Fluorescent Nano-Probes to Image Plant Cell Walls by Super-Resolution STED Microscopy
Abstract
:1. Introduction
2. Results
2.1. Sample Preparation
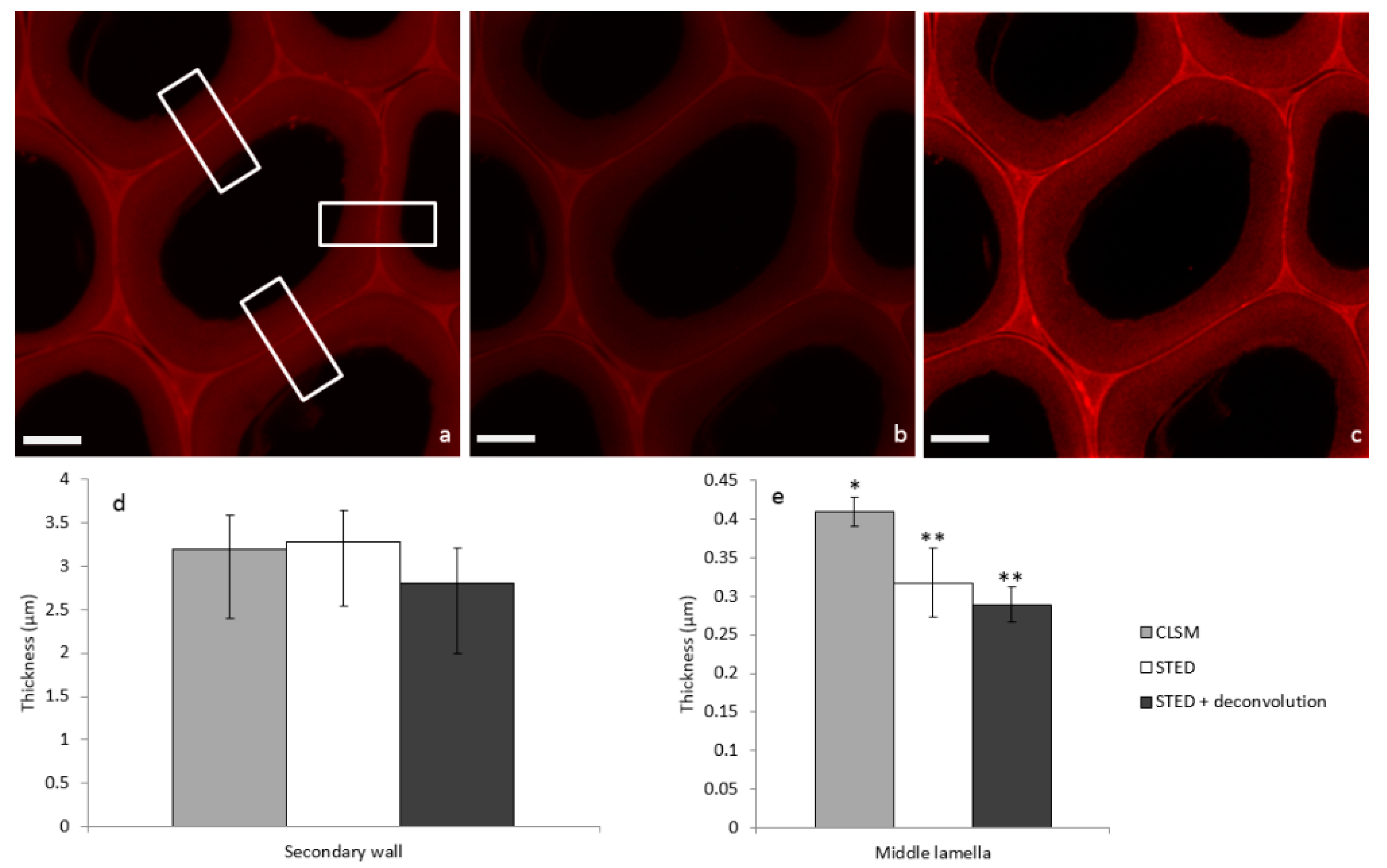
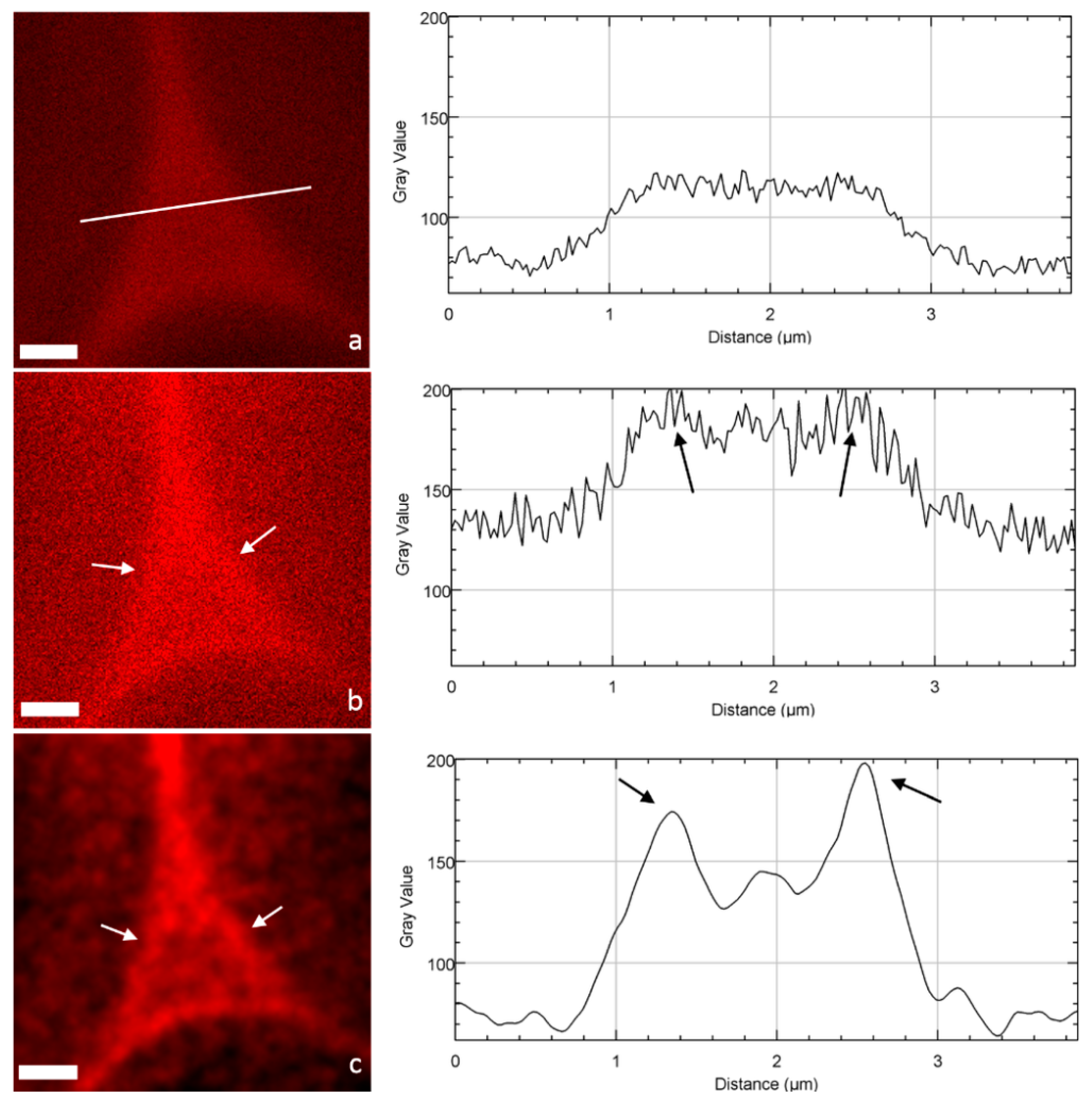
2.2. Image Acquisition and Analysis
3. Discussion
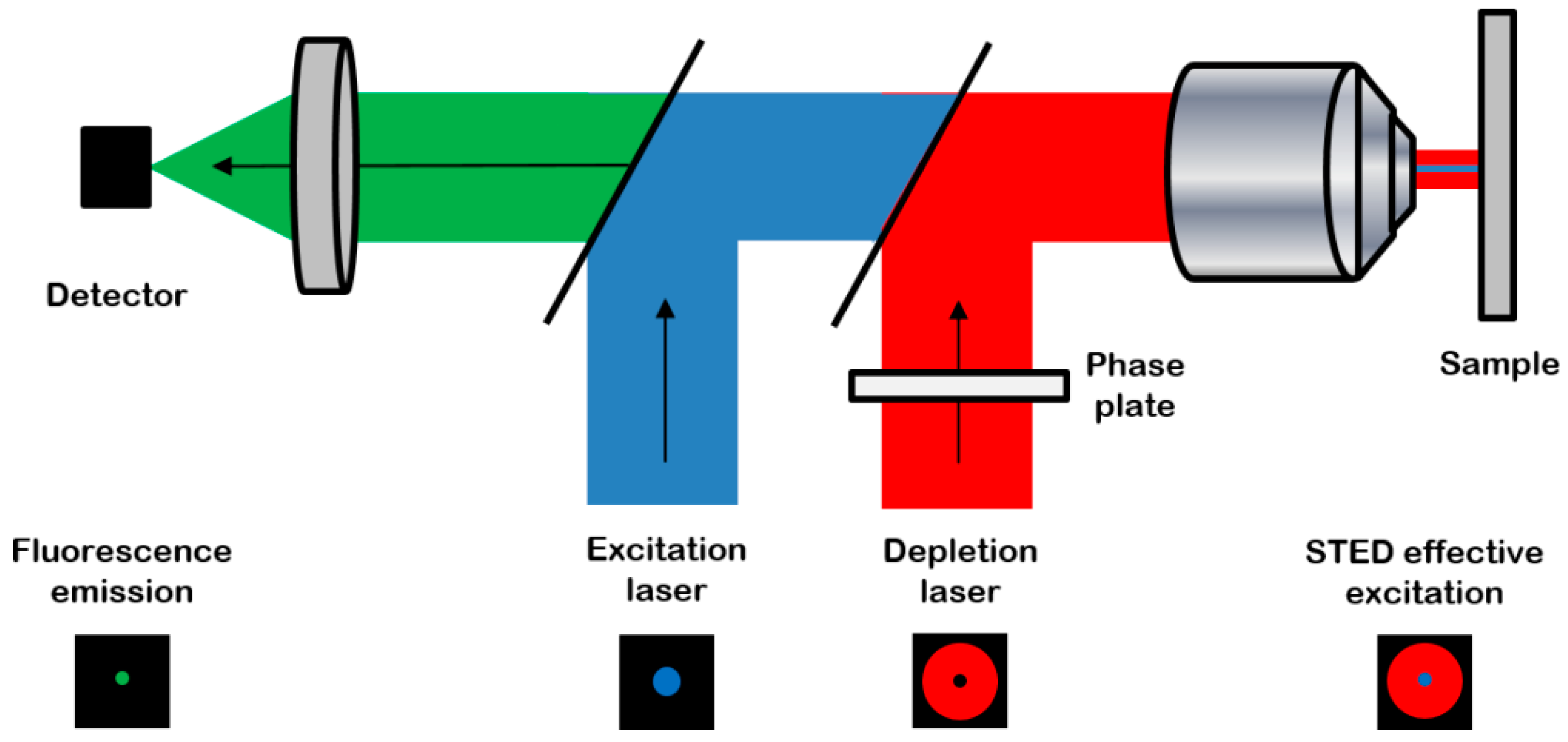
4. Materials and Methods
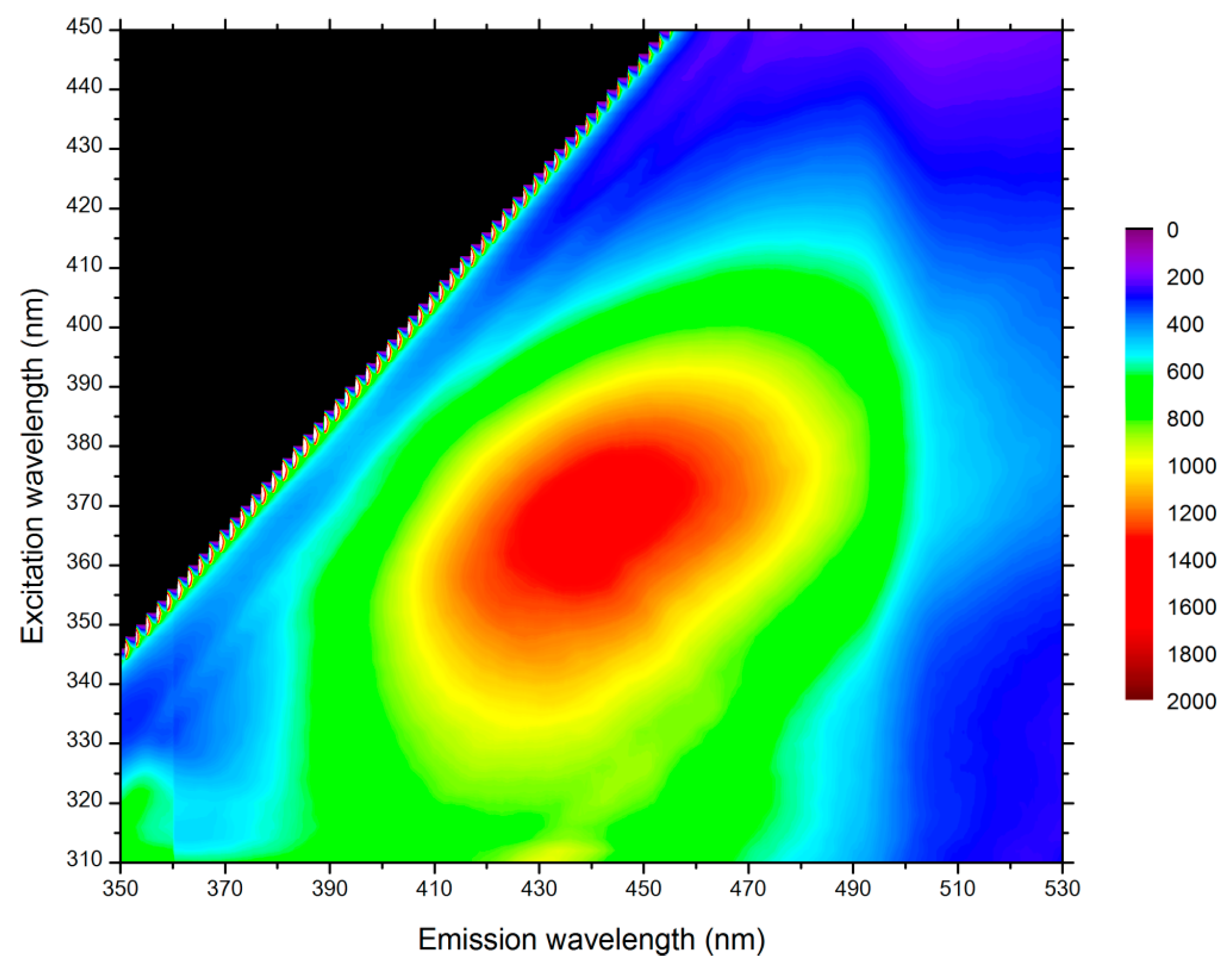
4.1. Fluorescent Probe Characterization
4.2. Poplar Sample Preparation for Microscopy
4.3. Confocal and STED Imaging
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Komis, G.; Šamajová, O.; Ovečka, M.; Šamaj, J. Super-resolution microscopy in plant cell imaging. Trends Plant Sci. 2015, 20, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Y.; Liu, Y.S.; Zeng, Y.N.; Himmel, M.E.; Baker, J.O.; Bayer, E.A. How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science 2012, 338, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Inouye, H.; Zhang, Y.; Yang, L.; Venugopalan, N.; Fischetti, R.F.; Gleber, S.C.; Vogt, S.; Fowle, W.; Makowski, B.; Tucker, M.; et al. Multiscale deconstruction of molecular architecture in corn stover. Sci. Rep. 2014, 4, 3756. [Google Scholar] [CrossRef] [PubMed]
- Chundawat, S.P.S.; Donohoe, B.S.; Sousa, L.D.; Elder, T.; Agarwal, U.P.; Lu, F.C.; Ralph, J.; Himmel, M.E.; Balan, V.; Dale, B.E. Multi-scale visualization and characterization of lignocellulosic plant cell wall deconstruction during thermochemical pretreatment. Energy Environ. Sci. 2011, 4, 973–984. [Google Scholar] [CrossRef]
- Liesche, J.; Ziomkiewicz, I.; Schulz, A. Super-resolution imaging with Pontamine Fast Scarlet 4BS enables direct visualization of cellulose orientation and cell connection architecture in onion epidermis cells. BMC Plant Biol. 2013, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Schermelleh, L.; Heintzmann, R.; Leonhardt, H. A guide to super-resolution fluorescence microscopy. J. Cell Biol. 2010, 190, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Blom, H.; Bates, M. Nanoscopy-imaging life at the nanoscale: A Nobel Prize achievement with a bright future. Phys. Scr. 2015, 90, 108010. [Google Scholar] [CrossRef]
- Leica Microsystems. Confocal Application Letter-Quick Guide to STED Sample Preparation; Leica Microsystems CMS GmbH: Mannheim, Germany, 2014. [Google Scholar]
- Blom, H.; Brismar, H. STED microscopy: Increased resolution for medical research? J. Intern. Med. 2014, 276, 560–578. [Google Scholar] [CrossRef] [PubMed]
- Hell, S.W.; Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: Stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994, 19, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Coltharp, C.; Xiao, J. Superresolution microscopy for microbiology. Cell. Microbiol. 2012, 14, 1808–1818. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.A.; Newman, R.H.; Campion, S.H.; Suckling, I.D. Strength of adsorption of polyethylene glycol on pretreated Pinus radiata wood and consequences for enzymatic saccharification. Biomass Bioenergy 2014, 70, 339–346. [Google Scholar] [CrossRef]
- Meng, X.; Ragauskas, A.J. Recent advances in understanding the role of cellulose accessibility in enzymatic hydrolysis of lignocellulosic substrates. Curr. Opin. Biotechnol. 2014, 27, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Paës, G. Fluorescent probes for exploring plant cell wall deconstruction: A review. Molecules 2014, 19, 9380–9402. [Google Scholar] [CrossRef] [PubMed]
- Paës, G.; Habrant, A.; Ossemond, J.; Chabbert, B. Exploring accessibility of pretreated poplar cell walls by measuring dynamics of fluorescent probes. Biotechnol. Biofuels 2017, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.A.; Newman, R.H.; Vaidya, A. Nanoscale interactions of polyethylene glycol with thermo-mechanically pre-treated Pinus radiata biofuel substrate. Biotechnol. Bioeng. 2014, 111, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.A.; Kroese, H.W.; Hill, S.J.; Franich, R.A. Detection of wood cell wall porosity using small carbohydrate molecules and confocal fluorescence microscopy. J. Microsc. 2015, 259, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Fong, M.; Berrin, J.G.; Paës, G. Investigation of the binding properties of a multi-modular GH45 cellulase using bioinspired model assemblies. Biotechnol. Biofuels 2016, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Luterbacher, J.S.; Parlange, J.Y.; Walker, L.P. A pore-hindered diffusion and reaction model can help explain the importance of pore size distribution in enzymatic hydrolysis of biomass. Biotechnol. Bioeng. 2013, 110, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Frankenstein, C.; Schmitt, U.; Koch, G. Topochemical studies on modified lignin distribution in the xylem of poplar (Populus spp.) after wounding. Ann. Bot. 2006, 97, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.A. Lignification and lignin topochemistry-an ultrastructural view. Phytochemistry 2001, 57, 859–873. [Google Scholar] [CrossRef]
- Pilate, G.; Chabbert, B.; Cathala, B.; Yoshinaga, A.; Leplé, J.-C.; Laurans, F.; Lapierre, C.; Ruel, K. Lignification and tension wood. C. R. Biol. 2004, 327, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, A.; Kusumoto, H.; Laurans, F.; Pilate, G.; Takabe, K. Lignification in poplar tension wood lignified cell wall layers. Tree Physiol. 2012, 32, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ding, D.; Ma, J.; Ji, Z.; Zhang, X.; Xu, F. Ultrastructure and topochemistry of plant cell wall by transmission electron microscopy. In The Transmission Electron Microscope-Theory and Applications; Maaz, K., Ed.; InTech: Vienna, Austria, 2015. [Google Scholar]
- Foston, M.; Hubbell, C.A.; Samuel, R.; Jung, S.; Fan, H.; Ding, S.-Y.; Zeng, Y.; Jawdy, S.; Davis, M.; Sykes, R.; et al. Chemical, ultrastructural and supramolecular analysis of tension wood in Populus tremula x alba as a model substrate for reduced recalcitrance. Energy Environ. Sci. 2011, 4, 4962. [Google Scholar] [CrossRef]
- Gierlinger, N.; Schwanninger, M. Chemical imaging of poplar wood cell walls by confocal Raman microscopy. Plant Physiol. 2006, 140, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Y.; Xu, Q.; Ali, M.K.; Baker, J.O.; Bayer, E.A.; Barak, Y.; Lamed, R.; Sugiyama, J.; Rumbles, G.; Himmel, M.E. Versatile derivatives of carbohydrate-binding modules for imaging of complex carbohydrates approaching the molecular level of resolution. Biotechniques 2006, 41, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, T.; Karita, S.; Araki, Y.; Watanabe, S.; Oyadomari, M.; Takada, R.; Tanaka, F.; Abe, K.; Watanabe, T.; Honda, Y. Analysis of exposed cellulose surfaces in pretreated wood biomass using Carbohydrate-Binding Module (CBM)-Cyan Fluorescent Protein (CFP). Biotechnol. Bioeng. 2010, 105, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Beaugrand, J.; Paës, G.; Reis, D.; Takahashi, M.; Debeire, P.; O'Donohue, M.; Chabbert, B. Probing the cell wall heterogeneity of micro-dissected wheat caryopsis using both active and inactive forms of a GH11 xylanase. Planta 2005, 222, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Vetharaniam, I.; Kelly, W.; Attwood, G.; Harris, P. A 3-D model of a perennial ryegrass primary cell wall and its enzymatic degradation. Computation 2014, 2, 23–46. [Google Scholar] [CrossRef]
- Balzarotti, F.; Eilers, Y.; Gwosch, K.C.; Gynnå, A.H.; Westphal, V.; Stefani, F.D.; Elf, J.; Hell, S.W. Nanometer resolution imaging and tracking of fluorescent molecules with minimal photon fluxes. Science 2016, 355, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paës, G.; Habrant, A.; Terryn, C. Fluorescent Nano-Probes to Image Plant Cell Walls by Super-Resolution STED Microscopy. Plants 2018, 7, 11. https://doi.org/10.3390/plants7010011
Paës G, Habrant A, Terryn C. Fluorescent Nano-Probes to Image Plant Cell Walls by Super-Resolution STED Microscopy. Plants. 2018; 7(1):11. https://doi.org/10.3390/plants7010011
Chicago/Turabian StylePaës, Gabriel, Anouck Habrant, and Christine Terryn. 2018. "Fluorescent Nano-Probes to Image Plant Cell Walls by Super-Resolution STED Microscopy" Plants 7, no. 1: 11. https://doi.org/10.3390/plants7010011




