Next-Generation Genome Sequencing of Sedum plumbizincicola Sheds Light on the Structural Evolution of Plastid rRNA Operon and Phylogenetic Implications within Saxifragales
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
2.2. Genome Assembly, Gene Annotation, and Sequence Analyses
2.3. Phylogenetic Analysis
3. Results and Discussion
3.1. General Features of S. plumbizincicola Plastome
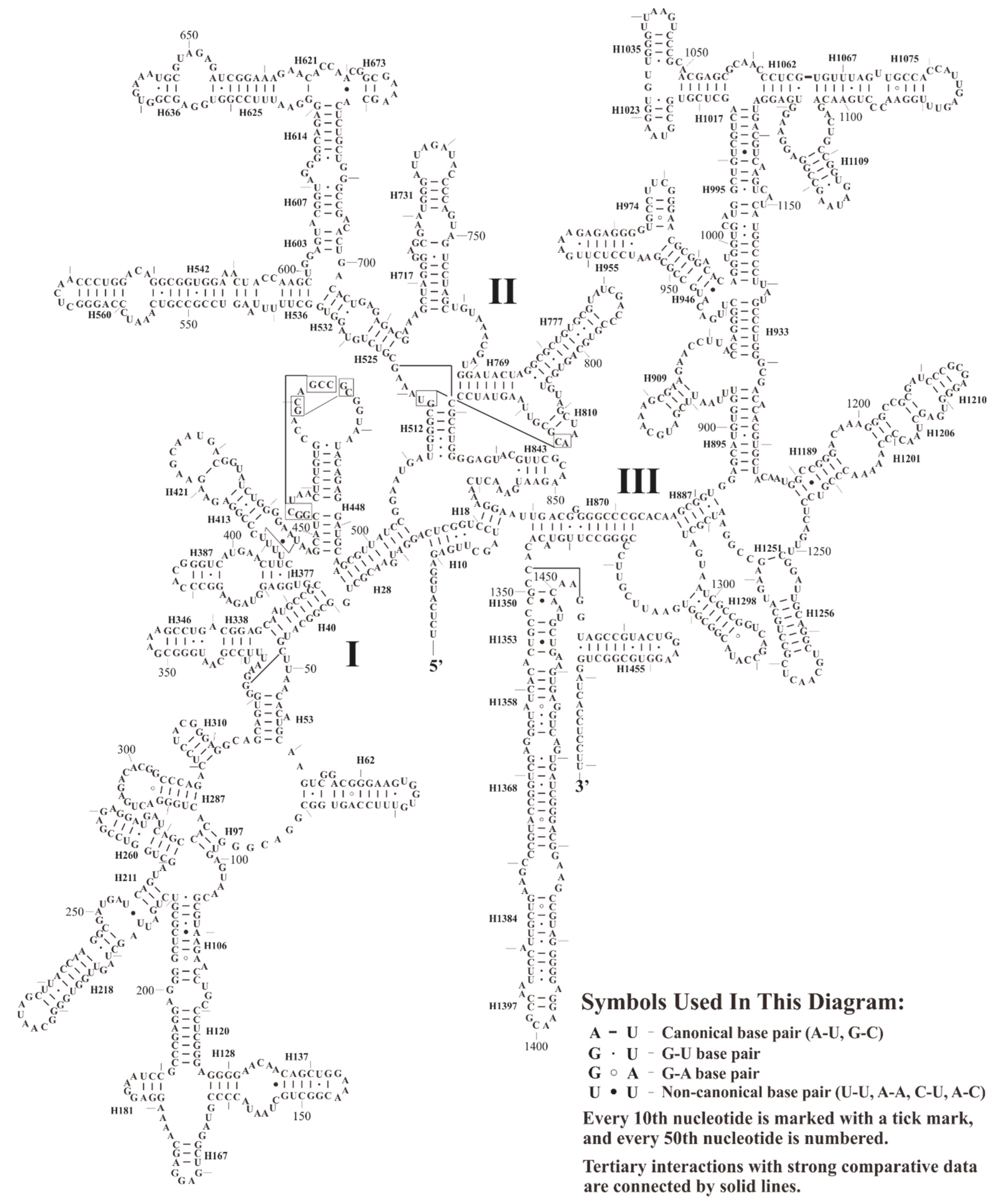
3.2. Structure Analyses of Plastid Ribosomal RNA Operon
3.2.1. Structure of 16S rRNA
3.2.2. Structure of 23S rRNA and 4.5S rRNA
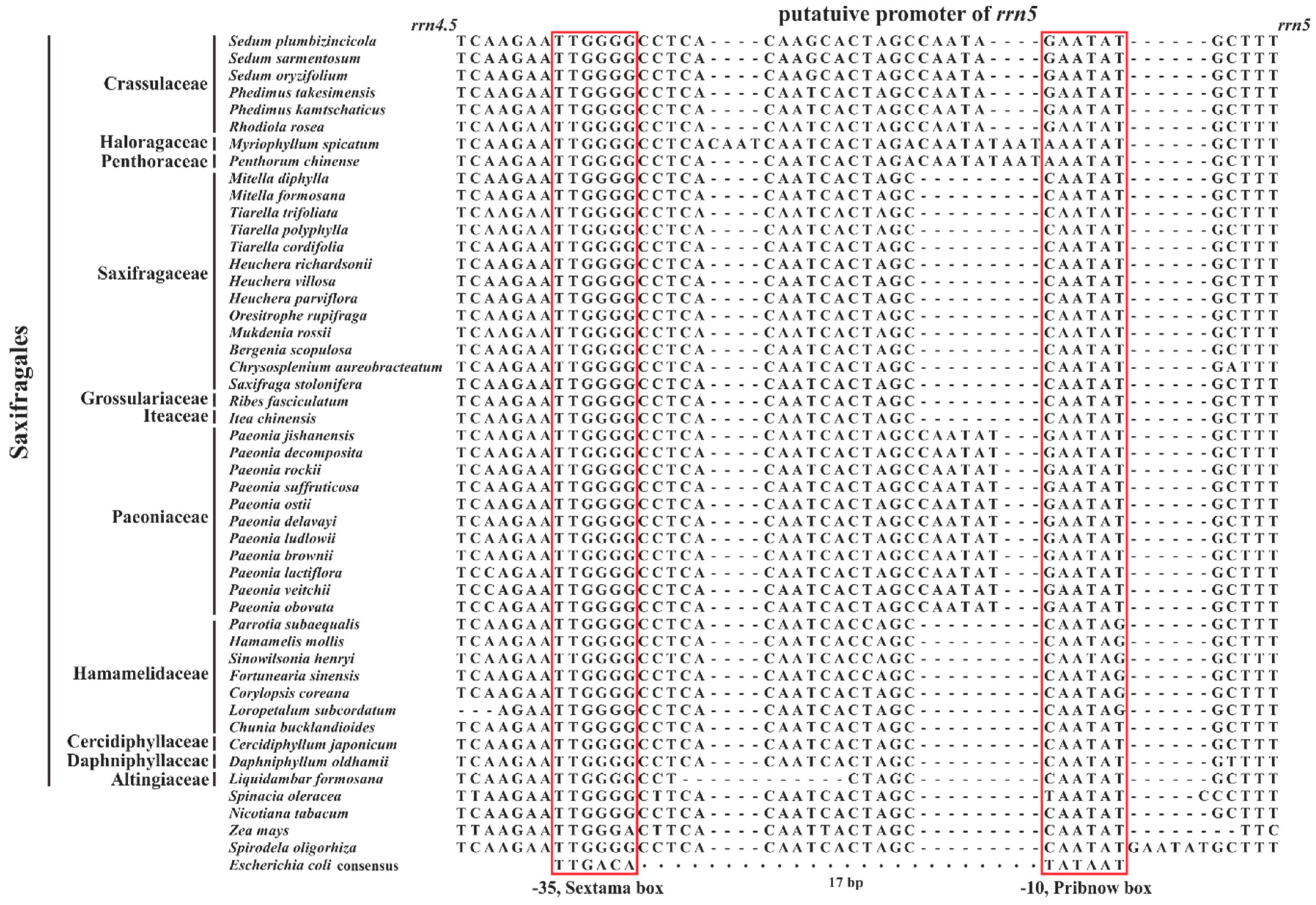
3.2.3. Structure of 5S rRNA and Evolutionary Implications of Its Putative Promoter
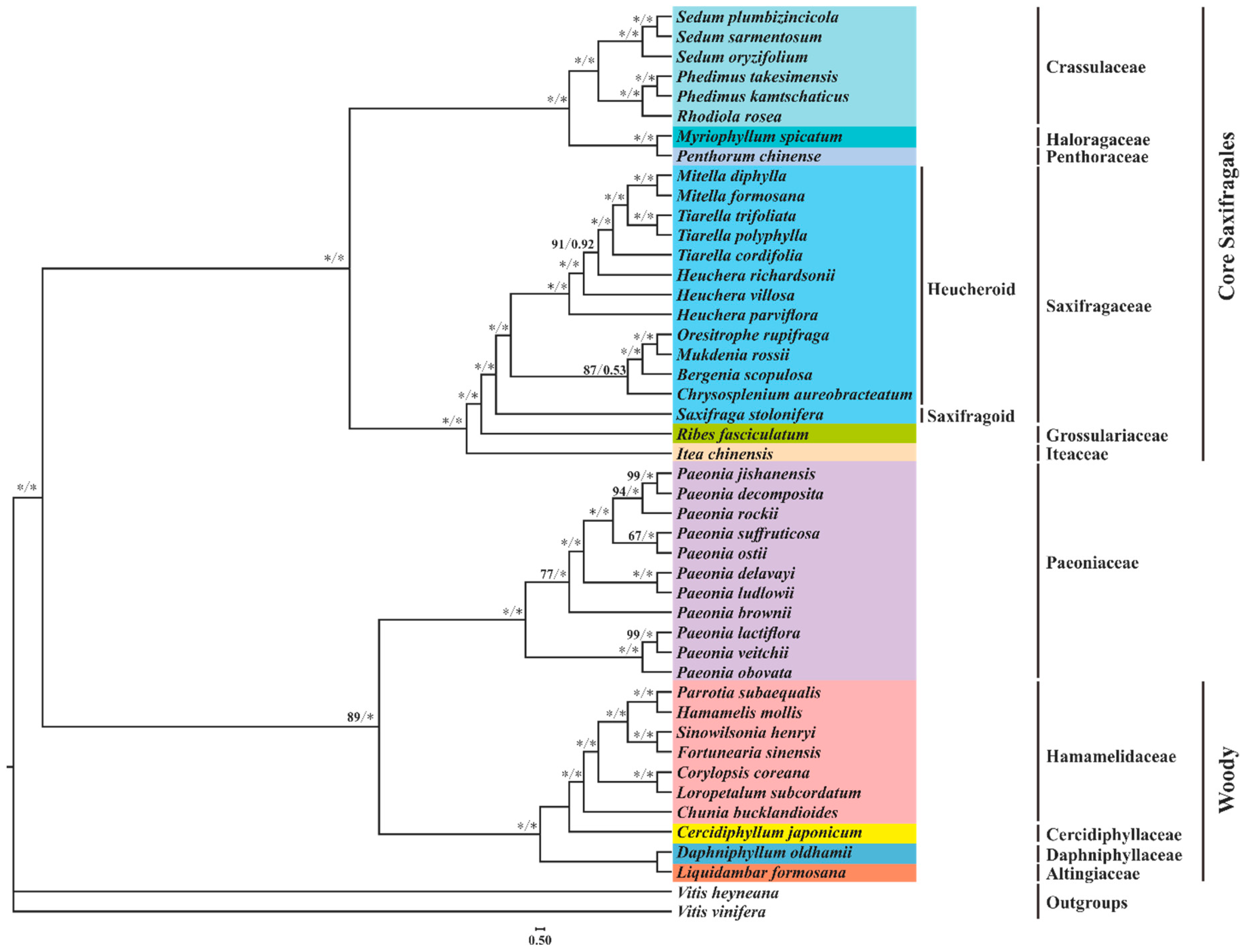
3.3. Phylogenetic Implications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nikulin, V.Y.; Gontcharova, S.B.; Stephenson, R.; Gontcharov, A.A. Phylogenetic relationships between Sedum L. and related genera (Crassulaceae) based on ITS rDNA sequence comparisons. Flora 2016, 224, 218–229. [Google Scholar] [CrossRef]
- Thiede, J.; Eggli, U. Crassulaceae. In The Families and Genera of Vascular Plants; Kubitzki, K., Ed.; Springer: Hamburg, Germany, 2007; pp. 83–118. [Google Scholar]
- Zhang, J.; Meng, S.; Wen, J.; Rao, G. Phylogenetic relationships and character evolution of Rhodiola (Crassulaceae) based on nuclear ribosomal ITS and plastid trnL-F and psbA-trnH sequences. Syst. Bot. 2014, 39, 441–451. [Google Scholar] [CrossRef]
- Berger, A. Crassulaceae. In Die Natürlichen Pflanzenfamilien, 2nd ed.; Engler, A., Prantl, K., Eds.; Verlag Wilhelm Engelmann: Leipzig, Germany, 1930; pp. 352–483. [Google Scholar]
- Borissova, A. Crassulaceae. Flora USSR 1939, 9, 8–134. [Google Scholar]
- Ohba, H. Generic and infrageneric classification of the Old World Sedoideae (Crassulaceae). J. Fac. Sci. Univ. Tokyo 3 1978, 12, 138–198. [Google Scholar]
- Dong, W.; Xu, C.; Cheng, T.; Lin, K.; Zhou, S. Sequencing angiosperm plastid genomes made easy: A complete set of universal primers and a case study on the phylogeny of Saxifragales. Genome Biol. Evol. 2013, 5, 989–997. [Google Scholar] [CrossRef]
- Dong, W.; Xu, C.; Wu, P.; Cheng, T.; Yu, J.; Zhou, S.; Hong, D.-Y. Resolving the systematic positions of enigmatic taxa: Manipulating the chloroplast genome data of Saxifragales. Mol. Phylogenet. Evol. 2018, 126, 321–330. [Google Scholar] [CrossRef]
- Fishbein, M.; Hibsch-Jetter, C.; Soltis, D.E.; Hufford, L. Phylogeny of Saxifragales (angiosperms, eudicots): Analysis of a rapid, ancient radiation. Syst. Biol. 2001, 50, 817–847. [Google Scholar] [CrossRef]
- Fishbein, M.; Soltis, D.E. Further resolution of the rapid radiation of Saxifragales (angiosperms, eudicots) supported by mixed-model Bayesian analysis. Syst. Bot. 2004, 29, 883–891. [Google Scholar] [CrossRef]
- Jian, S.; Soltis, P.S.; Gitzendanner, M.A.; Moore, M.J.; Li, R.; Hendry, T.A.; Qiu, Y.-L.; Dhingra, A.; Bell, C.D.; Soltis, D.E. Resolving an ancient, rapid radiation in Saxifragales. Syst. Biol. 2008, 57, 38–57. [Google Scholar] [CrossRef]
- Soltis, D.E.; Mort, M.E.; Latvis, M.; Mavrodiev, E.V.; O’Meara, B.C.; Soltis, P.S.; Burleigh, J.G.; Rubio de Casas, R. Phylogenetic relationships and character evolution analysis of Saxifragales using a supermatrix approach. Am. J. Bot. 2013, 100, 916–929. [Google Scholar] [CrossRef]
- Cui, Y.; Nie, L.; Sun, W.; Xu, Z.; Wang, Y.; Yu, J.; Song, J.; Yao, H. Comparative and Phylogenetic Analyses of Ginger (Zingiber officinale) in the Family Zingiberaceae Based on the Complete Chloroplast Genome. Plants 2019, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, Z.; Huang, S.; An, W.; Li, J.; Zheng, X. Comprehensive Analysis of Rhodomyrtus tomentosa Chloroplast Genome. Plants 2019, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, Y.; An, W.; Zheng, X.; Huang, S.; Liang, L. Sequencing and Structural Analysis of the Complete Chloroplast Genome of the Medicinal Plant Lycium chinense Mill. Plants 2019, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Eickbush, T.H.; Eickbush, D.G. Finely orchestrated movements: Evolution of the ribosomal RNA genes. Genetics 2007, 175, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.Z.; Wang, S.S.; Ding, X.; Wang, X.Q. Structural evolution of nrDNA ITS in Pinaceae and its phylogenetic implications. Mol. Phylogenet. Evol. 2007, 44, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Rooney, A.P. Concerted and birth-and-death evolution of multigene families. Annu. Rev. Genet. 2005, 39, 121–152. [Google Scholar] [CrossRef] [PubMed]
- Delp, G.; Kössel, H. rRNAs and rRNA genes of plastids. In Cell Culture and Somatic Cell Genetics of Plants; Bogorad, L., Vasil, I.K., Eds.; Academic Press: New York, NY, USA, 1991; pp. 139–167. [Google Scholar]
- Turmel, M.; Otis, C.; Lemieux, C. The complete chloroplast DNA sequence of the green alga Nephroselmis olivacea: Insights into the architecture of ancestral chloroplast genomes. Proc. Natl. Acad. Sci. USA 1999, 96, 10248–10253. [Google Scholar] [CrossRef]
- De Cambiaire, J.-C.; Otis, C.; Lemieux, C.; Turmel, M. The complete chloroplast genome sequence of the chlorophycean green alga Scenedesmus obliquus reveals a compact gene organization and a biased distribution of genes on the two DNA strands. BMC Evol. Biol. 2006, 6, 37. [Google Scholar] [CrossRef]
- Turmel, M.; Otis, C.; Lemieux, C. The chloroplast genome sequence of Chara vulgaris sheds new light into the closest green algal relatives of land plants. Mol. Biol. Evol. 2006, 23, 1324–1338. [Google Scholar] [CrossRef]
- Pombert, J.-F.; Otis, C.; Lemieux, C.; Turmel, M. The chloroplast genome sequence of the green alga Pseudendoclonium akinetum (Ulvophyceae) reveals unusual structural features and new insights into the branching order of chlorophyte lineages. Mol. Biol. Evol. 2005, 22, 1903–1918. [Google Scholar] [CrossRef]
- Yan, D.; Wang, Y.; Murakami, T.; Shen, Y.; Gong, J.; Jiang, H.; Smith, D.R.; Pombert, J.-F.; Dai, J.; Wu, Q. Auxenochlorella protothecoides and Prototheca wickerhamii plastid genome sequences give insight into the origins of non-photosynthetic algae. Sci. Rep. 2015, 5, 14465. [Google Scholar] [CrossRef]
- Ren, T.; Yang, Y.; Zhou, T.; Liu, Z.-L. Comparative plastid genomes of primula species: Sequence divergence and phylogenetic relationships. Int. J. Mol. Sci. 2018, 19, 1050. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Zheng, W.; Han, K.; Zeng, S.; Zhao, J.; Liu, Z.-L. Characterization of the complete chloroplast genome sequence of Lysionotus pauciflorus (Gesneriaceae). Conserv. Genet. Resour. 2017, 9, 185–187. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Zhou, S.; Guo, F.; Bi, D.; Guo, X.; Baker, A.; Smith, J.; Luo, Y. Sedum plumbizincicola XH Guo et SB Zhou ex LH Wu (Crassulaceae): A new species from Zhejiang Province, China. Plant Syst. Evol. 2013, 299, 487–498. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data (2010). Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 23 August 2019).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-J.; Yu, W.-B.; Yang, J.-B.; Song, Y.; Yi, T.-S.; Li, D.-Z. GetOrganelle: A simple and fast pipeline for de novo assembly of a complete circular chloroplast genome using genome skimming data. bioRxiv 2018. [Google Scholar] [CrossRef]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq–versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [PubMed]
- Cannone, J.J.; Subramanian, S.; Schnare, M.N.; Collett, J.R.; D’Souza, L.M.; Du, Y.; Feng, B.; Lin, N.; Madabusi, L.V.; Müller, K.M. The comparative RNA web (CRW) site: An online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. BMC Bioinform. 2002, 3, 2. [Google Scholar]
- Silvestro, D.; Michalak, I. raxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2012, 12, 335–337. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hu, R.; Yin, H.; Jenkins, J.; Shu, S.; Tang, H.; Liu, D.; Weighill, D.A.; Yim, W.C.; Ha, J. The Kalanchoë genome provides insights into convergent evolution and building blocks of crassulacean acid metabolism. Nat. Commun. 2017, 8, 1899. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Duan, N.; Wang, M.-B.; Liu, B.-B. Complete chloroplast genome of Cercidiphyllum japonicum (Cercidiphyllaceae), a tertiary relic endangered tree. Conserv. Genet. Resour. 2018, 11, 113–115. [Google Scholar] [CrossRef]
- Seo, H.-S.; Kim, S.-C. The complete chloroplast genome sequence of Phedimus Kamtschaticus (Crassulaceae) in Korea. Mitochondrial DNA Part B 2018, 3, 227–228. [Google Scholar] [CrossRef]
- Zhao, D.-N.; Zhang, J.-Q. Characterization of the complete chloroplast genome of the traditional medicinal plants Rhodiola rosea (Saxifragales: Crassulaceae). Mitochondrial DNA Part B 2018, 3, 753–754. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, Y.; Dai, J.; Wang, F.; Wu, W.; Fan, Q.; Zhou, R.; Ng, W.L. The chloroplast genome of Chunia bucklandioides (Hamamelidaceae): A rare tree endemic to Hainan, China. Conserv. Genet. Resour. 2018. [Google Scholar] [CrossRef]
- Choi, K.S.; Ha, Y.-H.; Jeong, K.S.; Joo, M.; Chang, K.S.; Choi, K. The complete chloroplast genome of Corylopsis coreana (Hamamelidaceae). Conserv. Genet. Resour. 2019, 11, 291–293. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, T.-W.; Zhao, N.; Li, T.; Liu, T.-J.; Yan, H.-F. Characterization of the complete plastid genome of an endangered species Fortunearia sinensis (Hamamelidaceae). Mitochondrial DNA Part B 2019, 4, 1432–1434. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, H.; Dong, J.; Gong, W.; Li, P.; Wang, Z. The complete chloroplast genome of Loropetalum subcordatum, a national key protected species in China. Conserv. Genet. Resour. 2018. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Q.; Sun, L.; Ng, W.L.; Zhou, R.; Wu, W. The chloroplast genome of Paeonia decomposita (Paeoniaceae), an endangered wild tree peony from Sichuan, China. Conserv. Genet. Resour. 2019, 11, 59–61. [Google Scholar] [CrossRef]
- Li, H.; Guo, Q.; Zheng, W. Characterization of the complete chloroplast genomes of two sister species of Paeonia: Genome structure and evolution. Conserv. Genet. Resour. 2018, 10, 209–212. [Google Scholar] [CrossRef]
- Zhou, X.-J.; Song, L.-L.; Peng, Z.-F.; Sun, S.-S.; Ya, H.-Y.; Cheng, Y.-W.; Zhang, Y.-Z. The complete chloroplast genome sequence of Paeonia jishanensis (Paeoniaceae), a rare wild tree peony. Mitochondrial DNA Part B 2019, 4, 503–504. [Google Scholar]
- Samigullin, T.H.; Logacheva, M.D.; Degtjareva, G.V.; Efimov, S.V.; Terentieva, E.I.; Vallejo-Roman, C.M. Complete plastome sequence of Paeonia lactiflora Pall. (Paeoniaceae: Saxifragales). Mitochondrial DNA Part B 2018, 3, 1110–1111. [Google Scholar] [CrossRef]
- Bai, G.; Guo, H.; Zhao, N.; Li, S.; Zhang, Y. The complete chloroplast genome of Paeonia rockii (Paeoniaceae), an endangered endemic species to China. Conserv. Genet. Resour. 2018, 10, 453–456. [Google Scholar] [CrossRef]
- Bai, G.; Fang, L.; Li, S.; Cui, X. Characterization of the complete chloroplast genome sequence of Bergenia scopulosa (Saxifragales: Saxifragaceae). Conserv. Genet. Resour. 2018, 10, 363–366. [Google Scholar]
- Kim, Y.-I.; Lee, J.-H.; Kim, Y.-D. The complete chloroplast genome of a Korean endemic plant Chrysosplenium aureobracteatum YI Kim & YD Kim (Saxifragaceae). Mitochondrial DNA Part B 2018, 3, 380–381. [Google Scholar]
- Folk, R.A.; Mandel, J.R.; Freudenstein, J.V. A protocol for targeted enrichment of intron--containing sequence markers for recent radiations: A phylogenomic example from Heuchera (Saxifragaceae). Appl. Plant Sci. 2015, 3, 1500039. [Google Scholar]
- Liu, L.; Wang, Y.; He, P.; Li, P.; Lee, J.; Soltis, D.E.; Fu, C. Chloroplast genome analyses and genomic resource development for epilithic sister genera Oresitrophe and Mukdenia (Saxifragaceae), using genome skimming data. BMC Genom. 2018, 19, 235. [Google Scholar] [CrossRef]
- Keane, T.M.; Creevey, C.J.; Pentony, M.M.; Naughton, T.J.; Mclnerney, J.O. Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evol. Biol. 2006, 6, 29. [Google Scholar] [CrossRef]
- Millen, R.S.; Olmstead, R.G.; Adams, K.L.; Palmer, J.D.; Lao, N.T.; Heggie, L.; Kavanagh, T.A.; Hibberd, J.M.; Gray, J.C.; Morden, C.W. Many parallel losses of infA from chloroplast DNA during angiosperm evolution with multiple independent transfers to the nucleus. Plant Cell 2001, 13, 645–658. [Google Scholar]
- Jansen, R.K.; Ruhlman, T.A. Plastid genomes of seed plants. In Genomics of Chloroplasts and Mitochondria, Advances in Photosynthesis and Respiration; Bock, R., Knoop, V., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 103–126. [Google Scholar]
- Liu, J.; Qi, Z.-C.; Zhao, Y.-P.; Fu, C.-X.; Xiang, Q.-Y.J. Complete cpDNA genome sequence of Smilax china and phylogenetic placement of Liliales-Influences of gene partitions and taxon sampling. Mol. Phylogenet. Evol. 2012, 64, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Fujimoto, M.; Arimura, S.-I.; Murata, J.; Tsutsumi, N.; Kadowaki, K.-I. Loss of the rpl32 gene from the chloroplast genome and subsequent acquisition of a preexisting transit peptide within the nuclear gene in Populus. Gene 2007, 402, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jansen, R.K.; Park, S. Complete plastome sequence of Thalictrum coreanum (Ranunculaceae) and transfer of the rpl32 gene to the nucleus in the ancestor of the subfamily Thalictroideae. BMC Plant Biol. 2015, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, H.-W.; Kim, Y.-K.; Sohn, J.-Y.; Cheon, S.-H.; Kim, K.-J. The complete plastome of tropical fruit Garcinia mangostana (Clusiaceae). Mitochondrial DNA Part B 2017, 2, 722–724. [Google Scholar] [CrossRef]
- Dong, W.; Xu, C.; Cheng, T.; Zhou, S. Complete chloroplast genome of Sedum sarmentosum and chloroplast genome evolution in Saxifragales. PLoS ONE 2013, 8, e77965. [Google Scholar] [CrossRef]
- Deng, J.-B.; Drew, B.T.; Mavrodiev, E.V.; Gitzendanner, M.A.; Soltis, P.S.; Soltis, D.E. Phylogeny, divergence times, and historical biogeography of the angiosperm family Saxifragaceae. Mol. Phylogenet. Evol. 2015, 83, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Downie, S.R.; Olmstead, R.G.; Zurawski, G.; Soltis, D.E.; Soltis, P.S.; Watson, J.C.; Palmer, J.D. Six independent losses of the chloroplast DNA rpl2 intron in dicotyledons: Molecular and phylogenetic implications. Evolution 1991, 45, 1245–1259. [Google Scholar] [CrossRef]
- Gu, C.; Ma, L.; Wu, Z.; Chen, K.; Wang, Y. Comparative analyses of chloroplast genomes from 22 Lythraceae species: Inferences for phylogenetic relationships and genome evolution within Myrtales. BMC Plant Biol. 2019, 19, 281. [Google Scholar] [CrossRef]
- Gu, C.; Tembrock, L.R.; Johnson, N.G.; Simmons, M.P.; Wu, Z. The complete plastid genome of Lagerstroemia fauriei and loss of rpl2 intron from Lagerstroemia (Lythraceae). PLoS ONE 2016, 11, e0150752. [Google Scholar] [CrossRef]
- Gu, C.; Tembrock, L.R.; Wu, Z. Chloroplast genome sequence of Lagerstroemia guilinensis (Lythraceae, Myrtales), a species endemic to the Guilin limestone area in Guangxi Province, China. Genome Announc. 2016, 4, e00341-16. [Google Scholar] [CrossRef]
- Nevill, P.G.; Howell, K.A.; Cross, A.T.; Williams, A.V.; Zhong, X.; Tonti-Filippini, J.; Boykin, L.M.; Dixon, K.W.; Small, I. Plastome-wide rearrangements and gene losses in carnivorous Droseraceae. Genome Biol. Evol. 2019, 11, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Njuguna, A.W.; Li, Z.-Z.; Saina, J.K.; Munywoki, J.M.; Gichira, A.W.; Gituru, R.W.; Wang, Q.-F.; Chen, J.-M. Comparative analyses of the complete chloroplast genomes of nymphoides and menyanthes species (menyanthaceae). Aquat. Bot. 2019, 156, 73–81. [Google Scholar] [CrossRef]
- Rabah, S.O.; Lee, C.; Hajrah, N.H.; Makki, R.M.; Alharby, H.F.; Alhebshi, A.M.; Sabir, J.S.; Jansen, R.K.; Ruhlman, T.A. Plastome sequencing of ten nonmodel crop species uncovers a large insertion of mitochondrial DNA in cashew. Plant Genome 2017, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sloan, D.B.; Alverson, A.J.; Wu, M.; Palmer, J.D.; Taylor, D.R. Recent acceleration of plastid sequence and structural evolution coincides with extreme mitochondrial divergence in the angiosperm genus Silene. Genome Biol. Evol. 2012, 4, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Wicke, S.; Wang, H.; Jin, J.-J.; Chen, S.-Y.; Zhang, S.-D.; Li, D.-Z.; Yi, T.-S. Plastid genome evolution in the early-diverging legume subfamily Cercidoideae (Fabaceae). Front. Plant Sci. 2018, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Wicke, S.; Müller, K.F.; de Pamphilis, C.W.; Quandt, D.; Wickett, N.J.; Zhang, Y.; Renner, S.S.; Schneeweiss, G.M. Mechanisms of functional and physical genome reduction in photosynthetic and nonphotosynthetic parasitic plants of the broomrape family. Plant Cell 2013, 25, 3711–3725. [Google Scholar] [CrossRef]
- Xu, C.; Dong, W.; Li, W.; Lu, Y.; Xie, X.; Jin, X.; Shi, J.; He, K.; Suo, Z. Comparative analysis of six Lagerstroemia complete chloroplast genomes. Front. Plant Sci. 2017, 8, 15. [Google Scholar] [CrossRef]
- Xue, Z.-Q.; Xue, J.-H.; Victorovna, K.M.; Ma, K.-P. The complete chloroplast DNA sequence of Trapa maximowiczii Korsh. (Trapaceae), and comparative analysis with other Myrtales species. Aquat. Bot. 2017, 143, 54–62. [Google Scholar] [CrossRef]
- Zhang, J.; Ruhlman, T.A.; Sabir, J.S.; Blazier, J.C.; Weng, M.-L.; Park, S.; Jansen, R.K. Coevolution between nuclear-encoded DNA replication, recombination, and repair genes and plastid genome complexity. Genome Biol. Evol. 2016, 8, 622–634. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, D.; Han, R.; Wang, L.; Qin, P. The complete chloroplast genome sequence of the shrubby cinquefoil Dasiphora fruticosa (Rosales: Rosaceae). Conserv. Genet. Resour. 2018, 10, 675–678. [Google Scholar] [CrossRef]
- Fink, G.R. Pseudogenes in yeast? Cell 1987, 49, 5–6. [Google Scholar] [CrossRef]
- Dujon, B. Group I introns as mobile genetic elements: Facts and mechanistic speculations—A review. Gene 1989, 82, 91–114. [Google Scholar] [CrossRef]
- Nickrent, D.L.; Duff, R.J.; Konings, D. Structural analyses of plastid-derived 16S rRNAs in holoparasitic angiosperms. Plant Mol. Biol. 1997, 34, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Gutell, R.R. Collection of small subunit (16S-and 16S-like) ribosomal RNA structures: 1994. Nucleic Acids Res. 1994, 22, 3502–3507. [Google Scholar] [CrossRef] [PubMed]
- Gutell, R.R. Comparative studies of RNA: Inferring higher-order structure from patterns of sequence variation. Curr. Opin. Struct. Biol. 1993, 3, 313–322. [Google Scholar] [CrossRef]
- Zeiner, M.; Cindrić, I.J. Review–trace determination of potentially toxic elements in (medicinal) plant materials. Anal. Methods 2017, 9, 1550–1574. [Google Scholar] [CrossRef]
- Kumar, N.; Soni, H. Characterization of heavy metals in vegetables using inductive coupled plasma analyzer (ICPA). J. Appl. Sci. Environ. Manag. 2007, 11, 75–79. [Google Scholar] [CrossRef]
- Gutell, R.R.; Gray, M.W.; Schnare, M.N. A compilation of large subunit (23S and 23S-like) ribosomal RNA structures: 1993. Nucleic Acids Res. 1993, 21, 3055. [Google Scholar] [CrossRef]
- MacKay, R.M. The origin of plant chloroplast 4.5 S ribosomal RNA. FEBS Lett. 1981, 123, 17–18. [Google Scholar] [CrossRef] [Green Version]
- Bowman, C.M.; Dyer, T. 4.5 S ribonucleic acid, a novel ribosome component in the chloroplasts of flowering plants. Biochem. J. 1979, 183, 605–613. [Google Scholar] [CrossRef]
- Whitfeld, P.R.; Leaver, C.J.; Bottomley, W.; Atchison, B. Low-molecular-weight (4.5 S) ribonucleic acid in higher-plant chloroplast ribosomes. Biochem. J. 1978, 175, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, I.; Pieler, T.; Subramanian, A.R.; Erdmann, V.A. Nucleotide sequence and secondary structure analysis of spinach chloroplast 4.5 S RNA. J. Biol. Chem. 1982, 257, 12924–12928. [Google Scholar] [PubMed]
- Takaiwa, F.; Kusuda, M.; Sugiura, M. The nucleotide sequence of chloroplast 4.5 S rRNA from a fern, Dryopteris acuminata. Nucleic Acids Res. 1982, 10, 2257–2260. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.; Kdssel, H. The rRNA operon from Zea mays chloroplasts: Nucleotide sequence of 23S rDNA and its homology with E. coli 23S rDNA. Nucleic Acids Res. 1981, 9, 2853–2869. [Google Scholar] [CrossRef]
- Clark, C.G.; Gerbi, S.A. Ribosomal RNA evolution by fragmentation of the 23S progenitor: Maturation pathway parallels evolutionary emergence. J. Mol. Evol. 1982, 18, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, M.; Zielezinski, A.; Barciszewski, J.; Erdmann, V.A.; Karlowski, W.M. 5SRNAdb: An information resource for 5S ribosomal RNAs. Nucleic Acids Res. 2015, 44, D180–D183. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, M.; Barciszewska, M.Z.; Erdmann, V.A.; Barciszewski, J. 5S ribosomal RNA database. Nucleic Acids Res. 2002, 30, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Delihas, N.; Andersen, J. Generalized structures of the 5S ribosomal RNAs. Nucleic Acids Res. 1982, 10, 7323–7344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Audren, H.; Bisanz-Seyer, C.; Briat, J.-F.; Mache, R. Structure and transcription of the 5S rRNA gene from spinach chloroplasts. Curr. Genet. 1987, 12, 263–269. [Google Scholar] [CrossRef]
- Hawley, D.K.; McClure, W.R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983, 11, 2237–2255. [Google Scholar] [CrossRef]
- Strittmauer, G.; Kössel, H. Cotranscription and processing of 23S, 4.5 S and 5S rRNA in chloroplasts from Zea mays. Nucleic Acids Res. 1984, 12, 7633–7647. [Google Scholar] [CrossRef] [PubMed]
- Leal-Klevezas, D.S.; Martı́nez-Soriano, J.P.; Nazar, R.N. Cotranscription of 5S rRNA–tRNAArg (ACG) from Brassica napus chloroplasts and processing of their intergenic spacer. Gene 2000, 253, 303–311. [Google Scholar] [CrossRef]
- Chase, M.W.; Christenhusz, M.; Fay, M.; Byng, J.; Judd, W.S.; Soltis, D.; Mabberley, D.; Sennikov, A.; Soltis, P.S.; Stevens, P.F. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [Green Version]
- Moore, M.J.; Hassan, N.; Gitzendanner, M.A.; Bruenn, R.A.; Croley, M.; Vandeventer, A.; Horn, J.W.; Dhingra, A.; Brockington, S.F.; Latvis, M. Phylogenetic analysis of the plastid inverted repeat for 244 species: Insights into deeper-level angiosperm relationships from a long, slowly evolving sequence region. Int. J. Plant Sci. 2011, 172, 541–558. [Google Scholar] [CrossRef]
- Ohba, H.; Bartholomew, B.M.; Turland, N.J.; Kunjun, F.; Kun-Tsun, F. New combinations in Phedimus (crassulaceae). Novon 2000, 10, 400–402. [Google Scholar] [CrossRef]
- Tkach, N.; Röser, M.; Miehe, G.; Muellner-Riehl, A.N.; Ebersbach, J.; Favre, A.; Hoffmann, M.H. Molecular phylogenetics, morphology and a revised classification of the complex genus Saxifraga (Saxifragaceae). Taxon 2015, 64, 1159–1187. [Google Scholar] [CrossRef]
- Xiang, Q. Molecular Systematics and Biogeography of Cornus L. and Putative Relatives. Ph.D. Thesis, Washington State University, Pullman, WA, USA, 1995. [Google Scholar]
- Soltis, D.E.; Soltis, P.S.; Collier, T.G.; Edgerton, M.L. Chloroplast DNA variation within and among genera of the Heuchera group (Saxifragaceae): Evidence for chloroplast transfer and paraphyly. Am. J. Bot. 1991, 78, 1091–1112. [Google Scholar] [CrossRef]
- Wen, J. Evolution of the eastern Asian and eastern North American disjunct pattern: Insights from phylogenetic studies. Korean J. Plant Taxon. 1998, 28, 63–81. [Google Scholar] [CrossRef]
- Soltis, D.E.; Kuzoff, R.K. Discordance between nuclear and chloroplast phylogenies in the Heuchera group (Saxifragaceae). Evolution 1995, 49, 727–742. [Google Scholar] [CrossRef]
- Kawabe, A.; Nukii, H.; Furihata, H. Exploring the history of chloroplast capture in Arabis using whole chloroplast genome sequencing. Int. J. Mol. Sci. 2018, 19, 602. [Google Scholar] [CrossRef] [PubMed]
- Healey, A.; Lee, D.J.; Furtado, A.; Henry, R.J. Evidence of inter-sectional chloroplast capture in Corymbia among sections Torellianae and Maculatae. Aust. J. Bot. 2018, 66, 369–378. [Google Scholar] [CrossRef]
- Ogishima, M.; Horie, S.; Kimura, T.; Yamashiro, T.; Dohzono, I.; Kawaguchi, L.; Nagano, A.J.; Maki, M. Frequent chloroplast capture among Isodon (Lamiaceae) species in Japan revealed by phylogenies based on variation in chloroplast and nuclear DNA. Plant Spec. Biol. 2019, 24, 127–137. [Google Scholar] [CrossRef]
- Hughes, M.; Peng, C.-I.; Lin, C.-W.; Rubite, R.R.; Blanc, P.; Chung, K.-F. Chloroplast and nuclear DNA exchanges among Begonia sect. Baryandra species (Begoniaceae) from Palawan Island, Philippines, and descriptions of five new species. PLoS ONE 2018, 13, e0194877. [Google Scholar] [CrossRef] [PubMed]
- Olsson, S.; Grivet, D.; Vian, J.C. Species-diagnostic markers in the genus Pinus: Evaluation of the chloroplast regions matK and ycf1. For. Syst. 2018, 27, 2. [Google Scholar] [CrossRef]






| Family | Species | Accession No. | Reference |
|---|---|---|---|
| Altingiaceae | Liquidambar formosana | NC_023092.1 | [7] |
| Cercidiphyllaceae | Cercidiphyllum japonicum | NC_037940.1 | [37] |
| Crassulaceae | Phedimus kamtschaticus | NC_037946.1 | [38] |
| Crassulaceae | Phedimus takesimensis | NC_026065.1 | Unpublished |
| Crassulaceae | Rhodiola rosea | NC_041671.1 | [39] |
| Crassulaceae | Sedum oryzifolium | NC_027837.1 | Unpublished |
| Crassulaceae | Sedum plumbizincicola | MN185459.1 | This study |
| Crassulaceae | Sedum sarmentosum | NC_023085.1 | [7] |
| Daphniphyllaceae | Daphniphyllum oldhamii | NC_037883.1 | [8] |
| Grossulariaceae | Ribes fasciculatum | MH191388.1 | [8] |
| Haloragaceae | Myriophyllum spicatum | NC_037885.1 | [8] |
| Hamamelidaceae | Chunia bucklandioides | NC_041163.1 | [40] |
| Hamamelidaceae | Corylopsis coreana | NC_040141.1 | [41] |
| Hamamelidaceae | Fortunearia sinensis | NC_041487.1 | [42] |
| Hamamelidaceae | Hamamelis mollis | NC_037881.1 | [8] |
| Hamamelidaceae | Loropetalum subcordatum | NC_037694.1 | [43] |
| Hamamelidaceae | Parrotia subaequalis | NC_037243.1 | Unpublished |
| Hamamelidaceae | Sinowilsonia henryi | NC_036069.1 | Unpublished |
| Paeoniaceae | Paeonia brownii | NC_037880.1 | [8] |
| Paeoniaceae | Paeonia decomposita | NC_039425.1 | [44] |
| Paeoniaceae | Paeonia delavayi | NC_035718.1 | [45] |
| Paeoniaceae | Paeonia jishanensis | MG991935.1 | [46] |
| Paeoniaceae | Paeonia lactiflora | NC_040983.1 | [47] |
| Paeoniaceae | Paeonia ludlowii | NC_035623.1 | [45] |
| Paeoniaceae | Paeonia obovata | NC_026076.1 | Unpublished |
| Paeoniaceae | Paeonia ostii | NC_036834.1 | Unpublished |
| Paeoniaceae | Paeonia rockii | NC_037772.1 | [48] |
| Paeoniaceae | Paeonia suffruticosa | NC_037879.1 | [8] |
| Paeoniaceae | Paeonia veitchii | NC_032401.1 | Unpublished |
| Penthoraceae | Penthorum chinense | NC_023086.1 | [7] |
| Iteaceae | Itea chinensis | NC_037884.1 | [8] |
| Saxifragaceae | Bergenia scopulosa | NC_036061.1 | [49] |
| Saxifragaceae | Chrysosplenium aureobracteatum | NC_039740.1 | [50] |
| Saxifragaceae | Heuchera parviflora | KR478645.1 | [51] |
| Saxifragaceae | Heuchera richardsonii | NC_042923.1 | Unpublished |
| Saxifragaceae | Heuchera villosa | NC_042924.1 | Unpublished |
| Saxifragaceae | Mitella diphylla | NC_042925.1 | Unpublished |
| Saxifragaceae | Mitella formosana | NC_042926.1 | Unpublished |
| Saxifragaceae | Mukdenia rossii | NC_037495.1 | Unpublished |
| Saxifragaceae | Oresitrophe rupifraga | NC_037514.1 | [52] |
| Saxifragaceae | Saxifraga stolonifera | NC_037882.1 | [8] |
| Saxifragaceae | Tiarella cordifolia | NC_042927.1 | Unpublished |
| Saxifragaceae | Tiarella polyphylla | NC_042928.1 | Unpublished |
| Saxifragaceae | Tiarella trifoliata | NC_042929.1 | Unpublished |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Zhu, R.; Dong, J.; Bi, D.; Jiang, L.; Zeng, J.; Huang, Q.; Liu, H.; Xu, W.; Wu, L.; et al. Next-Generation Genome Sequencing of Sedum plumbizincicola Sheds Light on the Structural Evolution of Plastid rRNA Operon and Phylogenetic Implications within Saxifragales. Plants 2019, 8, 386. https://doi.org/10.3390/plants8100386
Ding H, Zhu R, Dong J, Bi D, Jiang L, Zeng J, Huang Q, Liu H, Xu W, Wu L, et al. Next-Generation Genome Sequencing of Sedum plumbizincicola Sheds Light on the Structural Evolution of Plastid rRNA Operon and Phylogenetic Implications within Saxifragales. Plants. 2019; 8(10):386. https://doi.org/10.3390/plants8100386
Chicago/Turabian StyleDing, Hengwu, Ran Zhu, Jinxiu Dong, De Bi, Lan Jiang, Juhua Zeng, Qingyu Huang, Huan Liu, Wenzhong Xu, Longhua Wu, and et al. 2019. "Next-Generation Genome Sequencing of Sedum plumbizincicola Sheds Light on the Structural Evolution of Plastid rRNA Operon and Phylogenetic Implications within Saxifragales" Plants 8, no. 10: 386. https://doi.org/10.3390/plants8100386
APA StyleDing, H., Zhu, R., Dong, J., Bi, D., Jiang, L., Zeng, J., Huang, Q., Liu, H., Xu, W., Wu, L., & Kan, X. (2019). Next-Generation Genome Sequencing of Sedum plumbizincicola Sheds Light on the Structural Evolution of Plastid rRNA Operon and Phylogenetic Implications within Saxifragales. Plants, 8(10), 386. https://doi.org/10.3390/plants8100386





