Abstract
In this review, the relevance of the plant species belonging to the Pedicularis L. genus has been considered from different points of view. Particular emphasis was given to phytochemistry and ethnopharmacology, since several classes of natural compounds have been reported within this genus and many of its species are well known to be employed in the traditional medicines of many Asian countries. Some important conclusions on the chemotaxonomic and chemosystematic aspects of the genus have also been provided for the first time. Actually, this work represents the first total comprehensive review on this genus.
1. Systematics
Pedicularis L. is a genus of hemiparasitic plants, originally included in the Scrophulariaceae family but now belonging to the Orobanchaceae family [1]. The rest of the systematic classification is the following: order Scrophulariales, subclass Asteridae, class Magnoliopsida, division Magnoliophyta, superdivision Spermatophyta, subkingdom Tracheobionta. The genus comprises 568 accepted species, 335 synonymous species, and 450 unresolved species [2].
2. Etymology of the Name
The etymology of the genus name is Latin, with the term “pediculus” meaning “louse”, which refers to the fact that, according to an ancient English belief, cattle which grazed on these plants were soon found to be infested with lice [3].
3. Botany
Plants of the genus Pedicularis are generally herbaceous and perennial, with a height which can reach up to 50 cm. Annual or biennial species are quite rare but present. From the morphological standpoint, these species are characterized by big and fleshy roots, often taproots, which contain specific organs (haustoria) for their feeding on the lymph of the near plants. The stem is erect and ascendant and may be simple or branched (Figure 1). The leaves are basal and cauline. The former ones are disposed to form a rosette and are petiolate. The latter ones are opposite, alternated or verticillated, and sessile, instead. Both of these have a lanceolate shape and dentate margins which are rarely entire. Bracts are also present and are similar to the cauline leaves, even if they are smaller (Figure 1). More or less dense terminal spikes generally constitute the inflorescence. The flowers are big, hermaphrodite, zigomorphic, and tetrameric or pentameric. They can be sessile or pedunculated. The floreal formula is X, K(5), [C (2+3), A 2+2], G (2), (superior), capsule. The calyx is gamosepalous, formed by five lobes that may be dentate or not. The corolla is gamopetalous and bilabiate with a cylindrical shape slightly compressed on its sides. Its color ranges from pink to white, passing through red, purple, and yellow. The androecium possesses four didinamous stamens with the filaments well included into the base of the corolla. The anthers are hidden among dense hairs and may be mucronate. The pollen maturation is contemporaneous to the stigma. The ovary is superior, formed by two carpels, and bilocular. The stylus is inserted in the ovary apex and is filiform. The stigma is simple and protruded beyond the corolla hat in order to avoid self-pollination (Figure 1). The fruit is an acuminated bivalve capsule with an oval-lanceolate shape (Figure 1). The seeds are numerous or not and present an angular geometry. Reproduction occurs through pollination by insects or dispersion [4,5].
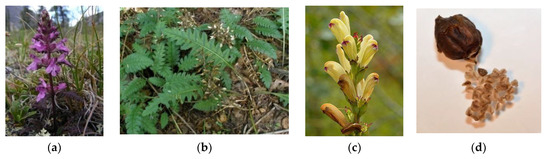
Figure 1.
Examples of the morphological features of Pedicularis species—stem (a), leaves (b), flowers (c), fruits (d) [source Google images].
4. Distribution and Habitat
The species of this genus are distributed in Europe, especially in the mountainous areas of the Mediterranean Basin, and in Northern Asia and America (Figure 2). The highest biodiversity is present in Europe, with about 70 species, India, with about 83 species, and China, with about 350 species, 271 of which are endemic [6,7,8]. In North America, the present species are 36 with two endemisms [9]. These species have been reported in Africa and Australia only as imported plants. The preferred habitat is a temperate mountainous one. The soil must be quite acidic and little- draining. The typical areas where these species can be found are meadows and lawns with little other vegetation [3].
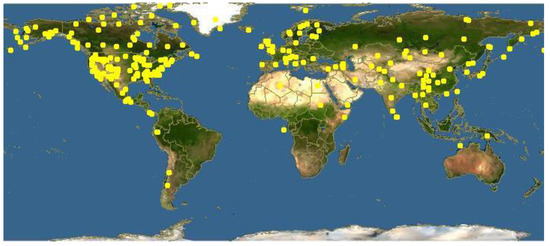
Figure 2.
Worldwide distribution of Pedicularis species [source Google images].
5. Phytochemistry
The genus Pedicularis is a rich source of different secondary metabolites mainly belonging to the polar fraction. In fact, Pedicularis species are poor essential oil producers. Only three species have been investigated as to this aspect, i.e., Pedicularis condensata M.Bieb. (u.n.), P. sibthorpii Boiss. (a.n.), and P. wilhelmsiana Fisch. ex M.Bieb (a.n.). The first one was collected in Turkey and showed the presence of several typical components of essential oils, i.e., more or less oxidized hydrocarbon derivatives and volatile terpenes [10]. The same composition was also observed in the accession of P. wilhelmsiana collected in Iran [11]. Indeed, an important difference was found between the two studied exemplars of P. sibthorpii, both collected in Iran but in two different regions. In fact, the work by Khodaie et al. [12] did not absolutely evidence the presence of sesquiterpenes, while the work by Morteza-Semnani et al. [13] reported these constituents in high amounts, representing 35.4% of all the identified components. This may actually have been explained by the different environmental growth conditions of the two studied species, which, once again, highlight how essential oil composition is greatly affected by external factors and does not depend only on genetics [14].
Among the polar fraction metabolites, several classes of natural compounds were found, i.e., fatty acids, alkaloids, steroids, lignans, neo-lignans, tannins, ionones, phenylpropanoid glycosides, phenylethanoid glycosides, flavonoids, xanthones, iridoids, seco-iridoids, phenyl-glycosides, organic acids, polyols, saccharides, and amino acids.
Table 1 reports on the components identified in all studied Pedicularis species as reported in literature, according to the species.

Table 1.
Phytochemical Compounds Reported in the Studied Pedicularis Species.
As Table 1 clearly shows, only 59 species have been studied for their phytochemical profiles, and, out of these, 12 have been studied only preliminarily, evidencing the presence of some classes of natural compounds but not the specific compounds.
The highest amounts of identified compounds have been recorded in 14 species, i.e., P. artselaeri, P. chinensis, P. decora, P. densispica, P. dolichocymba, P. kansuensis, P. longiflora, P. longiflora var. tubiformis, P. muscicola, P. rex, P. striata, P. torta, P. tricolor, and P. verticillata, while the lowest amounts have been recorded in 6 species, i.e., P. acmodonta, P. bracteosa, P. comosa, P. grayi, P. sarawchanica, and P. semibarbata. All the other species have been shown to biosynthesize metabolites in medium amounts. In only two cases, the data reported in literature have not specified the organs of the plant species that were studied, i.e., P. acmodonta and P. dolichorrhiza.
In general, the studied organs of the plants have been the aerial parts, the leaves, the flowers, or the whole plant, besides a few exceptions, such as P. chinensis and P. grayi, where the roots have been analyzed, and P. sarawchanica, where the fruits have been analyzed.
Indeed, for what concerns the other accepted, synonymous, and unresolved named species, no phytochemical data or even no data at allare reported in literature.
Table 2 reports, instead, on the components identified in all the studied Pedicularis species, as reported in literature, according to the compound.

Table 2.
Distribution of the Phytochemicals in the Various Pedicularis Species.
As Table 2 clearly shows, most of the phytochemicals identified in the Pedicularis genus belong to the class of natural metabolites known as iridoids. Phenylethanoid glycosides represent the second major class in this context. On the other hand, only one compound belonging to each of the natural classes of alkanes, fatty acids, and coumarins has been isolated from Pedicularis spp.
The iridoid acucubin and the phenylethanoid glycoside verbascoside are the two most common compounds in the entire genus, whereas some cases of specific compounds evidenced in only one species have also been observed.
As concerns the rest, the presence of other classes of natural metabolites has been shown to be at a medium level, along with their occurrence within the Pedicularis genus.
The structures of the majority of the identified compounds in Pedicularis species are reported in the figures below (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15, Figure 16, Figure 17, Figure 18, Figure 19, Figure 20 and Figure 21).
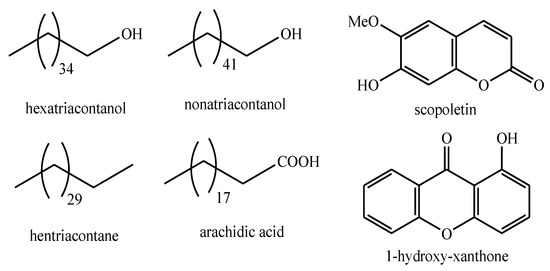
Figure 3.
Fatty acids, alkanes, alkyl alcohols, coumarins, and xanthones identified in Pedicularis species.
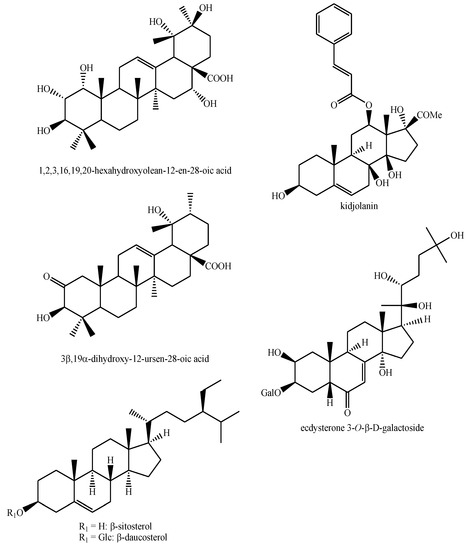
Figure 4.
Terpenoids identified in Pedicularis species.
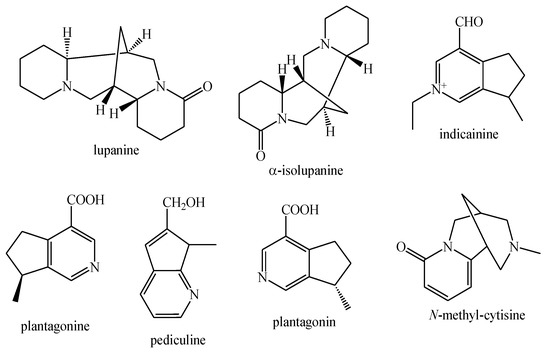
Figure 5.
Alkaloids identified in Pedicularis species—part 1.
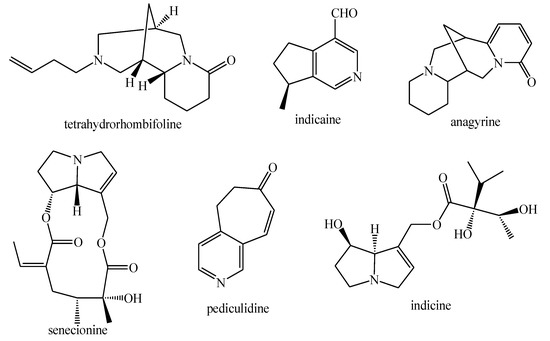
Figure 6.
Alkaloids identified in Pedicularis species—part 2.
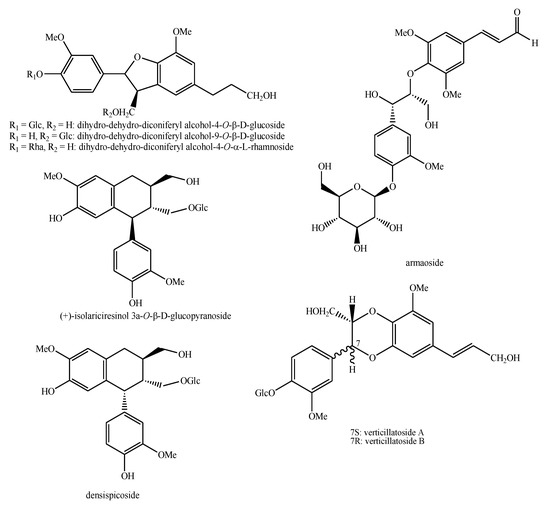
Figure 7.
Lignans and neo-lignans identified in Pedicularis species—part 1.
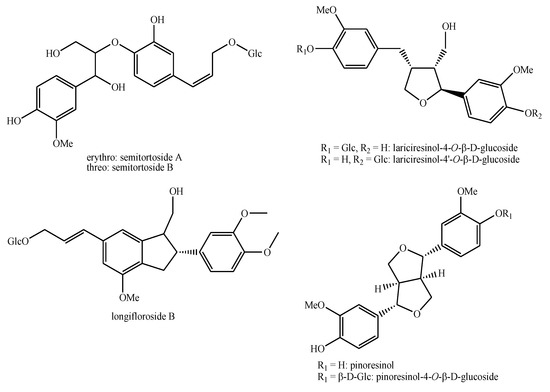
Figure 8.
Lignans and neo-lignans identified in Pedicularis species—part 2.
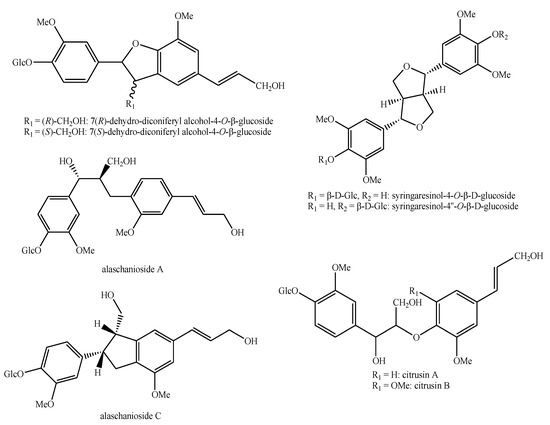
Figure 9.
Lignans and neo-lignans identified in Pedicularis species—part 3.
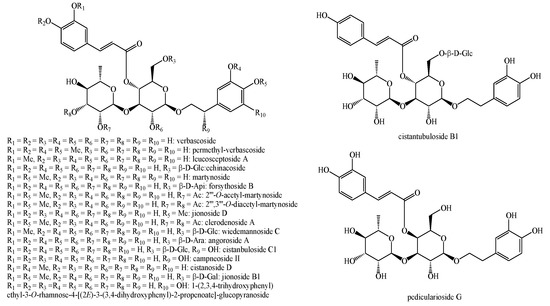
Figure 10.
Phenylethanoid glycosides identified in Pedicularis species—part 1.
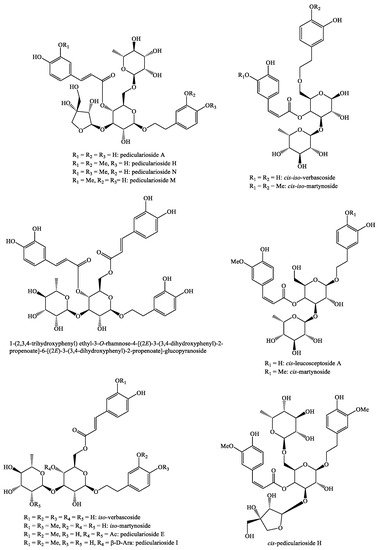
Figure 11.
Phenylethanoid glycosides identified in Pedicularis species—part 2.
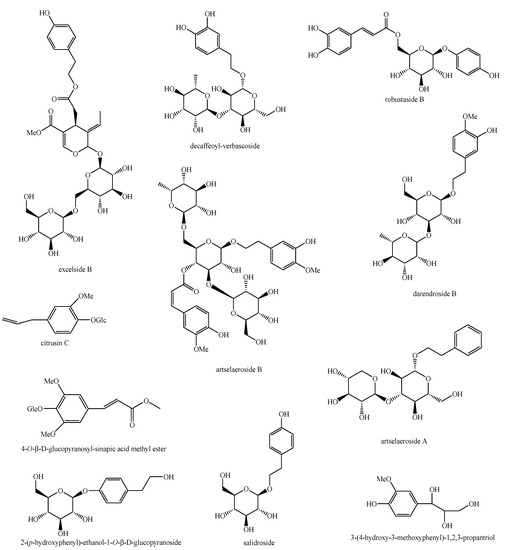
Figure 12.
Phenylethanoid glycosides identified in Pedicularis species—part 3.
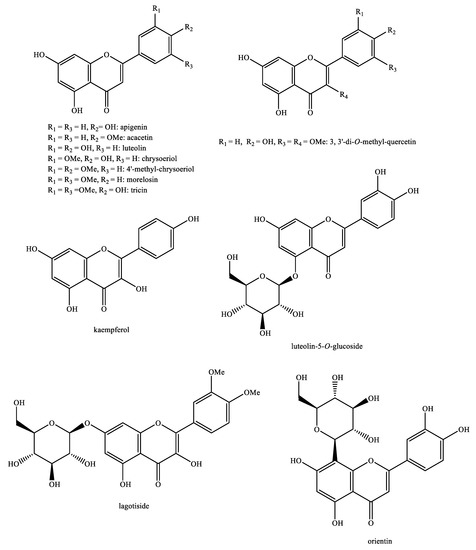
Figure 13.
Flavonoids identified in Pedicularis species—part 1.
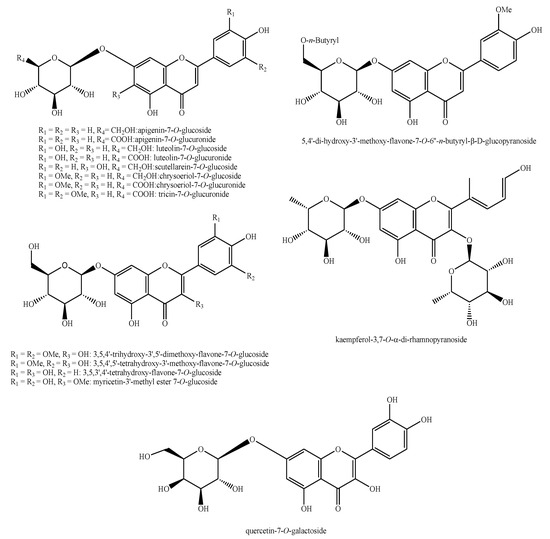
Figure 14.
Flavonoids identified in Pedicularis species—part 2.
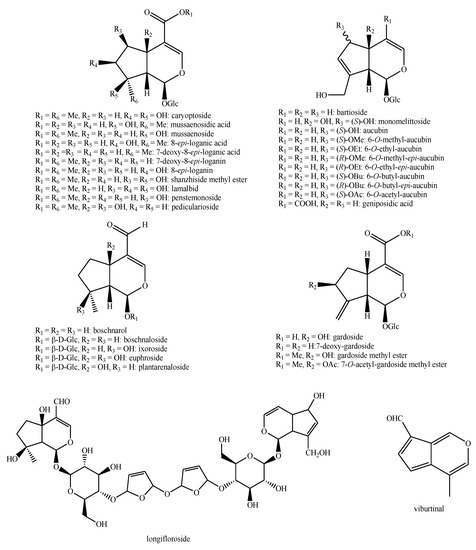
Figure 15.
Iridoids identified in Pedicularis species—part 1.
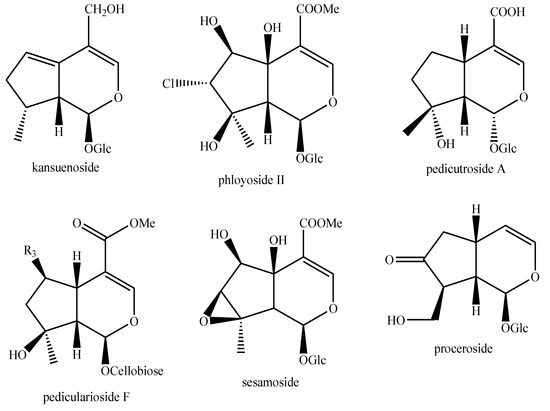
Figure 16.
Iridoids identified in Pedicularis species—part 2.
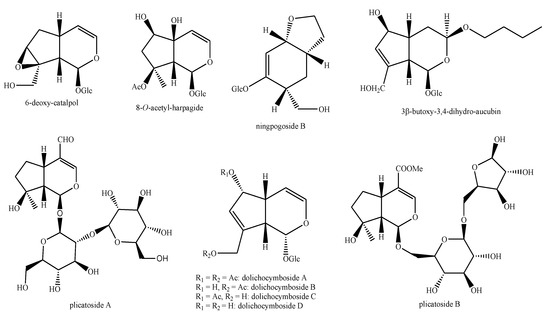
Figure 17.
Iridoids identified in Pedicularis species—part 3.
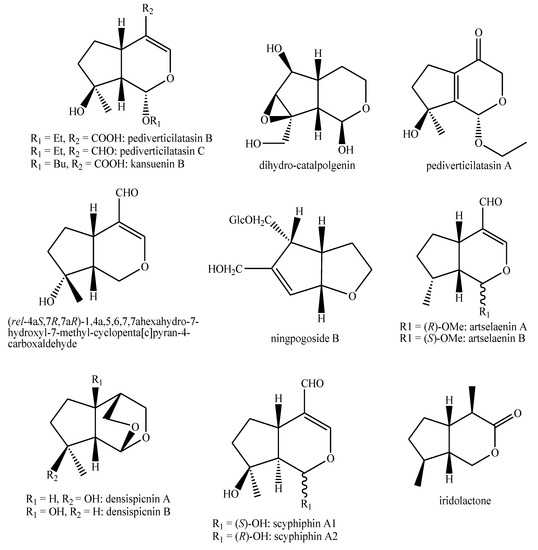
Figure 18.
Iridoids identified in Pedicularis species—part 4.
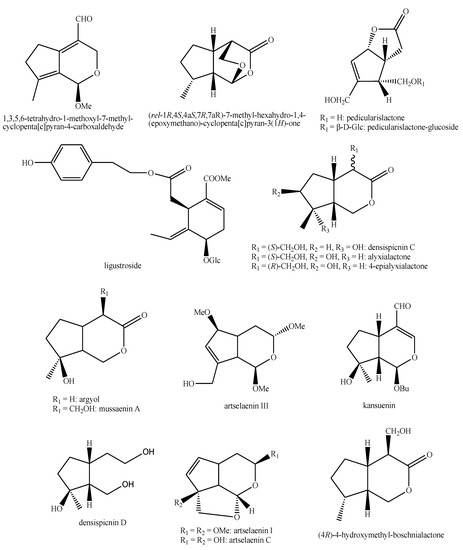
Figure 19.
Iridoids identified in Pedicularis species—part 5.
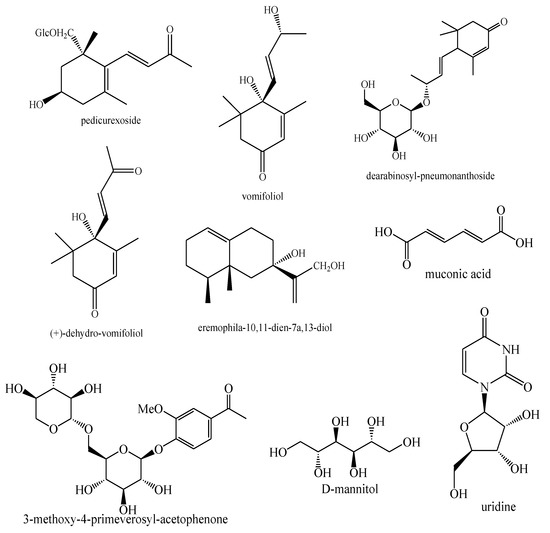
Figure 20.
Other compounds identified in Pedicularis species—part 1.
Figure 21.
Other compounds identified in Pedicularis species—part 2.
6. Corollary to Phytochemistry
After visualization of the relative structures of the identified compounds in Pedicularis spp., two important elements must be observed and highlighted.
The first one concerns the compound found in P. kansuensis by Zhang et al. [50]. According to the structure, the compound should not be named as 1,2,3,16,19,20-hexahydroxyolean-12-en-28-oic acid, but rather as 1,2,3,16,19,20-hexahydroxy-12-ursen-28-oic acid on the basis of the vicinal dimethyl functionalization in positions 19,20 of the pentacyclic triterpene skeleton, which indicates it as an ursane and not an oleane. A similar observation is valid for the 1-(2,3,4-trihydroxyphenyl)ethyl-3-O-rhamnose-4-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoate]-glucopyranoside and 1-(2,3,4-trihydroxyphenyl)ethyl-3-O-rhamnose-4-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoate]-6-[(2E)-3-(3,4-dihydroxy-phenyl)-2-propenoate]-glucopyranoside identified in P. kansuesnsis [49,50]. These names were given by the authors, but, actually, according to the routinal numeration of this kind of compounds, they should be named as 1-(,3,4,5-trihydroxyphenyl)ethyl-3-O-rhamnose-4-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoate]-glucopyranoside and 1-(3,4,5-trihydroxyphenyl)ethyl-3-O-rhamnose-4-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoate]-6-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoate]-glucopyranoside, respectively.
Finally, there are some problems with the correct association between the name of the iridoid longifloroside and its structure, since diverse possibilities are given in the literature. Anyway, in this case, longifloroside is considered to be the compound with the name: 5″-O-(4′-aucubinyl)-5′′′O-(4′-euphrosidyl)-(2″,2′′′-2,5H-furan-ether-(bis-iridoid glucoside), as reported in literature [55].
Moreover, for what concerns iridoids, some of those identified in Pedicularis spp. may indeed be artefacts due to the procedures applied during the phytochemical analysis. In particular, the two new iridoid glycosides 6-O-ethyl-aucubin and 6-O-ethyl-epi-aucubin, recognized from P. rex [69], and the three pediverticilatasins A–C isolated from P. verticillata [87], are likely due to the extraction with ethanol. The same has very likely happened for 6-O-methyl-aucubin, artselaenin III, and artselaenin I [19,20], all isolated from P. artselaeri after extraction with boiling methanol (at reflux), as well as the 3-butoxy-3,4,dihydroaucubin, 6-O-butyl-aucubin, and 6-O-butyl-epi-aucubin obtained from the n-butanol soluble fraction of P. chinensis [27]. In this context, the ethyl acetal function observable in the pediverticilatasins A–C and the butyl acetal function of kansuenin B observed in P. verticillata [87] are also suspect, in particular if considering that the majority of these compounds have the alcoxy function of the acetal group in α-configuration, which is the opposite of that generally observable for the saccharidic moieties in the glycosidic iridoids. Therefore, the presence of alcoxy acetals could possibly be due to an exchange between the saccharidic moiety and the alcohols present in solution as solvents (thus in high amount), favored by some specific conditions (i.e., acidity of the medium).
The possibility of generating this kind of artefacts from iridoids was one of the arguments of a recent review and of one editorial article [89,90] which reported about the reactivity of the hydroxyl substituent in allylic configuration, a functionalization very often present in several iridoid structures, like in the case of iridoids with an aucubin-like skeleton, as well as the possibility of addition of short-chain alcohols used as extractive solvents to the double bond in the 3,4-positions of the iridane skeleton. Unfortunately, the presence of such iridoid derivatives was not confirmed in the studied species by avoiding the possible causes of artefact formation. Therefore, the presence of these compounds remains doubtful without any further confirmation.
7. Methodologies for the Phytochemical Analysis
The phytochemical analyses of the studied plants were carried out by following the common procedures. In particular, the essential oils were studied through hydrodistillation and gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS) analysis [10,11,12,13].
For the study of the polar fraction metabolites, the starting plant material was mainly dried. The extraction was mainly at room temperature with ethanol, even if extractions with different solvents such as methanol, n-hexane, and dichloromethane were also performed. In some cases, these latter extractions were carried out in hot conditions using a Soxhlet apparatus. This extraction method is not the best choice, since the exposure of the extracts to high temperatures may be one cause of artefact formation, as reported in the previous section. Indeed, extraction with ethanol was often followed by a partitioning procedure with solvents at different polarity grades, such as ethyl acetate, n-butanol, diethyl ether, petroleum ether, and distilled water, and every different organic phase was separately subjected to further analysis. The separation of the metabolites from the phytocomplex was mainly achieved by means of column chromatography (CC), using silica gel and allumina as stationary phases and different mixtures of n-butanol and distilled water, chloroform or dichloromethane and methanol, or n-hexane and ethyl acetate at different concentrations as mobile phases. In a few cases, high performance liquid chromatography (HPLC) techniques were used for these purposes, using C18 columns and distilled water and acetonitrile more or less acidified with formic acid as eluting systems. Identification of the metabolites was mainly achieved by means of thin layer chromatography (TLC), infrared (IR), ultraviolet (UV), optical rotation (OR), nuclear magnetic resonance (NMR), and mass spectrometry (MS) techniques. Finally, preliminary analysis of the metabolite contents was performed via the Folin–Cocalteau test for the total phenolic content (TPC), the aluminium chloride colorimetric assay for the total flavonoid content (TFC), Dragendorff’s reagent test for the presence of alkaloids, and the ferric chloride test for the presence of tannins [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88].
Nonetheless, in a few cases, the methodology was partially or totally undescribed in the reported experimental sections.
At this point, it is extremely important to underline two facts. The first is that the phytochemical methods employed for analysis can deeply influence the results. The second is that works performed only by chromatographic evidence and reporting the generic presence of classes of constituents cannot be considered totally reliable. An example of this is the Dragendorff’s reagent test, which also results positive in the cases of α,β-unsaturated carbonyls.
For these reasons, phytochemical methods must be carefully considered.
8. Chemotaxonomy
The chemotaxonomy of the Pedicularis genus is quite complex, and involves several classes of natural compounds. In particular, its main chemotaxonomic marker is aucubin, and, in fact, it has been recognized in 25 of the studied species (Table 1). From a biogenetic standpoint, aucubin, like the other decarboxylated C-10 iridoids observed in species of the Lamiales order, derives from geranyl pyrophosphate. In particular, these follow the biosynthetic Route II, which involves epi-iridotrial and 8-epi-deoxy-loganic acid among its precursors, and leads to the biosynthesis of iridoids characterized by the α-configuration of the methyl function linked in the 8 position of the iridane skeleton. Its cyclization reaction occurs through a hydride nucleophillic attack on C1, which leads to the 1-O-carbonyl atom attack on C3 and then to the cyclic acetale [91]. Considering the biogenesis of iridoids in this genus, the actual presence of loganic acid recognized among the phytoconstituents of P. torta and P. longiflora is doubtful (see table for references). We are instead of the opinion that, without further confirmation, that compound was mistakenly reported instead of epi-loganic acid. In this context, studies on the biogenesis of iridoids in Pedicularis spp. by means of labelled precursors could be of help in delineating the biogenetic pathway and several products of that metabolite biosynthesis. This could also be an excellent analytical method by which to confirm the possible presence or not of compounds that appear to be in contrast with the biogenetic pathway of iridoids in this genus.
In fact, euphroside and mussaneoside are also minor chemotaxonomic markers of the genus, even if in several species the content of euphroside was shown to be higher than that of aucubin itself [22,51], and the amount of mussaenosidic acid was comparable with those of other iridoidic constituents [60]. Conversely, some iridoids are considered to be chemotaxonomic markers at the species level, since their presence has been reported only in one. The main example of this are pedicularioside for P. muscicola, kansuenin, kansuenin B, and kansuenoside for P. kansuensis, pliatosides A–B for P. plicata, and densispicnin A for P. densispica. In contrast with what is written in the previous paragraph concerning artefact iridoids, the presence of proceroside in P. procera [66], even if it presents a β-configuration in C-8 and therefore would seem to be derived from the Route I biogenetic pathway, is not an artefact and is not due to an erroneous interpretation of experimental data. In fact, the inversion of configuration at C-8 in proceroside is favored by the presence of a ketone function on the adjacent carbon (C-7), which is involved in a keto-enol equilibrium, and this may perfectly justify the β-configuration of the hydroxymethyl group at the position 8.
Seco-iridoids are metabolites that rarely derive from the biogenetic Route II. In fact, some derivatives have been observed in Lamium album [92], and their origin from 8-epi-deoxy-loganic acid, a precursor in the biogenetic Route II, has been fully confirmed. To date, the presence of seco-iridoids has been observed only in P. verticillata [70], and it could be of utmost interest to verify if these kinds of compounds are also present in other species of the genus. Obviously, it could also be interesting to investigate their possible biogenesis by suitable analytical methods.
Phenylethanoid glycosides (i.e., verbascoside and its derivatives) are considered to be other chemotaxonomic markers of the genus, since their presence has been evidenced in most of the studied species. However, these compounds are very common in all the Asteridae class, and, in fact, they have also been identified in other families such as Asteraceae [93], Caprifoliaceae [94], Lamiaceae [14], Oleaceae [95], Plantaginaceae [96], Scrophulariaceae [97], and Verbenaceae [98]. More specifically, the phenylethanoid glycosides have a chemotaxonomical relevance when co-occurring with iridoids [99]. This has been already observed in several species in the Lamiales order [100,101,102,103,104,105], as well as in the case of several Pedicularis spp. These compounds are also extremely common within the family Pedicularis genus belongs to (Orobanchaceae), and, in fact, they have already been reported in several genera, such as Orobanche L., Cistanche L., and Orthocarpus Nutt. [106]. For these reasons, phenylethanoid glycosides cannot actually be taken as general chemotaxonomic markers of the Pedicularis genus. Nevertheless, specific compounds can be useful chemotaxonomic markers, such as pediculariosides A, E, G, H, I, M, and N for the entire genus, permethyl-verbascoside for P. spicata, cis-iso-martynoside for P. kansuensis, cis-pedicularioside H for P. spicata, and artselaeroside B for P. artselaeri.
Lignans and derivatives are quite widespread in the genus, but also in the family Orobanchaceae and in many others [107]. However, semitortosides A and B can serve as chemotaxonomic markers for P. semitorta, striatosides A and B can serve as chemotaxonomic markers for P. striata, and longiflor B and longiflorides C and D can serve as chemotaxonomic markers for P. longiflora.
Flavones and, in particular, flavonols and glycosidic flavonoids presenting an apigenin, scutellarein, and isoscutellarein base moiety, are also considered to be chemotaxonomic markers of the genus. However, they are very common compounds in the plant kingdom, and for this reason, they are not particularly useful as chemotaxonomic markers. In particular, their presence can be easily evidenced in Lamiaceae species [14], as well as in many other families, such as Euphorbiaceae, Asteraceae, Compositae, and Hypericaceae [108,109,110,111,112,113,114].
In terms of alkaloids, pediculidine, pedicularidine, pediculine, and pediculinine have been evidenced only in Pedicularis species, and they can serve as chemotaxonomic markers at the genus level.
As for compounds belonging to classes of natural metabolites other than the ones already described, there have been no reports on them as chemotaxonomic markers of the Pedicularis genus or in general, since they are extremely common. Nevertheless, pedicurexoside, a sesquiterpene, may be suggested as a specific marker for P. rex, since it has been evidenced only in that species so far, while the polyol D-mannitol seems to be highly represented in hemiparasitic entities previously comprised in Scrophulariaceae and now classified as Orobanchaceae [51,70,102,115].
In this context, concerning phytochemistry and chemotaxonomy, it is of primary importance to also consider other aspects, together with the markers’ metabolite biogenesis, such as the ecology and hemiparasitic behaviour of the plant species, when the scope of the study is chemosystematics. In fact, the transfer of metabolites from the hosts to the hemiparasitic species has been observed in several cases, such in the cases of Euphrasia stricta D. Wolff [116], Euphrasia rostkoviana Hayne [117], and Odontites luteus Steven [118]. Therefore, it is suggested that the results from the phytochemical analysis of hemiparasitic plants should be carefully checked and subjected to the required criticism.
9. Ethnopharmacology
Pedicularis species are widely used in the traditional medicines of several countries around the world, especially Asian ones. The pharmacological activities exerted by these species are numerous and interesting, with one species often employed to treat more than one malady and vice versa.
Table 3 reports on the specific ethnopharmacological properties associated with every studied plant in this field. In addition, the organs of the plant species which show that medicinal activity are described, as well as the areas of the world where indigenous people employ these species in traditional medicine.

Table 3.
Ethnopharmacological Uses of Pedicularis Species as Reported in Literature.
10. Corollary for Ethnopharmacology
Some Pedicularis species have also been reported to have ethnopharmacological employments in certain areas of the world, but no specific medicinal and pharmacological properties have been reported in the literature. In particular, this concerns P. koengboensis Tsoong var. kongboensis (a.n.) in Nepal [151], P. heydei Prain (u.n.), P. nodosa Pennell (u.n.) and P. scullyana Prain ex. Maxim. (u.n.) in Tibet [152], and, finally, P. tristis L. (a.n.) in Mongolia [153]. Specific information concerning their specific way of employment is also lacking in the literature, which makes their uses doubtful but not certainly false, since their utilization may be only on a traditional local basis and favored by specialized people who may not be interested in sharing their knowledge. Regardless, phytochemical analysis of these species is also strongly suggested in the future.
11. Pharmacology
In spite of all the results reported in the previous section, for some Pedicularis species, only a few initial pharmacological properties have been assessed, and their ethnopharmacological employments have not yet been reported. This also concerns the species already used in the ethnopharmacological field but that have been studied for other possible employments.
Table 4 reports on these species and their relative pharmacological properties.

Table 4.
Pharmacological Activities of Pedicularis Species as Reported in Literature.
12. Relationships among Pharmacology, Ethnopharmacology, and Phytochemistry
Table 2 and Table 3 clearly show how fundamental Pedicularis species are in the ethnopharmacological and pharmacological fields. However, many Pedicularis species with ethnopharmacological and/or pharmacological uses are awaiting phytochemical analysis on their active constituents. Thus, their employment is strictly related to traditional uses, which are established on the basis of previous experiences. Conversely, for those species also presenting a well-established phytochemical profile, their ethnopharmacological and/or pharmacological uses can be obviously explained by their phytochemical compositions. In fact, phytochemical compounds (singularly or as a phytocomplex) are the major elements responsible for the pharmacological properties associated to every single species, and may justify their use in that sense from the phytochemical standpoint.
Several classes of natural compounds have been evidenced within the Pedicularis genus, and each of them exerts specific pharmacological activities. In particular, alkaloids have antimalarial, antitumor, antibacterial, and stimulant activities, among others [154,161], even if a particular subclass of them (pyrrolizidine alkaloids) are indeed known to cause severe genotoxicity, neurotoxicity, and tumourigenicity [162]. Lignans exert mainly antioxidant and anti-inflammatory properties [163]. Tannins are widely known for their astringent and antioxidant effects [164]. Phenylethanoid glycosides are good antioxidant, antibacterial, antiviral, antitumor, neuroprotective, and hepatoprotective compounds [106,165]. Flavonoids display, in particular, antioxidant, anti-inflammatory, anti-mutagenic, and anti-carcinogenic properties [166]. Xanthones are mainly insecticidal compounds [167]. Iridoids are widely used as antiviral, anti-inflammatory, hepatoprotective, antimicrobial, and antitumor agents [168]. Seco-iridoids are mainly anti-inflammatory and antifungal compounds [169]. Finally, fatty acids, organic acids, polyols, saccharides, nucleobases, and amino acids have several nutraceutical properties.
13. Other Uses
Some Pedicularis species are better known to have other uses different from those typical in the ethnopharmacological and pharmacological fields.
These uses all are reported in the table below (Table 5).

Table 5.
Other Uses of Pedicularis Species As Reported In Literature.
14. Curiosities
Some Pedicularis species present strange but interesting curiosities. In particular, although Pedicularis species are considered to be strong hemiparasitic plants, P. friderici-augusti Tomm. (a.n.), P. furbishiae S. Watson (a.n.), P. ishidoyana Koidz. & Ohwi (u.n.), P. kashmiriana Pennell (a.n.), P. petiolaris Ten. (a.n.), P. rainierensis Pennel & Warren (a.n.), P. rostratospicata Crantz (a.n.), P. siamensis P.C.Tsoong (u.n.), and P. thailandica T.Yamaz. (u.n.) are endangered species in their growth areas [173,174,175,176,177,178,179]. Moreover, P. porrecta Wall. (u.n.) grows only in arid areas [180], and the name P. stenantha Franch. (u.n.) is also often used to identify P. stenocorys Franch. (a.n.), but they are two different species [181].
15. Conclusions
This review has clearly evidenced and highlighted the importance of the plant species belonging to the Pedicularis genus from different points of view.
As it can be easily deduced, there is still much to discover and study, since the information about this genus is quite scarce as regards many specific arguments.
In particular, it could be interesting to investigate the biogenesis of iridoids, since, from a chemosystematic standpoint, they are the most important marker compounds in this genus. This may confirm or not the presence of unusual compounds such as derivatives with 8β-configurations and seco-iridoids, as well as potentially elucidate the key intermediates in their biosynthesis by means of labeled precursors.
On the other hand, for what concerns the bioactivity aspects of Pedicularis spp., we hope that this review will contribute to renewing the interest of researchers in deepening the general knowledge on the pharmacological potentials of Pedicularis extracts and pure constituents, in particular, their minor components.
Author Contributions
Conceptualization, C.F., A.B. and M.S.; All authors wrote and reviewed every section.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no potential conflict of interests in this work.
Abbreviations
| a.n. | accepted name |
| n.r. | none reported |
| n.s. | not specified |
| s.n. | synonym name |
| u.n. | unresolved name |
References
- The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Pedicularis genus. Available online: www.theplantlist.org (accessed on 15 May 2019).
- Nicolini, G. Enciclopedia Botanica Motta; Federico Motta: Milano, Italy, 1960; Volume 3, p. 236. [Google Scholar]
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1982; Volume 2, p. 590. [Google Scholar]
- Kadereit, J.W. The Families and Genera of Vascular Plants, Volume VII; Lamiales Sperling: Berlin, Germany, 2004; p. 424. [Google Scholar]
- Aeschimann, D.; Lauber, K.; Moser, D.M.; Theurillat, J.P. Flora Alpina; Zanichelli: Bologna, Italy, 2004; Volume 2, p. 264. [Google Scholar]
- Yatoo, M.I.; Dimri, U.; Gopalakrishan, A.; Saminathan, M.; Dhama, K.; Mathesh, K.; Saxena, A.; Gopinath, D.; Husain, S. Antidiabetic and Oxidative Stress Ameliorative Potential of Ethanolic Extract of Pedicularis longiflora Rudolph. Int. J. Pharmacol. 2016, 12, 177–187. [Google Scholar] [CrossRef]
- Li, M.X.; He, X.R.; Tao, R.; Cao, X. Phytochemistry and Pharmacology of the Genus Pedicularis Used in Traditional Chinese Medicine. Am. J. Chin. Med. 2014, 42, 1071–1098. [Google Scholar] [CrossRef] [PubMed]
- USDA (2019) Natural Resources Conservation Service Report. Available online: www. plants.usda.gov/java/ClassificationServlet?source=display&classid=PEDIC (accessed on 15 May 2019).
- Üçüncü, O.; Baltaci, C.; Ilter, S.M. Chemical composition, antimicrobial and antioxidant aactivities of essential oil from Pedicularis condensata BIEB. Hittite J. Sci. Engineer. 2016, 3, 105–109. [Google Scholar] [CrossRef]
- Khodaie, L.; Bamdad, S.; Delazar, A.; Nazemiyeh, H. Antioxidant, Total Phenol and Flavonoid Contents of Two Pedicularis L. Species from Eastern Azerbaijan, Iran. BioImpacts 2012, 2, 43–57. [Google Scholar]
- Khodaei, L.; Delazar, A.; Nazemiyeh, H.; Asnaashari, S.; Nahar, L.; Sarker, S.D. Composition of the Volatile Oils of the Aerial Parts of Pedicularis sibthorpii and P. wilhelmsiana Growing in Iran. J. Essent. Oil Bear. Plants 2012, 15, 352–356. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Saeedi, M.; Akbarzadeh, M. Chemical Composition of the Essential Oil of Pedicularis sibthorpii Boiss. J. Essent. Oil Bear. Plants 2014, 17, 1303–1307. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; Serafini, M.; Bianco, A. Phytochemistry, Chemotaxonomy, Ethnopharmacology, and Nutraceutics of Lamiaceae. Stud. Nat. Prod. Chem. 2019, 62, 125–178. [Google Scholar]
- Akdemir, Z.S.; Çalıs, I.; Doga, T.R. Iridoid and phenylpropanoid glycosides from Pedicularis comosa var. acmodonta Boiss. J. Pharma. 1992, 2, 63–70. [Google Scholar]
- Gao, J.J.; Jia, Z.J.; Liu, Z.M. Studies on the monoterpene iridoid glycosides from Pedicularis alaschanica. Chin. Chem. Lett. 1993, 14, 366–377. [Google Scholar]
- Gao, J.; Jia, Z.J. Lignan, iridoid and phenylpropanoid glycosides from Pedicularis alaschanica Maxim (Scrophulariaceae). Indian J. Chem. B 1995, 34, 466–468. [Google Scholar]
- Yuan, C.S.; Sun, X.B.; Zhao, P.H.; Cao, M.A. Antibacterial constituents from Pedicularis armata. J. Asian Nat. Prod. Res. 2007, 9, 673–677. [Google Scholar] [CrossRef]
- Su, B.N.; Zhai, J.J.; Jia, Z.J. New Iridoids from Pedicularis artselaeri. J. Asian Nat. Prod. Res. 1998, 1, 103–109. [Google Scholar] [CrossRef]
- Su, B.; Ma, L.; Jia, Z. Iridoid and Phenylpropanoid Glycosides from Pedicularis artselaeri. Planta Medica 1998, 64, 720–723. [Google Scholar] [CrossRef]
- Yatoo, M.I.; Dimri, U.; Gopalakrishnan, A.; Karthik, K.; Gopi, M.; Khandia, R.; Saminathan, M.; Saxena, A.; Alagawany, M.; Farag, M.R.; et al. Beneficial health applications and medicinal values of Pedicularis plants: A review. Biomed. Pharmacother. 2017, 95, 1301–1313. [Google Scholar] [CrossRef]
- Schneider, M.J.; Lynch, J.D.; Deutsch, L.; Duda, C.M.; Green, J.C.; Mcpeak, D. Iridoid glycosides of Pedicularis. Biochem. Syst. Ecol. 1996, 24, 793–794. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Suess, T.R.; Fink, N.H.; Puzziferri, N. Phytochemical Screening of Some Rocky Mountain Plants. J. Nat. Prod. 1981, 44, 693–695. [Google Scholar] [CrossRef]
- Jung, H.J.G.; Batzli, G.O.; Seigler, D.S. Patterns in the phytochemistry of arctic plants. Biochem. Syst. Ecol. 1979, 7, 203–209. [Google Scholar] [CrossRef]
- Yang, J.Q.; He, W.J.; Tan, N.H.; Chu, H.B.; Zhang, Y.M.; Mei, W.L.; Dai, H.F. Chemical constituents of Pedicularis cephalantha Franch and P. siphonantha Don. Nat. Prod. Res. Dev. 2009, 21, 600–603. [Google Scholar]
- Murai, Y.; Tsukasa, I. Phenolic compounds in the leaves of Pedicularis chamissonis in Japan Bull. Natl. Mus. Nat. Sci. Ser. B 2015, 41, 131–136. [Google Scholar]
- Yang, L.; Wang, C.Z.; Jia, Z.J. Iridoids in roots of Pedicularis chinensis. Phytochemistry 1995, 40, 491–494. [Google Scholar]
- Wang, C.Z.; Jia, Z.J. Iridoid, lignan and phenylpropanoid glycosides from Pedicularis chinensis. J. Lanzhou Univers. (Nat. Sci.) 1996, 32, 64–68. [Google Scholar]
- Akdemi̇r, Z.; Çali, H.; Junior, P. Iridoid and phenylpropanoid glycosides from Pedicularis condensata. Phytochemistry 1991, 30, 2401–2402. [Google Scholar]
- Schneider, M.J.; Stermitz, F.R. Uptake of host plant alkaloids by root parasitic Pedicularis species. Phytochemistry 1990, 29, 1811–1814. [Google Scholar] [CrossRef]
- An, B.L.; Liu, Q.G.; Geng, Z.; Cheng, Z.G. Amino acids and trace elements in Pedicularis decora franch. J. Shanxi. Norm. Univ. (Nat. Sci.) 1995, 23, 62–64. [Google Scholar]
- Guan, F.; Wang, X.J.; Yang, Y. Chemical studies on the Pedicularis decora Franch. J. Tradition. Chin. Med. 2004, 27, 920–921. [Google Scholar]
- Li, C.; Zhang, C.Z.; Liu, M. Phenol glycosides from Pedicularis dicora. Chin. Tradition. Herb. Drug 1998, 29, 11–12. [Google Scholar]
- Li, C.; Gu, D.S.; Zhang, C.Z.; Tao, B.Q. Iridoid glycosides from Pedicularis dicora Franch. China J. Chin. Mater. Med. 1999, 24, 40–41. [Google Scholar]
- Li, C.; Zhang, C.Z. Nor-monoterpene glucides from Pedicularis dicora. Chin. Tradition. Herb. Drug 1999, 30, 482–484. [Google Scholar]
- Zhang, C.Z.; Li, C.; Fen, S.L. Chemical studies on Pedicularis dicora. J. Lanzhou Med. Coll. 1991, 17, 199–200. [Google Scholar]
- Luo, D.Q.; Chu, H.B.; Tan, N.H.; Tian, X. Densispicnins A and B, Two Unusual Monoterpenes from Pedicularis densispica Franch. Heterocycles 2008, 75, 177. [Google Scholar] [CrossRef]
- Chu, H.; He, W.; Zhang, Y.; Ji, C.; Tan, N. Flavonoids and nor-sesquiterpenes of Pedicularis densispica. China J. Chin. Mater. Medica 2011, 36, 2672–2675. [Google Scholar]
- Chu, H.B.; Zeng, G.Z.; Zhu, M.J.; He, W.J.; Zhang, Y.M.; Tan, N.H. Chemical Constituents of Pedicularis densispica Franch. Z. Naturforsch. B 2011, 66, 641–646. [Google Scholar] [CrossRef]
- Chu, H.B.; Tan, N.H. Iridoid Glycosides from Pedicularis dolichocymba Hand.-Mazz. J. Integr. Plant. Biol. 2006, 48, 1250–1253. [Google Scholar] [CrossRef]
- Chu, H.B.; Tan, N.H.; Xiong, J.; Zhang, Y.M.; Ji, C.J. Chemical constituents of Pedicularis dolichocymba Hand.-Mazz. Nat. Prod. Res. Dev. 2007, 19, 584–587. [Google Scholar]
- Ubeav, K.; Yuldashev, P.K.; Yunusov, S.Y. Pedicularis olgae alkaloids. Uzbeksk. Khim. Zh. 1963, 7, 33–36. [Google Scholar]
- Karanjit, N.; Shakya, K.; Pradhan, N.B.; Gautam, L.; Shakya, D. Acompilation report of preliminary phytochemical and biological screening of some medicinal plants of Nepal. Bull. Dept. Pl. Res. 2007, 29, 79–96. [Google Scholar]
- Wangchuk, P.; Keller, P.A.; Pyne, S.G.; Taweechotipatr, M.; Tonsomboon, A.; Rattanajak, R.; Kamchonwongpaisan, S. Evaluation of an ethnopharmacologically selected Bhutanese medicinal plants for their major classes of Phytochemicals and biological activities. J. Ethnopharmacol. 2011, 137, 730–742. [Google Scholar] [CrossRef]
- Yin, J.G.; Yuan, C.S.; Jia, Z.J. A new iridoid and other chemical constituents from Pedicularis kansuensis forma albiflora Li. Arch. Pharmacal Res. 2007, 30, 431–435. [Google Scholar] [CrossRef]
- Yuan, C.S.; Zhang, Q.; Xie, W.D.; Yang, X.P.; Jia, Z.J. Iridoids from Pedicularis kansuensis forma albiflora. Pharmazie 2003, 34, 428–430. [Google Scholar] [CrossRef]
- Jiang, T.F.; Qing, Y.O.; Yan, P.S. Separation and determination of phenyl-propanoid glycosides from Pedicularis species by capillary electrophoresis. J. Chromatogr. A 2003, 986, 163–167. [Google Scholar] [CrossRef]
- Guarnaccia, R.; Madyastha, K.M.; Tegtmeyer, E.; Coscia, C.J. Geniposidic acid, an iridoid glucoside from Genipa americana. Tetrahedron Lett. 1972, 13, 5125–5127. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Xie, W.D.; Jia, Z.J. Glycosides from two Pedicularis species. Biochem. Syst. Ecol. 2008, 36, 467–472. [Google Scholar] [CrossRef]
- Zhang, B.B.; Shi, K.; Liao, Z.X.; Dai, Y.; Zou, Z.H. Phenylpropanoid glycosides and triterpenoid of Pedicularis kansuensis Maxim. Fitoterapia 2011, 82, 854–860. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Serafini, M.; Bianco, A. Iridoids and phenylethanoid from Pedicularis kerneri Dalla Torre growing in Dolomites, Italy. Nat. Prod. Res. 2016, 30, 327–331. [Google Scholar] [CrossRef]
- Berg, T.; Damtoft, S.; Jensen, S.R.; Nielsen, B.J.; Rickelt, L.F. Iridoid glucosides from Pedicularis. Phytochemistry 1985, 24, 491–493. [Google Scholar] [CrossRef]
- Jia, Z.J.; Liu, Z.M.; Wang, C.Z. Phenylpropanoid and iridoid glycosides from Pedicularis lasiophrys. Phytochemistry 1992, 31, 263–266. [Google Scholar]
- Wang, C.Z.; Jia, Z.J. Neolignan glycosides from Pedicularis longiflora. Planta Medica 1997, 63, 241–244. [Google Scholar] [CrossRef]
- Jia, Z.J.; Liu, Z.M. Phenylpropanoid and iridoid glycosides from Pedicularis longiflora. Phytochemistry 1992, 31, 3125–3127. [Google Scholar]
- Zhang, L.; Yue, H.L.; Zhao, X.H.; Li, J.; Shao, Y. Separation of Four Phenylpropanoid Glycosides from a Chinese Herb by HSCCC. J. Chromatogr. Sci. 2014, 53, 860–865. [Google Scholar] [CrossRef]
- Fujii, M.; Miyaichi, Y.; Tomiinori, T. Flavonoid, phenylethanoid and iridoid constituents of the whole plant of Pedicularis longiflora var. tubiformis. Planta Medica 1991, 61, 584. [Google Scholar] [CrossRef]
- Ma, Z.K.; Niu, B.J.; Zhang, B.B.; Liao, Z.X. Chemical constituents from Pedicularis longiflora var. tubiformis. Chin. Tradition. Herb. Drug 2013, 44, 403–407. [Google Scholar]
- Zhang, L.; Shao, Y.; Zhao, X.H.; Yue, H.L.; Chen, C.; Tao, Y.D.; Mei, L.J.; Zhou, G.Y. Chemical constituents of Pedicularis longiflora Rudolph. var. tubiformis (Klotz). Tsoong. Nat. Prod. Res. Dev. 2013, 25, 40–43. [Google Scholar]
- Kang, J.G.; Jia, Z.J. The chemical constituents of Pedicularis muscicola Maxim. J. Lanzhou Univ. (Nat. Sci.) 1997, 33, 69–74. [Google Scholar]
- Kang, J.; Jia, Z. Chemical constituents of Pedicularis muscicola Maxim. China J. Chin. Mater. Medica 1997, 22, 167–168. [Google Scholar]
- Yang, L.C.; Chen, L.D. Studies on the chemical constituents of Pedicularis muscicola Maxim. China J. Chin. Mater. Med. 1992, 17, 485–487. [Google Scholar]
- Akdemir, Z.; Caliş, I.; Junior, P. Iridoids and phenyipropanoid glycosides from Pedicularis nordmanniana. Planta Medica 1991, 57, 584–585. [Google Scholar] [CrossRef]
- Sadikov, Y.J. Biologically Active Substances of Wild Medicinal Plants of Tajikistan; Academy of Sciences of the Republic of Tajikistan: Dushanbe, Tajikistan, 2003; p. 320. [Google Scholar]
- Jia, Z.J.; Gao, J.J.; Liu, Z.M. Iridoid and phenylpropanoid glycosides from Pedicularis plicata Maxim. Indian J. Chem. B 1994, 33, 460–464. [Google Scholar]
- Schneider, M.J.; Green, J.C.; McPeak, D. Proceroside, an iridoid glucoside from Pedicularis procera. Phytochemistry 1997, 46, 1097–1098. [Google Scholar] [CrossRef]
- Schneider, M.J.; Romero, K.A. Iridoid glycosides from Pedicularis punctata. Abstr. Pap. Am. Chem. Soc. 1995, 210, 355. [Google Scholar]
- Bazzaz, B.S.F.; Haririzadeh, G.; Imami, A.S.; Rashed, M.H. Survey of Iranian Plants for Alkaloids, Flavonoids, Saponins, and Tannins (Khorasan Province). Int. J. Pharmacogn. 1997, 35, 17–30. [Google Scholar] [CrossRef]
- Chu, H.B.; Tan, N.H.; Zhang, Y.M. Chemical constituents from Pedicularis rex C. B. Clarke. Z. Naturforsch. B 2007, 62, 1465–1470. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Sciubba, F.; Foddai, S.; Serafini, M.; Nicoletti, M.; Bianco, A. Secoiridoids and other chemotaxonomically relevant compounds in Pedicularis: Phytochemical analysis and comparison of Pedicularis rostratocapitata Crantz and Pedicularis verticillata L. from Dolomites. Nat. Prod. Res. 2016, 30, 1698–1705. [Google Scholar] [CrossRef]
- Sharopov, F.; Setzer, W.N. Medicinal Plants of Tajikistan; Egamberdieva, D., Öztürk, M., Eds.; Vegetation of Central Asia and Environs; Springer: Berlin, Germany, 2019. [Google Scholar]
- Stermitz, F.R.; Belofsky, G.N.; Ng, D.; Singer, M.C. Quinolizidine alkaloids obtained by Pedicularis semibarbata (Scrophulariaceae), from Lupinus fulcratus (Leguminosae) fail to influence the specialist herbivore Euphydrya seditha (Lepidotera). J. Chem. Ecol. 1989, 15, 2521–2530. [Google Scholar] [CrossRef]
- Wang, C.Z.; Jia, Z.J.; Shen, X.M. Phenylpropanoid, neolignan and iridoid glycosides from Pedicularis semitorta. Indian J. Chem. 1997, 36, 150–153. [Google Scholar]
- Khodaie, L.; Delazar, A.; Lotfipour, F.; Nazemiyeh, H.; Asnaashari, S.; Moghadam, S.B.; Nahar, L.; Sarker, S.D. Phytochemistry and bioactivity of Pedicularis sibthorpii growing in Iran. Rev. Bras. Farm. 2012, 22, 1268–1275. [Google Scholar] [CrossRef]
- Jia, Z.J.; Liu, Z.; Wang, C. Phenypropanoid and iridoid glycosides from P. spicata. Phytochemistry 1991, 30, 3745–3747. [Google Scholar]
- Zheng, R.L.; Wang, P.F.; Li, J.; Liu, Z.M.; Jia, Z.J. Inhibition of the autoxidation of linoleic acid by phenylpropanoid glycosides from Pedicularis in micelles. Chem. Phys. Lipids 1993, 65, 151–154. [Google Scholar] [CrossRef]
- Liu, Z.M.; Jia, Z.J. Phenylpropanoid and iridoid glycosides from Pedicularis striata. Phytochemistry 1991, 30, 1341–1344. [Google Scholar]
- Liu, Z.M.; Wang, C.Z.; Jia, Z.J. Chemical constituents from Pedicularis striata. J. Lanzhou Univ. (Nat. Sci.) 1994, 30, 62–65. [Google Scholar]
- Mu, P.; Gao, X.; Jia, Z.J.; Zheng, R.L. Natural antioxidant pedicularioside G inhibits angiogenesis and tumourigenesis in vitro and in vivo. Basic Clin. Pharmacol. Toxicol. 2008, 102, 30–34. [Google Scholar] [CrossRef]
- Gao, J.J.; Jia, Z.J.; Codina, C. An eremophilane sesquiterpenoid from Pedicularis striata Pall ssp. arachnoidea. Phytochemistry 1996, 43, 1411–1412. [Google Scholar] [CrossRef]
- Gao, J.; Yang, L.; Jia, Z. A New Eremophilane Sesquiterpenoid and a New Iridoid from Pedicularis striata subsp. arachnoides. Planta Medica 1997, 63, 248–250. [Google Scholar] [CrossRef]
- Jia, Z.J.; Gao, J.J. Phenylpropanoid glycosides from Pedicularis striata pall ssp. arachnoidea. Phytochemistry 1993, 34, 1188–1190. [Google Scholar]
- Wang, C.Z.; Jia, Z.J. Neolignan glucosides from Pedicularis torta. Chin. Chem. Lett. 1996, 7, 141–144. [Google Scholar]
- Wang, C.Z.; Jia, Z.J. Lignan, phenylpropanoid and iridoid glycosides from Pedicularis torta. Phytochemistry 1997, 45, 159–166. [Google Scholar] [CrossRef]
- Yang, L.R.; Xiong, J.; Tan, N.H.; Chu, H.B.; Xu, L.; Li, M.Y. Chemical constituents of Pedicularis tricolor (Scrophulariaceae). Acta Bot. Yunnan 2006, 28, 553–557. [Google Scholar]
- Liu, L.F.; Yao, M.J.; Li, M.Y.; Wu, X.Z.; Yuan, C.S. Iridoid Derivatives with Cytotoxic Activity from Pedicularis uliginosa Bunge. Chem. Biodivers. 2019, 16, e1800524. [Google Scholar] [CrossRef]
- Shao, M.H.; Dai, W.; Yuan, S.W.; Lu, Y.; Chen, D.F.; Wang, Q.; Shao, M.; Yuan, S.; Chen, D. Iridoids from Pedicularis verticillata and Their Anti-Complementary Activity. Chem. Biodivers. 2018, 15, e1800033. [Google Scholar] [CrossRef]
- Su, B.N.; Yang, L.; Jia, Z.J. Neolignan, phenylpropanoid and iridoid glycosides from Pedicularis verticillata. Phytochemistry 1997, 45, 1271–1273. [Google Scholar] [CrossRef]
- Venditti, A. What is and what should never be: Artifacts, improbable phytochemicals, contaminants and natural products. Nat. Prod. Res. 2018, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A. Artifacts in natural products studies. An old and underestimated re-emerging problem. Nat. Prod. Res. 2018, 32, I–II. [Google Scholar] [CrossRef] [PubMed]
- McGarbey, D.; Croteau, R. Terpenoid Metabolism. Plant Cell 1995, 7, 1015–1026. [Google Scholar]
- Damtoft, S.; Jensen, S.R.; Nielsen, B.J. Biosynthesis of iridoid glucosides in Lamium album. Phytochemistry 1992, 31, 135–137. [Google Scholar] [CrossRef]
- Cheminat, A.; Zawatzky, R.; Becker, H.; Brouillard, R. Caffeoyl conjugates from Echinacea species: Structures and biological activity. Phytochemistry 1998, 27, 2787–2794. [Google Scholar] [CrossRef]
- Machida, K.; Nakano, Y.; Kikuchi, M. Phenolic glycosides from Viburnum dilatatum. Phytochemistry 1991, 30, 2013–2014. [Google Scholar] [CrossRef]
- Kitagawa, S.; Hisada, S.; Nishibe, S. Phenolic compounds from Forsythia leaves. Phytochemistry 1984, 23, 1635–1636. [Google Scholar] [CrossRef]
- Ravn, H.; Nishibe, S.; Sasahara, M.; Xuebo, L. Phenolic compounds from Plantago asiatica. Phytochemistry 1990, 29, 3627–3631. [Google Scholar] [CrossRef]
- Gardner, D.R.; Narum, J.; Zook, D.; Stermitz, F.R. New Iridoid Glucosides from Castilleja and Besseya: 6-Hydroxyadoxoside and 6-Isovanillylcatapol. J. Nat. Prod. 1987, 50, 485–489. [Google Scholar] [CrossRef]
- Lin, Y.L.; Kuo, Y.H. A New Glycoside, Brachynoside, Isolated from Clerodendron brachyanthum SCHAUER. Chem. Pharm. Bull. 1992, 40, 1928–1929. [Google Scholar] [CrossRef][Green Version]
- Jensen, S.R. Systematic Implications of the Distribution of Iridoids and Other Chemical Compounds in the Loganiaceae and Other Families of the Asteridae. Ann. Mo. Bot. Gard. 1992, 79, 284. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; Di Cecco, M.; Ciaschetti, G.; Serafini, M.; Bianco, A. Iridoids and phenylethanoid glycosides from the aerial parts of Ajuga tenorei, an endemic Italian species. Nat. Prod. Res. 2017, 31, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Frezza, C.; Venditti, A.; Matrone, G.; Serafini, I.; Foddai, S.; Bianco, A.; Serafini, M. Iridoid glycosides and polyphenolic compounds from Teucrium chamaedrys L. Nat. Prod. Res. 2018, 32, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Serrilli, A.M.; Bianco, A. Iridoids from Bellardia trixago (L.) All. Nat. Prod. Res. 2013, 27, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Bianco, A.; Nicoletti, M.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Vitali, L.A.; Petrelli, D.; Papa, F.; Vittori, S.; et al. Phytochemical analysis, biological evaluation and micromorphological study of Stachys alopecuros (L.) Benth. subsp. divulsa (Ten.) Grande endemic to central Apennines, Italy. Fitoterapia 2013, 90, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Frezza, C.; Celona, D.; Bianco, A.; Serafini, M.; Fiorini, D.; Maggi, F.; Celenza, G.; Ferraro, S.; Venditti, A.; Cianfaglione, K.; et al. Polar constituents, protection against reactive oxygen species, and nutritional value of Chinese artichoke (Stachys affinis Bunge). Food Chem. 2017, 221, 473–481. [Google Scholar]
- Venditti, A.; Frezza, C.; Bianco, A.; Serafini, M.; Cianfaglione, K.; Nagy, D.U.; Iannarelli, R.; Caprioli, G.; Maggi, F. Polar Constituents, Essential Oil and Antioxidant Activity of Marsh Woundwort (Stachys palustris L.). Chem. Biodivers. 2017, 14, e1600401. [Google Scholar] [CrossRef]
- Jiménez, C.; Riguera, R. Phenylethanoid glycosides in plants: Structure and biological activity. Nat. Prod. Rep. 1994, 11, 591–606. [Google Scholar] [CrossRef]
- Xu, W.H.; Zhao, P.; Wang, M.; Liang, Q. Naturally occurring furofuran lignans: Structural diversity and biological activities. Nat. Prod. Res. 2017. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; Sciubba, F.; Tomai, P.; Antonetti, M.; Franceschin, M.; Di Cocco, M.E.; Gentili, A.; Delfini, M.; Serafini, M.; et al. Phytochemical profile of Euphorbia peplus L. collected in Central Italy and NMR semi-quantitative analysis of the diterpenoid fraction. J. Pharm. Biomed. Anal. 2018, 160, 152–159. [Google Scholar] [CrossRef]
- Iwashina, T. The Structure and Distribution of the Flavonoids in Plants. J. Plant. Res. 2000, 113, 287–299. [Google Scholar] [CrossRef]
- Bohm, B.A.; Stuessy, T.F. Flavonoids of the Sunflower Family (Asteraceae); Springer Science and Business Media LLC: Berlin, Germany, 2001. [Google Scholar]
- Caprioli, G.; Alunno, A.; Beghelli, D.; Bianco, A.; Bramucci, M.; Frezza, C.; Iannarelli, R.; Papa, F.; Quassinti, L.; Sagratini, G.; et al. Polar Constituents and Biological Activity of the Berry-Like Fruits from Hypericum androsaemum L. Front. Plant. Sci. 2016, 7, 62. [Google Scholar] [CrossRef]
- Mesquita, A.A.; Corréa, D.D.B.; De Pádua, A.P.; Guedes, M.L.; Gottlieb, O.R. Flavonoids from four compositae species. Phytochemistry 1986, 25, 1255–1256. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Majd Zadeh, S.M.; Foddai, S.; Serafini, M.; Bianco, A. Secondary metabolites from Teucrium polium L. collected in Southern Iran. Arab. J. Med. Arom. Pl. 2017, 3, 108–123. [Google Scholar]
- Venditti, A.; Bianco, A. Secondary metabolites of Hypericum richer Vill. collected in Central Italy: Chemotaxonomy and ethnomedicinal relevance. Tr. Phytochem. Res. 2018, 2, 155–162. [Google Scholar]
- Venditti, A.; Ballero, M.; Serafini, M.; Bianco, A. Polar compounds from Parentucellia viscosa (L.) Caruel from Sardinia. Nat. Prod. Res. 2015, 29, 602–606. [Google Scholar] [CrossRef]
- Rasmussen, L.S.; Rank, C.; Jensen, S.R. Transfer of iridoid glucosides from host plant Galium verum to hemiparasitic Euphrasia stricta. Biochem. Syst. Ecol. 2006, 34, 763–765. [Google Scholar] [CrossRef]
- D’Ambrosio, M.; Ciocarlan, A.; Aricu, A. Minor acetylated metabolites from Euphrasia rostkoviana. Nat. Prod. Res. 2018, 1–6. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Foddai, S.; Serafini, M.; Nicoletti, M.; Bianco, A. Chemical Traits of Hemiparasitism in Odontites luteus. Chem. Biodivers. 2017, 14, e1600416. [Google Scholar] [CrossRef]
- Jiangsu New Medical College. Chinese Medicine Dictionary; Shanghai People’s Publishing House: Shanghai, China, 1977; p. 286. [Google Scholar]
- Manandhar, N.P. Ethnobotanical note on folk-lore remedies of Baglung district, Nepal. Contrib.Nepal Stud. J. 1993, 20, 183–196. [Google Scholar]
- Angmo, K.; Adhikari, B.S.; Rawat, G.S. Changing aspects of Traditional Healthcare System in Western Ladakh, India. J. Ethnopharmacol. 2012, 143, 621–630. [Google Scholar] [CrossRef]
- Balami, N.P. Ethnomedicinal uses of plants among the Newar community of Pharping village of Kathmandu district, Nepal. Tribhuvan Univers. J. 2004, 24, 1–10. [Google Scholar] [CrossRef]
- Duffy Davis, J.; Banack, S.A. Ethnobotany of the Kiluhikturmiut Inuinnait of Kugluktuk, Nunavut, Canada. Ethnobiol. Lett. 2012, 3, 78–90. [Google Scholar] [CrossRef]
- Ballabh, B.; Chaurasia, O.P. Medicinal plants of cold desert Ladakh used in the treatment of stomach disorders. Indian J. Tradition. Know. 2009, 8, 185–190. [Google Scholar]
- Ballabh, B.; Chaurasia, O.P. Herbal Formulations from Cold Desert Plants Used For Gynecological Disorders. Ethnobot. Res. Appl. 2011, 9, 59–66. [Google Scholar] [CrossRef]
- Çakır, E.A. Traditional knowledge of wild edible plants of Iğdır Province (East Anatolia, Turkey). Acta Soc. Bot. Pol. 2017, 86, 1–20. [Google Scholar]
- Wang, P.; Kang, J.; Zheng, R.; Yang, Z.; Lu, J.; Gao, J.; Jia, Z. Scavenging effects of phenylpropanoid glycosides from Pedicularis on superoxide anion and hydroxyl radical by the Spin trapping method. Biochem. Pharmacol. 1996, 51, 687–691. [Google Scholar] [CrossRef]
- Wangchuk, P.; Dorji, S.; Ugyen; Thinley, J.; Afaq, S.H. High altitude plants used in Bhutanese traditional medicine (gSo-ba-rig-pa). Ethnobot. 2008, 20, 54–64. [Google Scholar]
- Wali, R.; Rahman, K.; Raja, N.I.; Qureshi, R.; Mashwani, Z.U.R. A quantitative medico-botanical expedition of Fairy Meadows National Park, Diamir, Gilgit Baltistan, Pakistan. bioRxiv 2018, 507848. [Google Scholar]
- Malla, B.; Gauchan, D.P.; Chhetri, R.B. An ethnobotanical study of medicinal plants used by ethnic people in Parbat district of western Nepal. J. Ethnopharmacol. 2015, 165, 103–117. [Google Scholar] [CrossRef]
- Pande, P.C.; Tiwari, L.; Pande, H.C. Ethnoveterinary plants of Uttaranchal—A review. Indian J. Tradition. Know. 2007, 6, 444–458. [Google Scholar]
- Singh, G.; Rawat, G.S. Ethnomedicinal survey of Kedarnath wildlife sanctuary in Western Himalaya, India. Indian J. Fund Appl. Life Sci. 2011, 1, 35–46. [Google Scholar]
- Uprety, Y.; Lacasse, A.; Asselin, H. Traditional uses of medicinal plants from the Canadian Boreal Forest for the management of chronic pain syndromes. Pain Pract. 2016, 16, 459–466. [Google Scholar] [CrossRef]
- Mentsikhang, L.K. Himalayan Doctors and Healing Herbs: The Amchi Tradition and Medicinal Plants of Mustang; Mera Publications: Kathmandu, Nepal, 2005. [Google Scholar]
- Wangchuk, P. Bioactive alkaloids from medicinal plants of Bhutan. Ph.D. Thesis, Department of Chemistry, University of Wollongong, Wollongong, Australia, 2004. [Google Scholar]
- Rinchen, T.; Pant, S. Ethnopharmacological uses of plants among inhabitants surrounding Suru and Zanskar valleys of cold desert, Ladakh. Int. J. Pharma. Bio Sci. 2014, 5, 486–494. [Google Scholar]
- Shang, X.; Tao, C.; Miao, X.; Wang, D.; Tangmuke; Dawa; Wang, Y.; Yang, Y.; Pan, H. Ethno-veterinary survey of medicinal plants in Ruoergai region, Sichuan province, China. J. Ethnopharmacol. 2012, 142, 390–400. [Google Scholar] [CrossRef]
- O’Neill, A.R.; Rana, S.K. An ethnobotanical analysis of parasitic plants (Parijibi) in the Nepal Himalaya. J. Ethnobiol. Ethnomed. 2016, 12, 79. [Google Scholar] [CrossRef][Green Version]
- Kapahi, B.; Srivastava, T.; Sarin, Y. Traditional medicinal plants of gurez (kashmir)—An ethnobotanical study. Anc. Sci. Life 1993, 13, 119–124. [Google Scholar]
- Singh, K.; Lal, B. Ethnomedicines used against four common ailments by the tribal communities of Lahaul-Spiti in western Himalaya. J. Ethnopharmacol. 2008, 115, 147–159. [Google Scholar] [CrossRef]
- Devi, U.; Seth, M.K.; Sharma, P.; Rana, J.C. Study on ethnomedicinal plants of kibber wildlife sanctuary: A cold desert in Trans Himalaya, India. J. Med. Pl. Res. 2013, 7, 3400–3419. [Google Scholar]
- Dutt, H.C.; Bhagat, N.; Pandita, S. Oral traditional knowledge on medicinal plants in jeopardy among Gaddi shepherds in hills of northwestern Himalaya, J&K, India. J. Ethnopharmacol. 2015, 168, 337–348. [Google Scholar]
- Gairola, S.; Sharma, J.; Bedi, Y.S. A cross-cultural analysis of Jammu, Kashmir and Ladakh (India) medicinal plant use. J. Ethnopharmacol. 2014, 155, 925–986. [Google Scholar] [CrossRef]
- Salim, M.A.; Ranjitkar, S.; Hart, R.; Khan, T.; Ali, S.; Kiran, C.; Parveen, A.; Batool, Z.; Bano, S.; Xu, J. Regional trade of medicinal plants has facilitated the retention of traditional knowledge: Case study in Gilgit-Baltistan Pakistan. J. Ethnobiol. Ethnomed. 2019, 15, 1–33. [Google Scholar] [CrossRef]
- Singh, K.N. Traditional knowledge on ethnobotanical uses of plant biodiversity: A detailed study from the Indian western Himalaya. Biodivers. Res. Conserv. 2012, 28, 63–77. [Google Scholar] [CrossRef]
- Bano, A.; Ahmad, M.; Zafar, M.; Sultana, S.; Rashid, S.; Khan, M.A. Ethnomedicinal knowledge of the most commonly used plants from Deosai Plateau, Western Himalayas, Gilgit Baltistan, Pakistan. J. Ethnopharmacol. 2014, 155, 1046–1052. [Google Scholar] [CrossRef]
- Kayani, S.; Ahmad, M.; Sultana, S.; Shinwari, Z.K.; Zafar, M.; Yaseen, G.; Hussain, M.; Bibi, T. Ethnobotany of medicinal plants among the communities of Alpine and Sub-alpine regions of Pakistan. J. Ethnopharmacol. 2015, 164, 186–202. [Google Scholar] [CrossRef]
- Yoo, S.J.; Yim, D.S.; Lee, S.Y. Biological activities of Pedicularis resupinata var. oppositifolia. Korean J. Pharmacog. 1993, 24, 258–262. [Google Scholar]
- Marles, R.; Farnsworth, N. Antidiabetic plants and their active constituents. Phytomedicine 1995, 2, 137–189. [Google Scholar] [CrossRef]
- Toba, S. Plant names in Khaling: A study in ethnobotany and village economy. Kailash 1975, 3, 145–169. [Google Scholar]
- Rokaya, M.B.; Münzbergová, Z.; Shrestha, M.R.; Timsina, B. Distribution patterns of medicinal plants along an elevational gradient in central Himalaya, Nepal. J. Mt. Sci. 2012, 9, 201–213. [Google Scholar] [CrossRef]
- Wagner, A.; Kriechbaum, M.; Koch, M.A. Applied Vulnerability Assessment of Useful Plants: A case study of Tibetan Medicinal Plants from Nepal. Botanische Jahrbücher 2008, 127, 359–387. [Google Scholar] [CrossRef]
- Magsar, U.; Nyamsuren, K.; Khadbaatar, S.; Tovuudorj, M.E.; Baasansuren, E.; Indree, T.; Lkhagvadorj, K.; Kwon, O. Survey of medicinal plants in the Khuvsgul and Khangai Mountain regions of Mongolia. J. Ecol. Environ. 2017, 41, 1–5. [Google Scholar] [CrossRef]
- Qiu, S.; Sun, H.; Zhang, A.H.; Xu, H.Y.; Yan, G.L.; Han, Y.; Wang, X.J. Natural alkaloids: Basic aspects, biological roles, and future perspectives. Chin. J. Nat. Med. 2014, 12, 401–406. [Google Scholar] [CrossRef]
- Hu, L.L. Protection of Pedicularis on liver lesion of mice induced by Alloxan. J. Ankang Univ. 2007, 19, 72–74. [Google Scholar]
- Dulger, B.; Ugurlu, E. Evaluation of Antimicrobial Activity of Some Endemic Scrophulariaceae. Members from Turkey. Pharm. Biol. 2005, 43, 275–279. [Google Scholar] [CrossRef][Green Version]
- Yang, J.X.; Tian, J.W.; Li, F.R. Influence on antioxidative ability of Taibaishen in mice. Northwest Pharmaceut. J. 2001, 16, 209–211. [Google Scholar]
- Gao, M.; Li, Y.; Yang, J. Protective effect of Pedicularis decora Franch root extracts on oxidative stress and hepatic injury in alloxan-induced diabetic mice. J. Med. Pl. Res. 2011, 5, 5848–5856. [Google Scholar]
- Gonchig, E.; Erdenebat, S.; Togtoo, O.; Bataa, S.; Gendaram, O.; Kim, Y.S.; Ryu, S.Y. Antimicrobial activity of Mongolian medicinal plants. Nat. Prod. Sci. 2008, 14, 1–5. [Google Scholar]
- Moreno-Escobar, J.A.; Bazaldúa, S.; Villarreal, M.L.; Bonilla-Barbosa, J.R.; Mendoza, S.; López, V.R. Cytotoxic and antioxidant activities of selected Lamiales species from Mexico. Pharm. Biol. 2011, 49, 1243–1248. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef]
- Moreira, R.; Pereira, D.M.; Valentão, P.; Andrade, P.B. Pyrrolizidine Alkaloids: Chemistry, Pharmacology, Toxicology and Food Safety. Int. J. Mol. Sci. 2018, 19, 1668. [Google Scholar] [CrossRef]
- Korkina, L.; Kostyuk, V.; De Luca, C.; Pastore, S. Plant phenylpropanoids as emerging anti-inflammatory agents. Mini Rev. Med. Chem. 2011, 11, 823–835. [Google Scholar] [CrossRef]
- Muller-Harvey, I.; McAllan, A.B. Tannins: Their biochemistry and nutritional properties. Adv. Plant Cell Biochem. Biotechnol. 1992, 1, 151–217. [Google Scholar]
- Fu, G.; Pang, H.; Wong, Y.H. Naturally occurring phenylethanoid glycosides: Potential leads for new therapeutics. Curr. Med. Chem. 2008, 15, 2592–2613. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 1–15. [Google Scholar] [CrossRef]
- Steiner, L.F.; Summerland, S.A. Xanthone as an Ovicide and Larvicide for the Codling moth. J. Econ. Èntomol. 1943, 36, 435–439. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Statti, G.; Menichini, F. Biological and Pharmacological Activities of Iridoids: Recent Developments. Mini Rev. Med. Chem. 2008, 8, 399–420. [Google Scholar] [CrossRef]
- Ghisalberti, E. Biological and pharmacological activity of naturally occurring iridoids and secoiridoids. Phytomedicine 1998, 5, 147–163. [Google Scholar] [CrossRef]
- Li, X.X.; Zhou, Z.K. Endemic Wild Ornamental Plants from Northwestern Yunnan, China. HortScience 2005, 40, 1612–1619. [Google Scholar] [CrossRef]
- Clark, C. Inuit Ethnobotany and Ethnoecology in Nunavik and Nunatsiavut, Northeastern Canada. Ph.D. Thesis, Univeristy of Montreal, Montreal, QC, Canada, 2012. [Google Scholar]
- Tardío, J.; Morales, R.; Pardo-De-Santayana, M.; Pardo-De-Santayana, M.; Pardo-De-Santayana, M. Ethnobotanical review of wild edible plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- Menges, E.S. Population Viability Analysis for an Endangered Plant. Conserv. Biol. 1990, 4, 52–62. [Google Scholar] [CrossRef]
- Cho, W.B.; Choi, I.S.; Choi, B.H. Development of microsatellite markers for the endangered Pedicularisis hidoyana (Orobanchaceae) using next-generation sequencing source. Appl. Pl. Sci. 2003, 3, 1–3. [Google Scholar]
- Ali, H. Conservation Issues of High Altitude Medicinal Plants of Pakistan. In Proceedings and Abstract of the 2nd International Symposium on Kaz Mountains (Mount Ida) and Edremit; Edremit, Turkey, 3–5 May 2013, Ikes2013: Izmir, Turkey, 2013; pp. 82–93. Available online: https://www.academia.edu/4814159/Proceedings-The_Second_International_Symposium_on_Kaz_Mountains_and_Edremit-Human_-_Environment_Interactions (accessed on 15 July 2019).
- Liu, Y.Y.; Hu, Y.K.; Yu, J.M.; Li, K.H.; Gao, G.G.; Wang, X. Study on harmfulness of Pedicularis myriophylla and its control measures. Arid Zone Res. 2008, 25, 778–782. [Google Scholar]
- Smith-Kuebel, C.; Lillybridge, T.R. Report on Sensitive plants and noxious weeds of the Wenatchee National Forest; The Cultural Resources and Vegetation Management Division of the Bonneville Power Administration: Portland, OR, USA, 1993. [Google Scholar]
- Santisuk, T.; Chayamarit, K.; Pooma, R.; Suddee, S. Thailand Red Data: Plants; Office of Natural Resources and Environmental Policy and Planning: Bangkok, Thailand, 2006; pp. 1–256. [Google Scholar]
- Conti, F.; Bartolucci, F. Specie a Rischio in Abruzzo. Elenco Delle Piante di Interesse Conservazionistico. In La Biodiversità vegetale in Abruzzo. Tutela e conservazione del patrimonio vegetale abruzzese; One Group Ed.: L’Aquila, Italy, 2012; pp. 81–109. [Google Scholar]
- Kumar, A.; Mitra, M.; Adhikari, B.S.; Rawat, G.S. Flora of Niti Valley: A cold arid region of Nanda Devi Biosphere Reserve, Western Himalaya, India. Check List. 2016, 12, 1824. [Google Scholar] [CrossRef]
- Yu, W.B.; Wang, H.; Li, D.Z. (2204) Proposal to conserve Pedicularis stenocorys against P. stenantha (Orobanchaceae). TAXON 2013, 62, 1066–1067. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).