Progress toward Understanding the Molecular Basis of Fruit Response to Hypoxia
Abstract
:1. Introduction
2. Oxygen Exchange in Fruit Tissue
3. Effects of Oxygen Availability on Metabolism and Development
Physiological Effects of Low-Oxygen-Based Storage
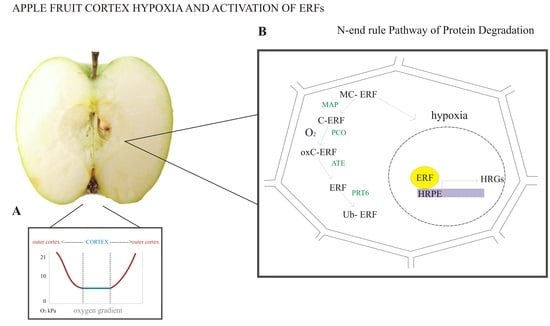
4. Activation of the Anaerobic Response by the N-End Rule Pathway
Oxygen-Dependent Degradation of Fruit-Specific ERF VII
5. Conclusions
Funding
Conflicts of Interest
References
- Barry, C.S.; Giovannoni, J.J. Ethylene and fruit ripening. J. Plant Growth Regul. 2007, 26, 143–159. [Google Scholar] [CrossRef]
- Bouzayen, M.; Latche, A.; Pech, J.C.; Nath, P. Mechanism of fruit ripening. In Plant Developmental Biology-Biotechnological Perspective; Pua, E.C., Davey, M.R., Eds.; Springer: Heidelberg/Berlin, Germany, 2010; Volume 1, pp. 319–339. [Google Scholar]
- Karlova, R.; Chapman, N.; David, K.; Angenent, G.C.; Seymour, G.B.; De Maagd, R.A. Transcriptional control of fleshy fruit development and ripening. J. Exp. Bot. 2014, 65, 4527–4541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Stacewiez-Sapuntzakis, M.; Duncan, C.; Sharifi, R.; Ghosh, L.; Breemen, R.; van Ashton, D.; Bowen, P.E. Oxidative DNA damage in prostate cancer patients consuming tomato saucebased entrees as a whole-food intervention. J. Natl. Cancer Inst. 2001, 93, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Mares-Perlman, J.A.; Millen, A.E.; Ficek, T.L.; Hankinson, S.E. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease. J. Nutr. 2002, 132, 5185–5245. [Google Scholar] [CrossRef] [PubMed]
- Biale, J.B.; Young, R.E. Respiration and ripening in fruits retrospective and prospect. In Recent Advances in the Biochemistry of Fruits and Vegetables; Friend, J., Rhodes, M.J.C., Eds.; Academic Press: New York, NY, USA, 1981; pp. 1–39. [Google Scholar]
- Lelievre, J.M.; Latche, A.; Jones, B.; Bouzayen, M.; Peach, J.C. Ethylene and fruit ripening. Physiol. Plant 1997, 101, 727–739. [Google Scholar] [CrossRef]
- Alexander, L.; Grierson, D. Ethylene biosynthesis and action in tomato: A model for climacteric fruit ripening. J. Exp. Bot. 2002, 53, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Yahia, E.M. Modified and Controlled Atmospheres for the Storage, Transportation, and Packaging of Horticultural Commodities, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 1–10. [Google Scholar]
- Thompson, K.A. Controlled Atmosphere Storage of Fruits and Vegetables, 2nd ed.; CABI: Cambridge, UK, 2010; pp. 26–43. [Google Scholar]
- Verboven, P.; Kerckhofs, G.; Mebatsion, H.K.; Ho, Q.T.; Temst, K.; Wevers, M.; Cloetens, P.; Nicolai, B.M. Three-Dimensional Gas Exchange Pathways in Pome Fruit Characterized by Synchrotron X-Ray Computed Tomography. Plant Physiol. 2008, 147, 518–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, Q.T.; Verboven, P.; Verlinden, B.E.; Nicolai, B.M. A model for gas transport in pear fruit at multiple scales. J. Exp. Bot. 2010, 61, 2071–2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burg, S.P.; Burg, E.A. Gas exchange in fruits. Plant Physiol. 1965, 18, 870–874. [Google Scholar] [CrossRef]
- Cameron, A.C.; Yang, S.F. A simple method for the determination of resistance to gas diffusion in plant organs. Plant Physiol. 1982, 70, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Solomos, T. Principles of gas exchange in bulky plant tissue. HortScience 1987, 22, 766–771. [Google Scholar]
- Schotsmans, W.; Verlinden, B.E.; Lammertyn, J.; Nicolaı, B.M. Simultaneous measurement of oxygen and carbon dioxide diffusivity in pear fruit tissue. Postharvest Biol. Technol. 2003, 29, 155–166. [Google Scholar] [CrossRef]
- Nobel, P.S. Physicochemical and Environmental Plant Physiology; Academic Press Inc.: London, UK, 1991; pp. 1–46. [Google Scholar]
- Hagenmaier, R. Method for measuring internal gases of citrus fruit and determining peel permeance. Proc. Fla. State Hortic. Soc. 2004, 116, 418–423. [Google Scholar]
- Hagenmaier, R. A comparison of ethane, ethylene and CO2 peel permeance for fruits with different coatings. Postharvest Biol. Technol. 2005, 37, 56–64. [Google Scholar] [CrossRef]
- Ben-Yehoshua, S. Gas exchange, transpiration and the commercial deterioration of stored orange fruit. J. Am. Soc. Hortic. Sci. 1969, 94, 524–526. [Google Scholar]
- Ho, Q.T.; Verboven, P.; Mebatsion, H.K.; Verlinden, B.E.; Vandewalle, S.; Nicolaï, B.M. Microscale mechanisms of gas exchange in fruit tissue. New Phytol. 2009, 182, 163–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, W. Aeration in higher plants. Adv. Bot. Res. 1979, 7, 225–332. [Google Scholar]
- Marcellin, P. Mesure de la diffusion des gas dans Ies organes vegetaux. Bull. Soc. Fr. Physiol. Veg. 1963, 9, 29. [Google Scholar]
- Salvatori, D.; Andrés, A.; Chiralt, A.; Fito, P. The response of some properties of fruit to vacuum impregnation. J. Food Process Eng. 1998, 21, 59–73. [Google Scholar] [CrossRef]
- Rajapakse, N.C.; Banks, N.H.; Hewett, E.W.; Cleland, D.J. Development of oxygen concentration gradients in flesh tissues of bulky plant organs. J. Am. Soc. Hortic. Sci. 1990, 115, 793–797. [Google Scholar]
- Sharifi, M.; Rafiee, S.; Keyhani, A.; Jafari, A.; Mobli, H.; Rajabipour, A.; Akram, A. Some physical properties of orange (var. Tompson). Int. Agrophys. 2007, 21, 391–397. [Google Scholar]
- Ho, Q.T.; Verboven, P.; Verlinden, B.E.; Lammertyn, J.; Vandewalle, S.; Nicolaï, B.M. A Continuum Model for Metabolic Gas Exchange in Pear Fruit. PLoS Comput. Biol. 2008, 4, e1000023. [Google Scholar] [CrossRef] [PubMed]
- Ho, Q.T.; Verboven, P.; Verlinden, B.E.; Herremans, E.; Wevers, M.; Carmeliet, J.; Nicolaï, B.M. A three-dimensional multiscale model for gas exchange in fruit. Plant Physiol. 2011, 155, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Mellidou, I.; Buts, K.; Hatoum, D.; Ho, Q.T.; Johnston, J.W.; Watkins, C.B.; Schaffer, R.J.; Gapper, N.E.; Giovannoni, J.J.; Rudell, D.R. Transcriptomics events associated with internal browning of apple during postharvest storage. BMC Plant Biol. 2014, 14, 328. [Google Scholar] [CrossRef] [PubMed]
- Ho, Q.T.; Verlinden, B.E.; Verboven, P.; Vandewalle, S.; Nicolaï, B.M. A permeation-diffusion-reaction model of gas transport in cellular tissue of plant materials. J. Exp. Bot. 2006, 57, 4215–4224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, Q.T.; Verlinden, B.E.; Verboven, P.; Nicolaï, B.M. Gas diffusion properties at different positions in the pear. Postharvest Biol. Technol. 2006, 41, 113–120. [Google Scholar] [CrossRef]
- Drew, M.C. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.J.; Chen, K.S.; Ferguson, I.B. Programmed cell death features in apple suspension cells under low oxygen culture. J. Zhejiang Univ. Sci. 2004, 5, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Paul, V.; Pandey, R. Role of internal atmosphere on fruit ripening and storability—A review. Int. J. Food Sci. Technol. 2014, 51, 1223–1250. [Google Scholar] [CrossRef] [PubMed]
- Saglio, P.H.; Rancillac, M.; Bruzau, F.; Pradet, A. Critical oxygen pressure for growth and respiration of excised and intact roots. Plant Physiol. 1984, 76, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.C. Soil aeration and plant-root metabolism. Soil Sci. 1992, 154, 259–268. [Google Scholar] [CrossRef]
- Kader, A.A. Biochemical and physiological basis for effects of controlled and modified atmospheres on fruits and vegetables. Food Technol. 1986, 40, 99–104. [Google Scholar]
- Boersig, M.R.; Kader, A.A.; Romani, R.J. Aerobic-anaerobic respiratory transition in pear fruit and cultured pear fruit cells. J. Am. Soc. Hortic. Sci. 1988, 111, 869–873. [Google Scholar]
- Nanos, G.D.; Romani, R.J.; Kader, A.A. Metabolic and other response of ‘Bartlett’ pear fruit and suspension cultured ‘Passe Crassane’ pear fruit cells held in 0.25% O2. J. Am. Soc. Hortic. Sci. 1992, 117, 934–940. [Google Scholar]
- Saquet, A.A.; Streif, J.; Bangerth, F. Changes in ATP, ADP and pyridine nucleotide levels related to the incidence of physiological disorders in ‘Conference’ pears and ‘Jonagold’ apples during controlled atmosphere storage. J. Hortic. Sci. Biotechnol. 2000, 75, 243–249. [Google Scholar] [CrossRef]
- Kerbel, E.L.; Keder, A.A.; Romani, R.S. Effect of elevated CO2 concentrations on glycolysis in intact ‘Bartlet’ pear fruit. Plant Physiol. 1988, 86, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Zhou, L.; Kader, A.A. Mode of oxygen and carbon dioxide action on strawberry ester biosynthesis. J. Am. Soc. Hortic. Sci. 1994, 119, 971–975. [Google Scholar]
- Shipway, M.R.; Bramlage, W.J. Effects of carbon dioxide on activity of apple mitochondria. Plant Physiol. 1973, 51, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Bulens, I.; Van de Poel, B.; Hertog, M.L.A.T.M.; Cristescu, S.M.; Harren, F.J.M.; De Proft, M.P.; Geeraerd, A.H.; Nicolai, B.M. Dynamic changes of the ethylene biosynthesis in “Jonagold” apple. Physiol. Plant 2014, 150, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.E.; Sørensen, H.; Cantwell, M. Changes in acetaldehyde, ethanol and amino acid concentrations in broccoli florets during air and controlled atmosphere storage. Postharvest Biol. Technol. 2001, 22, 227–237. [Google Scholar] [CrossRef]
- Xiao, Z.; Rogiers, S.Y.; Sadras, V.O.; Tyerman, S.D. Hypoxia in grape berries: The role of seed respiration and lenticels on the berry pedicel and the possible link to cell death. J. Exp. Bot. 2018, 69, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Cukrov, D.; Zermiani, M.; Brizzolara, S.; Cestaro, A.; Licausi, F.; Luchinat, C.; Santucci, C.; Tenori, L.; Van Veen, H.; Zuccolo, A.; et al. Extreme Hypoxic Conditions Induce Selective Molecular Responses and Metabolic Reset in Detached Apple Fruit. Front. Plant Sci. 2016, 7, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara, M.V.; Budde, C.O.; Porrini, L.; Borsani, J.; Murray, R.; Andreo, C.S.; Drincovich, M.F. Peach (Prunus persica) fruit response to anoxia: Reversible ripening delay and biochemical changes. Plant Cell Physiol. 2011, 52, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Choi, S.C.; Jung, S.; Cho, B.K.; Ahn, G.H.; Ryu, S.B. A Transcriptome Approach Toward Understanding Fruit Softening in Persimmon. Front. Plant Sci. 2017, 8, 1556. [Google Scholar] [CrossRef] [PubMed]
- Loulakakis, K.; Hassan, M.; Gerasopoulos, G.; Kanellis, A.K. Effects of low oxygen on in vitro translation products of poly(A)+ RNA, cellulase and alcohol dehydrogenase expression in preclimacteric and ripening-initiated avocado fruit. Postharvest Biol. Technol. 2016, 39, 29–37. [Google Scholar] [CrossRef]
- Kanellis, A.K.; Solomos, T.; Roubelakis-Angelakis, K.A. Supspecificities of cellulose and polygalacturonase isozymes in avocado fruit mesocarp subjected to low oxygen stress. Plant Physiol. 1991, 96, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.R.S.; Chase, T., Jr. Alcohol dehydrogenase and pyruvate decarboxylase induction in ripening and hypoxia tomato fruit. Plant Physiol. Biochem. 1993, 31, 875–885. [Google Scholar]
- Kader, A.A.; Zagory, D.; Kerbel, E.L. Modified atmosphere packaging of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 1989, 28, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Gorny, J.R.; Kader, A.A. Fresh-cut fruit products. In Fresh-Cut Products: Maintaining Quality and Safety; Cantwell, M., Ed.; University of California: San Diego, CA, USA, 1996; Volume 10, pp. 14–59. [Google Scholar]
- Wang, Z.; Dilley, D.R. Control of superficial scald of apples by low-oxygen atmospheres. Hortic. Sci. 1999, 34, 1145–1151. [Google Scholar]
- Lanahan, M.B.; Yen, H.C.; Giovannoni, J.J.; Klee, H.J. The never ripe mutation blocks ethylene perception in tomato. Plant Cell 1994, 6, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Pasentsis, K.; Falara, V.; Pateraki, I.; Gerasopoulos, D.; Kanellis, A.K. Identification and expression profiling of low oxygen regulated genes from Citrus flavedo tissues using RT-PCR differential display. J. Exp. Bot. 2007, 58, 2203–2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lumpkin, C.; Fellman, J.K.; Rudell, D.R.; Mattheis, J. Scarlett spur Red Delicious apple volatile production accompanying disorder development during low pO2 controlled atmosphere storage. J. Agric. Food Chem. 2014, 62, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Mustroph, A.; Zanetti, M.E.; Jang, C.J.H.; Holtan, H.E.; Repetti, P.P.; Galbraith, D.W.; Girke, T.; Bailey-Serres, J. Profiling translatomes of discrete cell populations resolves altered cellular priorities during hypoxia in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 18843–18848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustroph, A.; Lee, S.C.; Oosumi, T.; Zanetti, M.E.; Yang, H.; Ma, K.; Yaghoubi-Masihi, A.; Fukao, T.; Bailey-Serres, J. Cross-kingdom comparis on of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol. 2010, 152, 1484–1500. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Mustroph, A.; Sasidharan, R.; Vashisht, D.; Pedersen, O.; Oosumi, T.; Voesenek, L.A.C.J.; Bailey-Serres, J. Molecular characterization of the submergence response of the Arabidopsis thaliana ecotype Columbia. New Phytol. 2011, 190, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.J.; Lee, S.C.; Isa, N.M.; Gramuglia, S.; Fukao, T.; Bassel, G.W.; Correia, C.S.; Corbineau, F.; Theodoulou, F.L.; Bailey-Serres, J.; et al. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 2011, 479, 415–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licausi, F.; Kosmacz, M.; Weits, D.A.; Giuntoli, B.; Giorgi, F.M.; Voesenek, L.A.C.J.; Perata, P.; van Dongen, J.T. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 2011, 479, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Kosmacz, M.; Parlanti, S.; Schwarzländer, M.; Kragler, F.; Licausi, F.; van Dongen, J.T. The stability and nuclear localization of the transcription factor RAP2.12 are dynamically regulated by oxygen concentration. Plant Cell Environ. 2015, 38, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Gasch, P.; Fundinger, M.; Müller, J.T.; Lee, T.; Bailey-Serres, J.; Mustroph, A. Redundant ERF-VII transcription factors bind to an evolutionarily conserved cis-motif to regulate hypoxia-responsive gene expression in Arabidopsis. Plant Cell 2015, 28, 60–80. [Google Scholar] [CrossRef] [PubMed]
- Licausi, F.; van Dongen, J.T.; Giuntoli, B.; Novi, G.; Santaniello, A.; Geigenberger, P.; Perata, P. HRE1 and HRE2, two hypoxia-inducible ethylene response factors, affect anaerobic responses in Arabidopsis thaliana. Plant J. 2010, 62, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Büttner, M.; Singh, K.B. Arabidopsis thaliana ethylene-responsive element binding protein (AtEBP), an ethylene-inducible, GCC box DNA-binding protein interacts with an ocs element binding protein. Proc. Natl. Acad. Sci. USA 1997, 94, 5961–5966. [Google Scholar] [CrossRef] [PubMed]
- Papdi, C.; Abrahám, E.; Joseph, M.P.; Popescu, C.; Koncz, C.; Szabados, L. Functional identification of Arabidopsis stress regulatory genes using the controlled cDNA overexpression system. Plant Physiol. 2008, 147, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Wilson, I.W.; Yang, J.; Buerstenbinder, K.; Llewellyn, D.; Dennis, E.S.; Sauter, M.; Dolferus, R. Arabidopsis RAP2.2: An ethylene response transcription factor that is important for hypoxia survival. Plant Physiol. 2010, 153, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Chye, M.-L. Arabidopsis Acyl-CoA-binding protein ACBP2 interacts with an ethylene-responsive element-binding protein, AtEBP, via its ankyrin repeats. Plant Mol. Biol. 2004, 54, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Xiao, S.; Chye, M.-L. Ethylene- and pathogen-inducible Arabidopsis acyl-CoA-binding protein 4 interacts with an ethylene-responsive element binding protein. J. Exp. Bot. 2008, 59, 3997–4006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bui, L.T.; Giuntoli, B.; Kosmacz, M.; Parlanti, S.; Licausi, F. Constitutively expressed ERF-VII transcription factors redundantly activate the core anaerobic response in Arabidopsis thaliana. Plant Sci. 2015, 236, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.J.; Md Isa, N.; Movahedi, M.; Lozano-Juste, J.; Mendiondo, G.M.; Berckhan, S.; Marín-de la Rosa, N.; Vicente Conde, J.; Sousa Correia, C.; Pearce, S.P.; et al. Nitric oxide sensing in plants is mediated by proteolytic control of group VII ERF transcription factors. Mol. Cell 2014, 53, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Weits, D.A.; Giuntoli, B.; Kosmacz, M.; Parlanti, S.; Hubberten, H.M.; Riegler, H.; Hoefgen, R.; Perata, P.; Van Dongen, J.T.; Licausi, F. Plant cysteine oxidases control the oxygen-dependent branch of the N-end-rule pathway. Nat. Commun. 2014, 5, 3425. [Google Scholar] [CrossRef] [PubMed]
- Licausi, F.; Weits, D.A.; Pant, B.D.; Scheible, W.R.; Geigenberger, P.; van Dongen, J.T. Hypoxia responsive gene expression is mediated by various subset of transcription factors and miRNAs that are determined by the actual oxygen availability. New Phytol. 2011, 190, 442–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cukrov, D. Molecular Characterization of Fruit Responses to Low Oxygen. Doctoral Dissertation, Scuola Superiore Sant’Anna, Institute of Life Sciences, Pisa, Italy, 4 July 2016. [Google Scholar]
- Girardi, C.L.; Rombaldi, C.V.; Dal Cero, J.; Nobile, P.M.; Laurens, F.; Bouzayen, M.; Quecini, V. Genome-wide analysis of the AP2/ERF superfamily in apple and transcriptional evidence of ERF involvement in scab pathogenesis. Sci. Hortic. 2013, 151, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Pirrello, J.; Prasad, N.; Zhang, W.; Chen, K.; Mila, I.; Zouine, M.; Latché, A.; Pech, J.C.; Ohme-Takagi, M.; Regad, F.; et al. Functional analysis and binding affinity of tomato ethylene response factors provide insight on the molecular bases of plant differential responses to ethylene. BMC Plant Biol. 2012, 12, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Joung, J.G.; McQuinn, R.; Chung, M-Y.; Fei, Z.; Tieman, D.; Klee, H.; Giovannoni, J.J. Combined transcriptome, genetic diversity and metabolite profiling in tomato fruit reveals that the ethylene response factor SlERF6 plays an important role in ripening and carotenoid accumulation. Plant J. 2012, 70, 191–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Lima Gomes, B.; Mila, I.; Purgatto, E.; Peres, L.E.P.; Frasse, P.; Maza, E.; Zouine, M.; Roustan, J.P.; Bouzayen, M.; et al. Comprehensive profiling of Ethylene Response Factors expression identifies ripening-associated ERF genes and their link to key regulators of fruit ripening in tomato (Solanum lycopersicum). Plant Physiol. 2016, 33, 01859. [Google Scholar] [CrossRef] [PubMed]
- Meitha, K.; Agudelo-Romero, P.; Signorelli, S.; Gibbs, D.J.; Considine, J.A.; Foyer, C.H.; Considine, M.J. Developmental control of hypoxia during bud burst in grapevine. Plant Cell Environ. 2018, 41, 1154–1170. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Yin, X.R.; Shi, Y.N.; Luo, Z.R.; Yao, Y.C.; Grierson, D.; Ferguson, I.B.; Chen, K.S. Ethylene-responsive transcription factors interact with promoters of ADH and PDC involved in persimmon (Diospyros kaki) fruit de-astringency. J. Exp. Bot. 2012, 63, 6393–6405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, T.; Fang, F.; Ge, H.; Shi, Y.N.; Luo, Z.R.; Yao, Y.C.; Grierson, D.; Yin, X.R.; Chen, K.S. Two novel anoxia-induced ethylene response factors that interact with promoters of deastringency-related genes from persimmon. PLoS ONE 2014, 9, e97043. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Zhu, Q.G.; Deng, C.L.; Luo, Z.R.; Sun, N.J.; Grierson, D.; Yin, X.R.; Chen, K.S. Hypoxia-responsive ERFs involved in postdeastringency softening of persimmon fruit. Plant Biotechnol. J. 2017, 15, 1409–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.G.; Gong, Z.Y.; Wang, M.M.; Li, X.; Grierson, D.; Yin, X.R.; Chen, K.S. A transcription factor network responsive to high CO2/hypoxia is involved in deastringency in persimmon fruit. J. Exp. Bot. 2018, 69, 2061–2070. [Google Scholar] [CrossRef] [PubMed]


| Fruit | Porosity (%) | Respiration |
|---|---|---|
| Apple (Granny Smith) | 23.8 | Climacteric |
| Strawberry (Chandler) | 6.3 | Nonclimacteric |
| Peach (Miraflores) | 2.6 | Climacteric |
| Mango (Tommy Atkins) | 9.9 | Climacteric |
| Nectarine (Sunglo) | 4.0 | Climacteric |
| Orange (Thomson) | 48.5 | Nonclimacteric |
| Pear (Hosui) | 1.7 | Climacteric |
| Species | Gene ID | Symbol |
|---|---|---|
| Solanum lycopersicum | Solyc09g075420 | ERF2b (ERF.E.1) * |
| Solyc06g063070 | JERF1 (ERF.E.2) | |
| Solyc03g123500 | JERF3 (ERF.E.3) | |
| Solyc01g65980 | ERF 6 (ERF.E.4) | |
| Soyc12g049560 | ERF3 (ERF.E.5) | |
| Arabidopsis thaliana | AT1G53910.1 | RAP2.12 |
| AT1G72360.2 | HRE1 | |
| AT2G47520.1 | HRE2 | |
| AT3G14230.1 | RAP2.2 | |
| AT3G16770.1 | RAP2.3 | |
| Malus x domestica | MDP0000308922 | Mdo006699 |
| MDP0000403580 | Mdo006712 | |
| MDP0000679280 | - | |
| MDP0000566690 | - | |
| MDP0000413387 | Mdo002990 | |
| MDP0000128979 | Mdo003328 | |
| MDP0000848905 | Mdo002404 | |
| MDP0000288465 | Mdo002401 | |
| Vitis vinifera | VIT_07s0005g00820 | VvERF057 |
| VIT_05s077g01860 | VvERF058 | |
| VIT_09s0002g00470 | VvERF059 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cukrov, D. Progress toward Understanding the Molecular Basis of Fruit Response to Hypoxia. Plants 2018, 7, 78. https://doi.org/10.3390/plants7040078
Cukrov D. Progress toward Understanding the Molecular Basis of Fruit Response to Hypoxia. Plants. 2018; 7(4):78. https://doi.org/10.3390/plants7040078
Chicago/Turabian StyleCukrov, Dubravka. 2018. "Progress toward Understanding the Molecular Basis of Fruit Response to Hypoxia" Plants 7, no. 4: 78. https://doi.org/10.3390/plants7040078