, K.; Heilmann, I.; Sommarin, M. PIPKs are essential for rhizoid elongation and caulonemal cell development in the moss Physcomitrella patens. Plant J. 2011, 67, 635–647. [Google Scholar] [CrossRef]
- Ischebeck, T.; Seiler, S.; Heilmann, I. At the poles across kingdoms: Phosphoinositides and polar tip growth. Protoplasma 2010, 240, 13–31. [Google Scholar] [CrossRef]
- Ischebeck, T.; Stenzel, I.; Heilmann, I. Type B phosphatidylinositol-4-phosphate 5-kinases mediate pollen tube growth in Nicotiana tabacum and Arabidopsis by regulating apocal pectin secretion. Plant Cell 2008, 20, 3312–3330. [Google Scholar] [CrossRef]
- Kost, B. Spatial control of Rho (Rac-Rop) signaling in tip-growth plant cells. Trends Cell Biol. 2008, 18, 119–127. [Google Scholar] [CrossRef]
- Vermeer, J.E.M.; Thole, J.M.; Goedhart, J.; Nielsen, E.; Munnik, T.; Gadella, T.W.J. Imaging phophatidylinositol 4-phosphate dynamics in living plant cells. Plant J. 2009, 57, 356–372. [Google Scholar] [CrossRef]
- Preuss, M.L.; Schmitz, A.J.; Thole, J.M.; Bonner, H.K.; Otegui, M.S.; Nielsen, E. A role for the RabA4b effector protein PI-4Kbeta1 in polarized expansion of root hairs of Arabidopsis thaliana. J. Cell Biol. 2006, 172, 991–998. [Google Scholar] [CrossRef]
- Szumlanski, A.L.; Nielsen, E. The Rab GTPase RabA4d regulates pollen tube tip growth in Arabidopsis thaliana. Plant Cell 2009, 21, 526–544. [Google Scholar] [CrossRef]
- Munnik, T.; Nielsen, E. Green light for phosphoinositide signals in plants. Curr. Opin. Plant Biol. 2011, 14, 489–497. [Google Scholar] [CrossRef]
- Eklund, D.M.; Svensson, E.M.; Kost, B. Physcomitrella patens: A model to investigate the role of RAC/ROP GTPase signaling in tip growth. J. Exp. Bot. 2010, 61, 1917–1937. [Google Scholar] [CrossRef]
- Guo, F.; McCubben, A.G. The pollen-specific R-SNARE/longin PiVAMP726 mediates fusion of endo- and exocytic compartments in pollen tube tip growth. J. Exp. Bot. 2012, 63, 3083–3095. [Google Scholar] [CrossRef]
- Lycett, G. The role of Rab GTPases in cell wall metabolism. J. Exp. Bot. 2008, 59, 4061–4074. [Google Scholar] [CrossRef]
- Craddock, C.; Lavagi, I.; Yang, Z. New insights into Rho signaling from plant ROP/Rac GTPases. Trends Cell Biol. 2012, 22, 492–501. [Google Scholar] [CrossRef]
- Zonia, L. Spatial and temporal integration of signaling networks regulating pollen tube growth. J. Exp. Bot. 2010, 61, 1939–1957. [Google Scholar] [CrossRef]
- Hepler, P.K.; Winship, L.J. Calcium at the cell wall-cytoplast interface. J. Integr. Plant Biol. 2010, 52, 147–160. [Google Scholar] [CrossRef]
- Konrad, K.R.; Wudick, M.M.; Feijo, J.A. Calcium regulation of tip growth: New genes for old mechanisms. Curr. Opin. Plant Biol. 2011, 14, 721–730. [Google Scholar] [CrossRef]
- Cole, R.A.; Fowler, J.E. Polarized growth: Maintaining focus on the tip. Curr. Opin. Plant Biol. 2006, 9, 579–588. [Google Scholar] [CrossRef]
- Chen, T.; Wu, X.; Chen, Y.; Li, X.; Huang, M.; Zheng, M.; Baluska, F.; Samaj, J.; Lin, J. Combined proteomic and cytological analysis of Ca2+-calmodulin regulation in Piceae meyeri pollen tube growth. Plant Physiol. 2009, 149, 1111–1126. [Google Scholar]
- Rounds, C.M.; Lubeck, E.; Hepler, P.K.; Winship, L.J. Propidium iodide competes with Ca2+ to label pectin in pollen tubes and Arabidopsis root hairs. Plant Physiol. 2011, 157, 175–187. [Google Scholar] [CrossRef]
- Moonshausen, G.B.; Bibikova, T.N.; Messerli, M.A.; Gilroy, S. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc. Natl. Acad. Sci. USA 2007, 104, 20996–21001. [Google Scholar]
- Leliaert, F.; Smith, D.R.; Moreau, H.; Herron, M.; Verbruggen, H.; Delwiche, C.F.; de Clerck, O. Phylogeny and molecular evolution of the green algae. Crit. Rev. Plant Sci. 2011, 31, 1–46. [Google Scholar]
- Timme, R.E.; Bachvaroff, T.R.; Delwiche, C.F. Broad phylogenetic sampling and sister lineage of land plants. PLoS One 2012. [Google Scholar] [CrossRef]
- Wodniok, S.; Brinkmann, H.; Glockner, G.; Heidel, A.J.; Phillippe, H.; Melknonian, M.; Becker, B. Origin of land plants: Do conjugating green algae hold the key? BMC Evol. Biol. 2011, 11, 104. [Google Scholar]
- Meindl, U. Micrasterias cells as a model system for research on morphogenesis. Microbiol. Rev. 1993, 50, 415–433. [Google Scholar]
- Meindl, U.; Lancell, S.A.; Hepler, P.K. Vesicle production and fusion during lobe formation in Micrasterias visualized by high-pressure freeze fixation. Protoplasma 1992, 170, 104–114. [Google Scholar] [CrossRef]
- Eder, M.; Lutz-Meindl, U. Pectin-like carbohydrates in the green alga Micrasterias characterized by cytochemical analysis and energy filtering TEM. J. Microsc. 2008, 231, 201–214. [Google Scholar] [CrossRef]
- Geitmann, A. How to shape a cylinder: Pollen tube as a model system. Sex. Plant Reprod. 2010, 23, 63–71. [Google Scholar] [CrossRef]
- Eder, M.; Lutz-Meindl, U. Analyses and localization of pectin-like carbohydrates in cell wall and mucilage of the green alga Netrium digitus. Protoplasma 2010, 243, 25–38. [Google Scholar] [CrossRef]
- Vannerum, K.; Abe, J.; Sekimoto, H.; Inze, D.; Vyverman, W. Intracellular localization of an endogenous cellulose synthase of Micrasterias denticulata (Desmidiales, Chlorophyta) by means of transient genetic transformation. J. Phycol. 2010, 46, 839–845. [Google Scholar] [CrossRef]
- Vannerum, K.; Huysman, M.J.J.; de Rycke, R.; Vuylsteke, M.; Leliaert, F.; Pollier, J.; Lutz-Meindl, U.; Gillard, J.; de Veylder, L.; Goosens, A.; et al. Transcriptional analysis of cell growth and morphogenesis in the unicellular green alga Micrasterias (Streptophyta), with emphasis on the role of expansin. BMC Plant Biol. 2011, 11, 128. [Google Scholar] [CrossRef]
- Pflugl-Haill, M.; Vidali, L.; Vos, J.W.; Hepler, P.K.; Lutz-Meindl, U. Changes of the actin filament system in the green alga Micrasterias denticulata induced by different cytoskeletal inhibitors. Protoplasma 2000, 212, 206–216. [Google Scholar] [CrossRef]
- Hoftberger, M.; Url, T.; Meindl, U. Disturbance of the secretory pathway in Micrasterias denticulata by tunicamycin and cyclopiazonic acid. Protoplasma 1995, 189, 173–179. [Google Scholar] [CrossRef]
- Troxell, C.L.; Scheffey, C. Ionic currents flow through Micrasterias and Closterium cells during expansion of the primary cell wall. Planta 1991, 184, 218–225. [Google Scholar] [CrossRef]
- Vannerum, K.; de Rycke, R.; Pollier, J.; Goosens, A.; Inze, D.; Vyverman, W. Characterization of a RABE (RAS gene from rat brain E) GTPase expressed during morphogenesis in the unicellular green alga Micrasterias denticulata (Zygnematophyceae, Streptophyta). J. Phycol. 2012, 48, 682–692. [Google Scholar] [CrossRef]
- Domozych, D.S.; Brechka, H.; Britton, A.; Toso, M. Cell wall growth and modulation dynamics in a model unicellular green alga—Penium margaritaceum: Live cell labeling with monoclonal antibodies. J. Bot. 2011. [Google Scholar] [CrossRef]
- Domozych, D.S.; Lambiasse, L.; Kiemle, S.N.; Gretz, M.R. Cell-wall development and bipolar growth in the desmid Penium margaritaceum (Zygnematophyceae, Streptophyta). Asymmetry in a symmetric world. J. Phycol. 2009, 45, 879–893. [Google Scholar] [CrossRef]
- Domozych, D.S.; Serfis, A.; Kiemle, S.N.; Gretz, M.R. The structure and biochemistry of charophycean cell walls: I. Pectins of Penium margaritaceum. Protoplasma 2007, 230, 99–115. [Google Scholar] [CrossRef]
- Ikehaya, I.; Sonobe, S.; Murakami, K.; Shimmen, T. Rhizoid differentiation of Spirogyra is regulated by substratum. J. Plant Res. 2008, 121, 571–579. [Google Scholar] [CrossRef]
- Inoue, N.; Sonobe, S.; Nagata, Y.; Shimmen, T. Secretion of lectin-binding material in rhizoid differentiation of Spirogyra. Plant Cell Physiol. 1999, 40, 973–977. [Google Scholar] [CrossRef]
- Yamada, S.; Sonobe, S.; Shimmen, T. Synthesis of a callosic substance during differentiation in Spirogyra. Plant Cell Physiol. 2003, 44, 1225–1228. [Google Scholar] [CrossRef]
- Yoshida, K.; Inoue, N.; Sonobe, S.; Shimmen, T. Involvement of microtubules in rhizoid differentiation of Spirogyra species. Protoplasma 2003, 221, 227–235. [Google Scholar]
- Marchant, H.J. Ultrastructure, development and cytoplsmic rotation of seta-bearing cells of Coleochaete scutata (Chlorophyceae). J. Phycol. 1977, 13, 28–36. [Google Scholar]
- McBride, G.E. The seta-bearing cells of Coleochaete scutata (Chlorophyceae, Chaetophorales). Phycologia 1974, 13, 271–285. [Google Scholar] [CrossRef]
- McBride, G.; LaBounty, J.; Adams, J.; Berns, M. The totipotency and relationship of seta-bearing cells to thallus development in the green alga Coleochaete scutata. A laser microbeam study. Devel. Biol. 2004, 37, 90–99. [Google Scholar]
- Hodick, D.; Buchen, B.; Sievers, A. Statolith positioning by microfilaments in Chara rhizoids and protonemata. Adv. Space Res. 1998, 21, 1183–1189. [Google Scholar] [CrossRef]
- Braun, M.; Wastenys, G.O. Reorganization of the actin andmicrotubule cytoskeleton throughout blue-light-induced differentiation of characean protonemata into multicellular thalli. Protoplasma 1998, 202, 38–53. [Google Scholar] [CrossRef]
- Boot, K.J.M.; Libbenga, K.R.; Hille, S.C.; Offringa, R.; van Dujin, B. Polar auxin transport. J. Exp. Bot. 2012, 63, 4213–4218. [Google Scholar] [CrossRef]
- Klambt, D.; Knauth, B.; Dittman, I. Auxin dependent growth of rhizoids of Chara globularis. Physiol. Plant 1992, 85, 537–540. [Google Scholar] [CrossRef]
- Braun, M. Gravisensing in single-celled systems: Characean rhizoids and protonemata. Adv. Space Res. 2001, 27, 1031–1039. [Google Scholar] [CrossRef]
- Hodick, D. Negative gravitropism in Chara protonemata: A model integrating the opposite gravitropic responses of protonemata and rhizoids. Planta 1994, 195, 43–49. [Google Scholar] [CrossRef]
- Braun, M.; Richter, M. Relocalization of the calcium gradient and a dihydropyridine receptor is involved in upward bending by bulging Chara protonemata, but not in downward bending by bowing of Chara rhizoids. Planta 1999, 209, 414–423. [Google Scholar] [CrossRef]
- Braun, M.; Hauslage, J.; Czogalla, A.; Limbach, C. Tip-localized actin polymerization and remodeling, reflected by the localization of ADF, profilin and villin, are fundamental for gravity sensing and polar growth in characean rhizoids. Planta 2004, 219, 379–388. [Google Scholar]
- Proseus, T.E.; Boyer, J.S. Calcium deprivation disrupts enlargement of Chara corallina cells: Further evidence for the calcium pectate cycle. J. Exp. Bot. 2012, 63, 953–958. [Google Scholar]
- Menand, B.; Calder, G.; Dolan, L. Both chloronemal and caulonemal cells expand by tip growth in the moss Physcomitrella patens. J. Exp. Bot. 2007, 58, 1843–1849. [Google Scholar] [CrossRef]
- Furt, F.; Lemoi, K.; Tuzel, E.; Vidali, L. Quantitative analysis of organelle distribution and dynamics in Physcomitrella patens protonematal cells. BMC Plant Biol. 2012, 12, 70. [Google Scholar] [CrossRef]
- Vidali, L.; Bezanilla, M. Physcomitrella patens: A model for tip cell growth and differentiation. Curr. Opin. Plant Biol. 2012, 15, 1–7. [Google Scholar] [CrossRef]
- Vidali, L.; Augustine, R.C.; Kleinman, K.P.; Bezanilla, M. Profilin is essential for tip growth in the moss Physcomitrella patens. Plant Cell 2007, 19, 3705–3722. [Google Scholar] [CrossRef]
- Augustine, R.C.; Pattavina, K.A.; Tuzel, E.; Vidali, L.; Bezanilla, M. Actin interacting protein! An actin depolymerizing factor drives rapid actin dynamics in Physcomitrella patens. Plant Cell 2011, 23, 3696–3710. [Google Scholar] [CrossRef]
- Perroud, P.-F.; Quatrano, R.S. BRICK1 is required for apical cell growth in filaments of the moss but not for gametophore morphology. Plant Cell 2008, 20, 411–422. [Google Scholar] [CrossRef]
- Roberts, A.W.; Roberts, E.M.; Haigler, C.H. Moss cell walls: Structure and biosynthesis. Front. Plant Sci. 2012. [Google Scholar] [CrossRef]
- Moller, I.; Sorensen, I.; Bernal, A.J.; Blaukopf, C.; Lee, K.; Obro, J.; Pettolino, F.; Roberts, A.; Mikkelsen, J.D.; Knox, J.P.; et al. High-throughput mapping of cell-wall polymers within and between plants using novel microarrays. Plant J. 2007, 50, 1118–1128. [Google Scholar] [CrossRef]
- Geitmann, A.; Ortega, J.K.E. Mechanics and modeling of plant cell growth. Trends Plant Sci. 2009, 14, 467–478. [Google Scholar] [CrossRef]
- Jones, V.A.S.; Dolan, L. The evolution of root hairs and rhizoids. Ann. Bot. 2012, 110, 205–212. [Google Scholar] [CrossRef]
- Bushart, T.; Roux, S.J. Conserved features of germination and polarized cell growth: A few insights from a pollen-fern comparison. Ann. Bot. 2007, 99, 9–17. [Google Scholar] [CrossRef]
- Clark, G.B.; Turnwald, S.; Tirlapur, U.K.; Haas, C.J.; von der Mark, K.; Roux, S.J.; Scheuerlein, R. Polar distribution of annexin-like proteins during phytochrome-mediated initiation and growth of rhizoids in the ferns Dryopteris and Anemia. Planta 1995, 197, 376–384. [Google Scholar]
- Parton, R.M.; Dyer, A.F.; Read, N.D.; Trewavas, A.J. Apical structure of actively growing fern rhizoids examined by DIC and confocal microscopy. Ann. Bot. 2000, 85, 233–245. [Google Scholar] [CrossRef]
- Morris, K.E.; Poretrfield, D.M. Nitric oxide and cGMP signaling and gravity dependent cell polarity in Ceratopteris richardii. Grav. Space Biol. Bull. 2004, 18, 13. [Google Scholar]
- Yi, H.; Puri, V.M. Architecture-based multiscale computational modeling of plant cell wall mechanics to examine the hydrogen-bonding hypothesis of the cell wall network structure model. Plant Physiol. 2012, 160, 1281–1292. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Wu, H.-W. Structural and functional compartmentalization in pollen tubes. J. Exp. Bot. 2007, 58, 75–82. [Google Scholar] [CrossRef]
- Pietruszka, M.; Lipowczan, M.; Geitmann, A. Persistent symmetry frustration in pollen tubes. PLoS One 2010, 7, 1–9. [Google Scholar]
- McKenna, S.T.; Kunkel, J.G.; Bosch, M.; Rounds, C.M.; Vidali, L.; Winship, L.J.; Hepler, P.K. Exocytosis precedes and predicts the increase in growth in oscillating pollen tubes. Plant Cell 2009, 21, 3026–3040. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, Z. Rapid tip growth; Insights from Pollen tubes. Semin. Cell Dev. Biol. 2011, 22, 816–824. [Google Scholar] [CrossRef]
- Zerzour, R.; Kroeger, J.; Geitmann, A. Polar growth in pollen tubes is associated with spatially confined dynamic changes in cell mechanical properties. Dev. Biol. 2009, 334, 437–446. [Google Scholar] [CrossRef]
- Fernando, D.D.; Lazzaro, M.D.; Owens, J.N. Growth and Development of conifer pollen tubes. Sex. Plant Reprod. 2005, 13, 149–162. [Google Scholar] [CrossRef]
- Libault, M.; Brechenmacher, L.; Cheng, J.; Xu, D.; Stacey, G. Root hair systems biology. Trends Plant Sci. 2010, 15, 641–650. [Google Scholar] [CrossRef]
- Guimil, S.; Dunand, C. Cell growth and differentiation in Arabidopsis epidermal cells. J. Exp. Bot. 2007, 58, 3829–3840. [Google Scholar] [CrossRef]
- Fu, Y.; Gu, Y.; Zheng, Z.; Wastenys, G.; Yang, Z. Interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 2005, 120, 687–700. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Domozych, D.S.; Fujimoto, C.; LaRue, T. Polar Expansion Dynamics in the Plant Kingdom: A Diverse and Multifunctional Journey on the Path to Pollen Tubes. Plants 2013, 2, 148-173. https://doi.org/10.3390/plants2010148
Domozych DS, Fujimoto C, LaRue T. Polar Expansion Dynamics in the Plant Kingdom: A Diverse and Multifunctional Journey on the Path to Pollen Tubes. Plants. 2013; 2(1):148-173. https://doi.org/10.3390/plants2010148
Chicago/Turabian StyleDomozych, David S., Chelsea Fujimoto, and Therese LaRue. 2013. "Polar Expansion Dynamics in the Plant Kingdom: A Diverse and Multifunctional Journey on the Path to Pollen Tubes" Plants 2, no. 1: 148-173. https://doi.org/10.3390/plants2010148






