Effect of Gamma Irradiation on 2-Acetyl-1-pyrroline Content, GABA Content and Volatile Compounds of Germinated Rice (Thai Upland Rice)
Abstract
:1. Introduction
2. Results
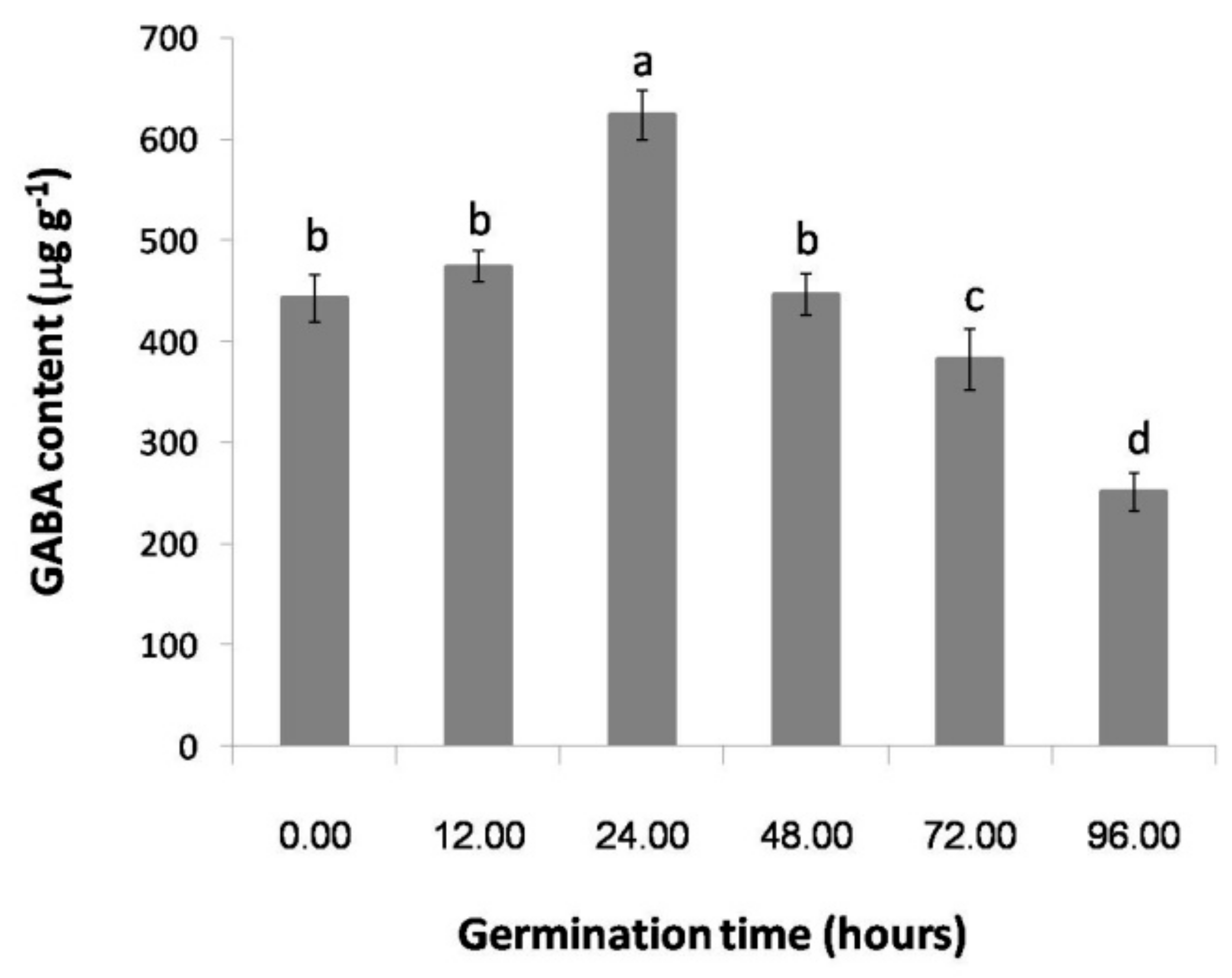
2.1. The Effect of Germination Time on the GABA Content of Thai Upland Rice
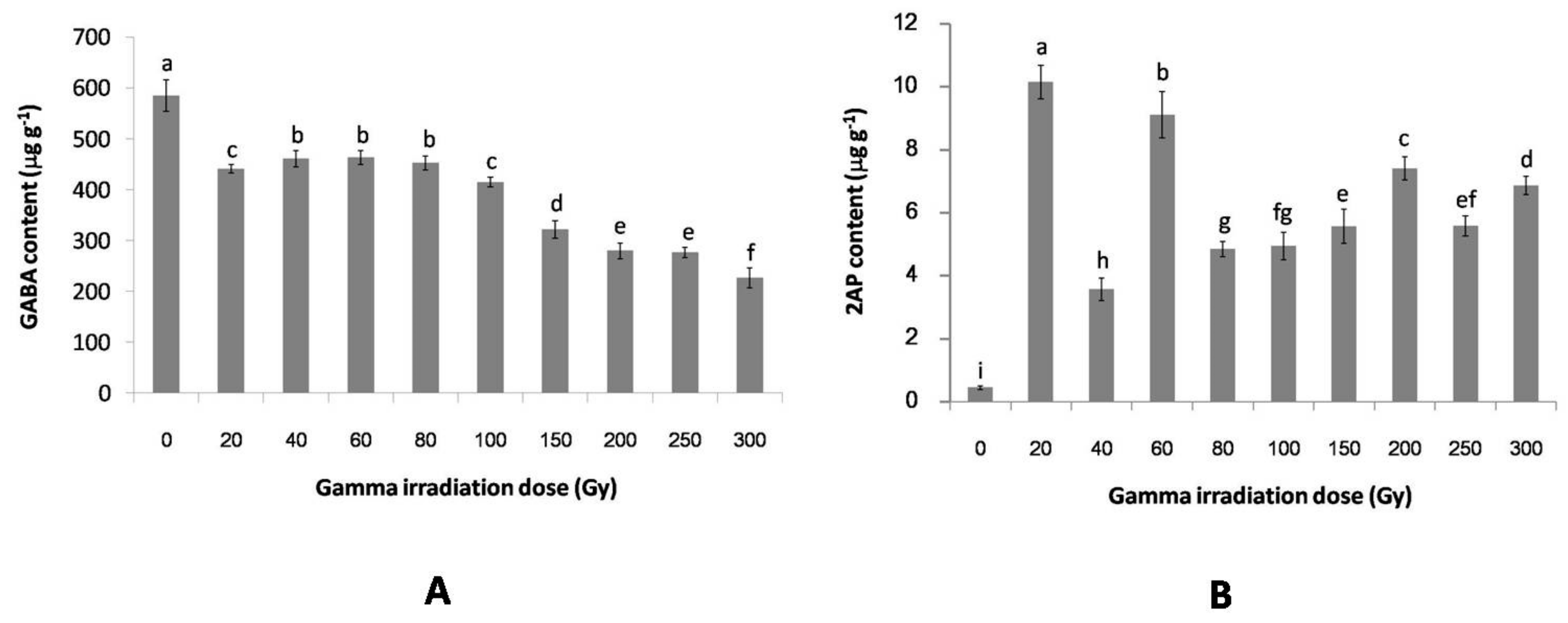
2.2. The Effect of Gamma Irradiation on the GABA and 2AP Contents of Non-Irradiated and Irradiated Rice
2.3. The Effect of Gamma Irradiation on Volatile Compounds of Non-Irradiated and Irradiated Rice
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Germination Time and GABA Content Quantification
4.3. Gamma Irradiation
4.4. The Effect of Gamma Irradiation on GABA Content, 2AP Content and Volatile Compounds
4.5. 2AP Quantification with Gas Chromatography-Mass Spectrometry (GC-MS)
4.6. Analysis of Volatile Compounds
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buttery, R.G.; Ling, L.C. 2-Acetyl-1-pyrroline: An important aroma component of cooked rice. Chem. Ind. 1982, 12, 958–959. [Google Scholar]
- Widjaja, R.; Craske, J.D.; Wootton, M. Comparative studies on volatile components of non-fragrant and fragrant rices. J. Sci. Food Agric. 1996, 70, 151–161. [Google Scholar] [CrossRef]
- Hinge, V.; Patil, H.; Nadaf, A. Comparative characterization of aroma volatiles and related gene expression analysis at vegetative and mature stages in basmati and non-basmati rice (Oryza sativa L.) cultivars. Appl. Biochem. Biotechnol. 2016, 178, 619–639. [Google Scholar] [CrossRef] [PubMed]
- Yoshihashi, T.; Huong, N.T.; Inatomi, H. Precursors of 2-acetyl-1-pyrroline, a potent flavor compound of an aromatic rice variety. J. Agric. Food Chem. 2002, 50, 2001–2004. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Li, W.; Pan, S.; Fitzgerald, T.L.; Xiao, F.; Tang, Y.; Wang, Y.; Duan, M.; Tian, H.; Tang, X. Shading during the grain filling period increases 2-acetyl-1-pyrrolinecontent in fragrant rice. Rice 2015, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, L.M.; Fitzgerald, T.L.; Henry, R.J.; Jin, Q.; Wates, D.L. The gene for fragrance in rice. Plant Biotechnol. J. 2005, 3, 3363–3370. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, L.M.; Gillies, S.A.; Brushett, D.J.; Waters, D.L.; Henry, R.J. Inactivation of an amino aldehyde dehydrogenase is responsible for fragrance in rice. Plant Mol. Biol. 2008, 68, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, Y.; Shi, W.; Ji, Q.; He, F.; Zhang, Z.; Cheng, Z.; Liu, X.; Xu, M. Badh2, encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell 2008, 20, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 2007, 13, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Yoshihashi, T.; Nguyen, T.; Kabaki, N. Area dependency of 2-acetyl-1-pyrroline content in an aromatic rice variety, Khao Dawk Mali 105. Jpn. Agric. Res. Q. 2004, 38, 105–109. [Google Scholar] [CrossRef]
- Gay, F.; Maraval, I.; Roque, S.; Gunata, Z.; Boulanger, R.; Audebert, A.; Mestres, C. Effect of salinity on yield and 2-acetyl-1-pyrroline content in the grains of three fragrant rice cultivars (Oryza sativa L.) in Camargue (France). Field Crops Res. 2010, 117, 154–160. [Google Scholar] [CrossRef]
- Hasthanasombut, S.; Supaibulwatana, K.; Mii, M.; Nakamura, I. Genetic manipulation of japonica rice using the OsBADH1 gene from indica rice to improve salinity tolerance. Plant Cell Tissue Organ Cult. 2011, 104, 79–89. [Google Scholar] [CrossRef]
- Poonlaphdecha, J.; Maraval, I.; Roques, S.; Audebert, A.; Boulanger, R.; Bry, X.; Gunata, Z. Effect of timing and duration of salt treatment during growth of a fragrant rice variety on yield and 2-acetyl-1-pyrroline, proline, and GABA Levels. J. Agric. Food Chem. 2012, 60, 3824–3830. [Google Scholar] [CrossRef] [PubMed]
- Bown, A.W.; Shelp, B.J. The metabolism and functions of [gamma]-aminobutyric acid. Plant Physiol. 1997, 115, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kinnersley, A.M.; Turano, F.J. Gamma aminobutyric acid (GABA) and plant responses to stress. Crit. Rev. Plant Sci. 2010, 19, 479–509. [Google Scholar] [CrossRef]
- Chung, H.J.; Jang, S.H.; Cho, H.Y.; Lim, S.T. Effects of steeping and anaerobic treatment on GABA (γ-aminobutyric acid) content in germinated waxy hull-less barley. LWT-Food Sci. Technol. 2009, 40, 1712–1716. [Google Scholar] [CrossRef]
- Shu, X.L.; Frank, T.; Shu, Q.Y.; Engel, K.H. Metabolite profiling of germinating rice seeds. J. Agric. Food Chem. 2008, 56, 11612–11620. [Google Scholar] [CrossRef] [PubMed]
- Khwanchai, P.; Chinprahast, N.; Pichyangkura, R.; Chaiwanichsiri, S. Gamma-aminobutyric acid and glutamic acid contents, and the GAD activity in germinated brown rice (Oryza sativa L.): Effect of rice cultivars. Food Sci. Biotechnol. 2014, 23, 73–379. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiang, J.; Zhang, L.; Zhu, X.; Evers, J.; Werf, W.; Duan, L. Optimizing soaking and germination conditions to improve gamma-aminobutyric acid content in japonica and indica germinated brown rice. J. Funct Foods 2014, 10, 283–291. [Google Scholar] [CrossRef]
- Roldan-Arjona, T.; Ariza, R.R. Repair and tolerance of oxidative DNA damage in plants. Mutat. Res. 2009, 681, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhang, L.; Feng, W.; Xu, H.; Wang, L.; Jiao, Z. ROS and ABA signaling are involved in the growth stimulation induced by low-dose gamma irradiation in Arabidopsis seedling. Appl. Biochem. Biotechnol. 2015, 175, 1490–1506. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.E.; Ahn, J.W.; Kwon, S.J.; Kim, J.B.; Kim, S.H.; Kang, S.Y.; Kim, D.S. Selection and molecular characterization of a high tocopherol accumulation rice mutant line induced by gamma irradiation. Mol. Biol. Rep. 2014, 41, 7671–7681. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, R.; Kalaiyarasi, R. Sensitivity of rice varieties to gamma irradiation. Electron. J. Plant Breed. 2010, 1, 885–889. [Google Scholar]
- Morita, R.; Kusaba, M.; Iida, S.; Yamaguchi, H.; Nishio, T.; Nishimura, M. Molecular characterization of mutations induced by gamma irradiation in rice. Genes Genet. Syst. 2009, 84, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Sansenya, S.; Hua, Y.; Chumanee, S.; Winyakul, C. Effect of gamma irradiation on the 2-acetyl-1-pyrroline content during growth of Thai black glutinous rice (Upland rice). Aust. J. Crop Sci. 2017, 11, 631–637. [Google Scholar]
- Karladee, D.; Suriyong, S. γ-aminobutyric acid (GABA) content in different varieties of brown rice during germination. ScienceAsia 2012, 38, 13–17. [Google Scholar] [CrossRef]
- Marcu, D.; Damian, G.; Cosma, C.; Cristea, V. Gamma radiation effects on seed germination, growth and pigment content, and ESR study of induced free radicals in maize (Zea mays). J. Biol. Phys. 2013, 39, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Preuss, S.B.; Britt, A.B. A DNA-damage-induced cell cycle checkpoint in Arabidopsis. Genetics 2003, 164, 323–334. [Google Scholar] [PubMed]
- Kiong, A.L.P.; Lai, A.G.; Hussein, S.; Harun, A.R. Physiological responses of orthosiphon stamineus plantles to gamma irradiation. Am. Eurasian J. Sustain. Agric. 2008, 2, 135–149. [Google Scholar]
- Sukhonthara, S.; Theerakulkait, C.; Miyazawa, M. Characterization of volatile aroma compounds from red and black rice bran. J. Oleo Sci. 2009, 58, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; Shewfelt, R.L.; Lee, K.S.; Kays, S.J. Comparison of odor-active compounds from six distinctly different rice flavor types. J. Agric. Food Chem. 2008, 56, 2780–2787. [Google Scholar] [CrossRef] [PubMed]
- Champagne, E.T. Rice aroma and flavor: A literature review. Cereal Chem. 2008, 85, 445–454. [Google Scholar] [CrossRef]
- Lam, H.S.; Proctor, A. Milled rice oxidation volatiles and odor development. J. Food Sci. 2003, 68, 2676–2681. [Google Scholar] [CrossRef]
- Mansoor, M.A.; Proctor, A. Volatile components analysis of commercially milled head and brown rice. J. Food Sci. 2004, 69, 632–636. [Google Scholar] [CrossRef]
- Maraval, I.; Mestres, C.; Penin, K.; Ribeyre, F.; Boulanges, R.; Guichard, E.; Gunata, Z. Odor-active compounds in cooked rice cultivars from camargue (France) analyzed by GC-O and GC-MS. J. Agric. Food Chem. 2008, 56, 5291–5298. [Google Scholar] [CrossRef] [PubMed]
- Mathure, A.V.; Jawali, N.; Thengane, R.J.; Nadaf, A.B. Comparative quantitative analysis of headspace volatiles and their association with BADH2 marker in non-basmati scented, basmati and non-scented rice (Oryza sativa L.) cultivars of India. Food Chem. 2014, 142, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Palta, J.P.; Whitaker, B.D.; Weiss, L.S. Plasma membrane lipids associated with genetic variability in freezing tolerance and cold acclimation of Solanum Specie. Plant Physiol. 1993, 103, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Zhukov, A.V. Palmitic acid and its role in the structure and functions of plant cell membranes. Russ. J. Plant Physl. 2015, 62, 706–713. [Google Scholar] [CrossRef]
| RT (min) | Volatile Compounds | 0 (Gy) | 20 (Gy) | 40 (Gy) | 60 (Gy) | 80 (Gy) | 100 (Gy) | 150 (Gy) | 200 (Gy) | 250 (Gy) | 300 (Gy) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 21.62 | Hexanal | + | + | + | + | + | + | + | + | + | + |
| 22.37 | Undecane | + | + | + | + | + | + | + | + | + | + |
| 27.58 | 5-Methyl-2-hexanone | − | − | − | − | − | − | − | − | − | + |
| 27.64 | Heptanal | − | − | − | + | − | + | + | + | + | + |
| 28.52 | Dodecane | + | + | + | + | + | + | + | + | + | + |
| 29.56 | Butyl butanoate | − | − | − | − | − | − | + | + | − | − |
| 30.17 | 2-Pentylfuran | + | + | + | + | + | + | + | + | + | + |
| 33.13 | Octanal | + | + | + | + | + | + | + | + | + | + |
| 33.74 | Tridecane | + | + | + | + | + | + | + | + | + | + |
| 34.20 | 1-Butyl-2-pentyl-cyclopentane | − | − | − | + | − | − | − | − | − | − |
| 34.84 | (Z)-2-Heptenal | + | + | + | + | + | + | + | + | + | + |
| 35.57 | 2-Acetyl-1-pyrroline | + | + | + | + | + | + | + | + | + | + |
| 35.73 | Cyclododecane | − | − | − | + | − | − | − | − | − | + |
| 36.43 | 1-Hexanol | − | − | − | + | − | − | − | − | − | − |
| 37.99 | Heptylcyclohexane | − | − | − | + | − | − | + | + | + | + |
| 38.16 | Nonanal | + | + | + | + | + | + | + | + | + | + |
| 38.30 | Tetradecane | + | + | + | + | + | + | + | + | + | + |
| 38.83 | 3-Octen-2-one | + | + | + | + | + | + | + | + | + | + |
| 39.18 | Hexyl butanoate | − | − | − | − | + | − | − | − | − | − |
| 39.79 | 8-Methyloctahydrocoumarin | + | + | + | − | + | + | − | − | + | + |
| 40.31 | n-Pentadecanol | + | + | + | + | + | − | − | − | − | − |
| 40.75 | 1-Octen-3-ol | + | + | + | + | + | + | + | + | + | + |
| 41.06 | 2-Ethyl-1-decanol | − | − | − | − | + | + | + | + | − | − |
| 41.07 | Isobutyl tridecyl carbonate | − | − | − | + | − | − | − | − | − | − |
| 41.08 | 2-Butoxyethyl acetate | + | + | + | − | − | − | − | − | − | − |
| 42.57 | 1-Hexadecanol | + | + | + | − | + | + | + | + | + | + |
| 42.58 | Cyclotetradecane | − | − | − | + | − | − | − | − | − | − |
| 42.76 | 4-(1-Acetyl-cyclopentyl)-but-3-en-2-one | + | + | + | − | − | − | − | − | − | − |
| 43.87 | Benzaldehyde | + | + | + | + | + | + | + | + | + | + |
| 44.62 | n-Heptadecanol-1 | + | + | + | − | − | + | + | + | + | + |
| 44.84 | 4-Ethyl-Tetradecane | + | + | + | − | + | − | − | − | − | − |
| 44.85 | 3-Methyl-Pentadecane | − | − | − | + | − | − | − | − | − | − |
| 45.37 | Octylformate | + | + | + | + | + | + | + | + | + | + |
| 46.19 | [S-(R*,R*)]-2,3-Butanediol, | + | + | + | − | + | − | − | − | + | − |
| 46.68 | 5,5-Diethyltridecane | + | + | + | + | + | + | + | + | + | − |
| 47.14 | n-Nonylcyclohexane | + | + | + | + | + | + | + | + | + | + |
| 48.02 | 2-(2-ethoxyethoxy)-Ethanol | + | + | + | − | + | + | + | + | − | − |
| 49.74 | 2-Butyl-2-Octenal | + | + | + | + | + | + | + | + | + | + |
| 51.03 | Undecane | + | + | + | + | + | + | + | + | + | + |
| 53.03 | Methyl N-hydroxybenzenecarboximidate | + | + | + | + | + | + | + | + | + | + |
| 57.03 | 2-Ethyl-3-hydroxyhexyl 2-methylpropanoate | + | + | + | + | + | + | + | + | + | + |
| 57.32 | Benzyl alcohol | + | + | + | + | + | + | + | + | + | + |
| 57.63 | 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | + | + | + | + | + | + | + | + | + | − |
| 58.31 | Butylatedhydroxytoluene | − | − | − | + | − | − | − | − | − | − |
| 61.15 | Phenol | − | − | − | − | + | + | + | + | + | − |
| 65.36 | Nonanoic acid | − | − | − | − | + | − | − | − | − | − |
| 62.58 | Octanoic acid | + | + | + | − | − | − | − | − | − | − |
| 64.37 | 6,10,14-Trimethylpentadecan-2-one | + | + | + | − | − | − | − | − | − | − |
| 70.77 | 2,3-Dihydrobenzofuran | + | + | + | + | + | + | + | + | + | + |
| 74.13 | n-Hexadecanoic acid | − | − | − | − | − | + | − | − | − | − |
| No. | Compound | Peak Area (%)2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 (Gy) | 20 (Gy) | 40 (Gy) | 60 (Gy) | 80 (Gy) | 100 (Gy) | 150 (Gy) | 200 (Gy) | 250 (Gy) | 300 (Gy) | ||
| 1 | 2-Acetyl-1-pyrroline | 2.76 | 2.98 | 2.98 | 3.42 | 3.22 | 4.83 | 3.36 | 3.52 | 3.74 | 3.72 |
| 2 | Octanal | 2.72 | 3.08 | 2.78 | 1.75 | 3.32 | 2.13 | 2.54 | 1.92 | 2.87 | 4.10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sansenya, S.; Hua, Y.; Chumanee, S.; Phasai, K.; Sricheewin, C. Effect of Gamma Irradiation on 2-Acetyl-1-pyrroline Content, GABA Content and Volatile Compounds of Germinated Rice (Thai Upland Rice). Plants 2017, 6, 18. https://doi.org/10.3390/plants6020018
Sansenya S, Hua Y, Chumanee S, Phasai K, Sricheewin C. Effect of Gamma Irradiation on 2-Acetyl-1-pyrroline Content, GABA Content and Volatile Compounds of Germinated Rice (Thai Upland Rice). Plants. 2017; 6(2):18. https://doi.org/10.3390/plants6020018
Chicago/Turabian StyleSansenya, Sompong, Yanling Hua, Saowapa Chumanee, Kannika Phasai, and Chanun Sricheewin. 2017. "Effect of Gamma Irradiation on 2-Acetyl-1-pyrroline Content, GABA Content and Volatile Compounds of Germinated Rice (Thai Upland Rice)" Plants 6, no. 2: 18. https://doi.org/10.3390/plants6020018





