Biochemical and Molecular Investigation of In Vitro Antioxidant and Anticancer Activity Spectrum of Crude Extracts of Willow Leaves Salix safsaf
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Chemicals and Supplies
2.3. Sample Collection and Preparation
2.4. Preparation of Crude Fractions/Extracts of Salix safsaf Leaves
2.5. Cell Lines and Culturing Conditions
2.6. Screening for Antiproliferative Activity by MTT Assay
2.7. Apoptosis and Cell Cycle Analysis by Flow Cytometry
2.8. Gene Expression Profiling by RT-qPCR
2.9. Extraction of Protein and Western Blot Analysis
2.10. Orbitrap Mass Spectrometry
2.11. Statistical Analyses
3. Results
3.1. Effect of the Different Fractions and Extracts on the Proliferation of Cancerous Cells
3.2. Identification of Phenolic Compounds Present in Chloroform (F2) and Ethyl Acetate (F3) Soluble Fractions of Willow Leaves
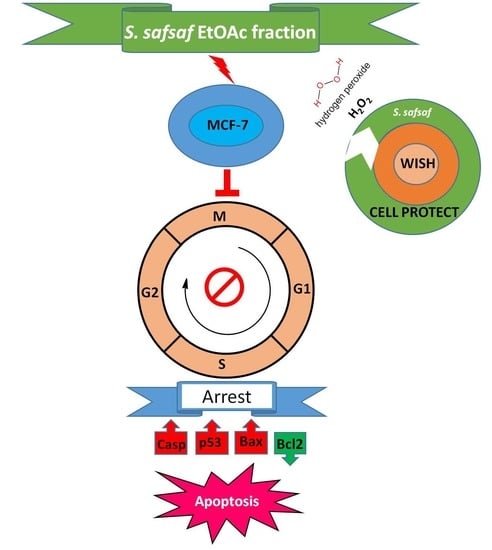
3.3. Organic Fractions F2 and F3 of Willow Leaves Induce Apoptosis in MCF-7 Cells
3.4. Cyto-Protective Effects of Ethyl Acetate Fraction (F3) of Willow Leaves against Oxidative Stress in H2O2-Exposed WISH Cells
3.5. Ethyl Acetate Fraction (F3) of Willow Leaves Does Not Elicit ROS Elevation in MCF-7 Cell Line
3.6. Apoptotic Marker Protein and mRNA Expression in F3-Treated MCF-7 Cells
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Afshin, A.; Agrawal, A.; et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Cancer Facts & Figures 2019. Available online: https://www.cancer.org/.../2019/cancer-facts-and-figures-2019.pdf (accessed on 11 July 2020).
- International Agency for Research on Cancer. World Cancer Report 2014; Stewart, B.W., Wild, C.P., Eds.; International Agency for Research on Cancer, WHO Press: Geneva, Switzerland, 2014; ISBN 978-92-832-0443-5. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, M.S.; Robertson, A.A.B.; Cooper, M.A. Natural product and natural product derived drugs in clinical trials. Nat. Prod. Rep. 2014, 31, 1612–1661. [Google Scholar] [CrossRef] [PubMed]
- Mussarat, S.; Abdel-Salam, N.M.; Tariq, A.; Wazir, S.M.; Ullah, R.; Adnan, M. Use of Ethnomedicinal Plants by the People Living around Indus River. Evid. Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Sadia, S.; Pan, K.; Ullah, I.; Mussarat, S.; Sun, F.; Abiodun, O.O.; Batbaatar, A.; Li, Z.; Song, D.; et al. A systematic review on ethnomedicines of anti-cancer plants. Phytother. Res. 2017, 31, 202–264. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.; Hmadi, R.; Kareh, M.; Tohme, R.; Darwiche, N. Cell death mechanisms of plant-derived anticancer drugs: Beyond apoptosis. Apoptosis 2015, 20, 1531–1562. [Google Scholar] [CrossRef]
- Hassan, B. Plants and Cancer Treatment. Med. Plants Use Prev. Treat. Dis. IntechOpen 2020. [Google Scholar] [CrossRef] [Green Version]
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [Green Version]
- Mahdi, J.G.; Mahdi, A.J.; Bowen, I.D. The historical analysis of aspirin discovery, its relation to the willow tree and antiproliferative and anticancer potential. Cell Prolif. 2006, 39, 147–155. [Google Scholar] [CrossRef] [PubMed]
- El-Shemy, H.A.; Aboul-Enein, A.M.; Aboul-Enein, M.I.; Issa, S.I.; Fujita, K. The effect of willow leaf extracts on human leukemic cells in vitro. J. Biochem. Mol. Biol. 2003, 36, 387–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shemy, H.A.; Aboul-Enein, A.M.; Aboul-Enein, K.M.; Fujita, K. Willow Leaves’ Extracts Contain Anti-Tumor Agents Effective against Three Cell Types. PLoS ONE 2007, 2, e178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboul-Soud, M.A.; Al-Amri, M.Z.; Kumar, A.; Al-Sheikh, Y.; Ashour, A.E.; El-Kersh, T.A. Specific Cytotoxic Effects of Parasporal Crystal Proteins Isolated from Native Saudi Arabian Bacillus thuringiensis Strains against Cervical Cancer Cells. Molecules 2019, 24, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashour, A.E.; Ahmed, A.F.; Kumar, A.; Zoheir, K.M.; Aboul-Soud, M.A.; Ahmad, S.F.; Attia, S.M.; Abd-Allah, A.R.A.; Cheryan, V.T.; Rishi, A.K. Thymoquinone inhibits growth of human medulloblastoma cells by inducing oxidative stress and caspase-dependent apoptosis while suppressing NF-κB signaling and IL-8 expression. Mol. Cell. Biochem. 2016, 416, 141–155. [Google Scholar] [CrossRef]
- Lombardo, L.J.; Lee, F.Y.; Chen, P.; Norris, D.; Barrish, J.C.; Behnia, K.; Castaneda, S.; Cornelius, L.A.M.; Das, J.; Doweyko, A.M.; et al. Discovery ofN-(2-Chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a Dual Src/Abl Kinase Inhibitor with Potent Antitumor Activity in Preclinical Assays. J. Med. Chem. 2004, 47, 6658–6661. [Google Scholar] [CrossRef]
- El-Hallouty, S.M.; Soliman, A.A.; Nassrallah, A.A.; Salamatullah, A.; AlKaltham, M.S.; Kamal, K.Y.; Hanafy, E.A.; Gaballa, H.S.; Aboul-Soud, M.A. Crude Methanol Extract of Rosin Gum Exhibits Specific Cytotoxicity against Human Breast Cancer Cells via Apoptosis Induction. AntiCancer Agents Med. Chem. 2020, 20, 1–27. [Google Scholar] [CrossRef]
- Saquib, Q.; Musarrat, J.; Siddiqui, M.; Dutta, S.; Dasgupta, S.; Giesy, J.P.; Alkhedhairy, A.A. Cytotoxic and necrotic responses in human amniotic epithelial (WISH) cells exposed to organophosphate insecticide phorate. Mutat. Res. Toxicol. Environ. Mutagen. 2012, 744, 125–134. [Google Scholar] [CrossRef]
- Saquib, Q.; Attia, S.M.; Siddiqui, M.; Aboul-Soud, M.A.; Alkhedhairy, A.A.; Giesy, J.P.; Musarrat, J. Phorate-induced oxidative stress, DNA damage and transcriptional activation of p53 and caspase genes in male Wistar rats. Toxicol. Appl. Pharmacol. 2012, 259, 54–65. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [Green Version]
- Solowey, E.; Lichtenstein, M.; Sallon, S.; Paavilainen, H.; Solowey, E.; Lorberboum-Galski, H. Evaluating Medicinal Plants for Anticancer Activity. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saklani, A.; Kutty, S.K. Plant-derived compounds in clinical trials. Drug Discov. Today 2008, 13, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Soud, M.A.M.; El-Shemy, H.A.; Aboul-Enein, K.M.; Mahmoud, A.M.; Al-Abd, A.M.; Lightfoot, D.A. Effects of plant-derived anti-leukemic drugs on individualized leukemic cell population profiles in Egyptian patients. Oncol. Lett. 2016, 11, 642–648. [Google Scholar] [CrossRef] [PubMed]
- El-Shemy, H.A.; Aboul-Soud, M.A.M.; Nassr-Allah, A.A.; Aboul-Enein, K.M.; Kabash, A.; Yagi, A. Antitumor Properties and Modulation of Antioxidant Enzymes Activity by Aloe vera Leaf Active Principles Isolated via Supercritical Carbon Dioxide Extraction. Curr. Med. Chem. 2010, 17, 129–138. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Aboul-Soud, M.A.; Han, J.; Al-Sheikh, Y.A.; Al-Abd, A.M.; El-Shemy, H.A. Transcriptional profiling of breast cancer cells in response to mevinolin: Evidence of cell cycle arrest, DNA degradation and apoptosis. Int. J. Oncol. 2016, 48, 1886–1894. [Google Scholar] [CrossRef]
- El-Desoky, G.E.; Abdel-Ghaffar, A.; Al-Othman, Z.A.; Habila, M.A.; Al-Sheikh, Y.A.; Ghneim, H.K.; Giesy, J.P.; Aboul-Soud, M.A.M. Curcumin protects against tartrazine-mediated oxidative stress and hepatotoxicity in male rats. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 635–645. [Google Scholar]
- Al-Sheikh, Y.A.; Ghneim, H.K.; Aljaser, F.S.; Aboul-Soud, M.A. Ascorbate ameliorates Echis coloratus venom-induced oxidative stress in human fibroblasts. Exp. Ther. Med. 2017, 14, 703–713. [Google Scholar] [CrossRef]
- Nassr-Allah, A.A.; Aboul-Enein, A.M.; Aboul-Enein, K.M.; Lightfoot, D.A.; Cocchetto, A.; El-Shemy, H.A. Anti-cancer and anti-oxidant activity of some Egyptian medicinal plants. J. Med. Plants Res. 2009, 3, 799–808. [Google Scholar]
- Enayat, S.; Ceyhan, M.Ş; Başaran, A.A.; Gursel, M.; Banerjee, S. Anticarcinogenic Effects of the Ethanolic Extract ofSalix aegyptiacain Colon Cancer Cells: Involvement of Akt/PKB and MAPK Pathways. Nutr. Cancer 2013, 65, 1045–1058. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Ballesteros-Vivas, D.; Parada-Alfonso, F.; Ibañez, E.; Cifuentes, A. Recent applications of high resolution mass spectrometry for the characterization of plant natural products. TrAC Trends Anal. Chem. 2019, 112, 87–101. [Google Scholar] [CrossRef]
- Qiao, X.; Li, R.; Song, W.; Miao, W.-J.; Liu, J.; Chen, H.; Guo, D.-A.; Ye, M. A targeted strategy to analyze untargeted mass spectral data: Rapid chemical profiling of Scutellaria baicalensis using ultra-high performance liquid chromatography coupled with hybrid quadrupole orbitrap mass spectrometry and key ion filtering. J. Chromatogr. A 2016, 1441, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Bailón-Moscoso, N.; Cevallos-Solorzano, G.; Romero-Benavides, J.C.; Orellana, M.I.R. Natural Compounds as Modulators of Cell Cycle Arrest: Application for Anticancer Chemotherapies. Curr. Genom. 2017, 18, 106–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, K.; Nomura, Y.; Sawajiri, M.; Mohapatra, P.K.; El-Shemy, H.A.; Nguyen, N.T.; Hosokawa, M.; Miyashita, K.; Maeda, T.; Saneoka, H.; et al. The extracts of Japanese willow tree species are effective forapoptotic desperation or differentiation of acute myeloid leukemia cells. Pharmacogn. Mag. 2014, 10, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.W.H.; Wong, S.C.; Letham, D.S.; Hocart, C.H.; Farquhar, G.D. Effects of Elevated [CO2] and Nitrogen Nutrition on Cytokinins in the Xylem Sap and Leaves of Cotton. Plant Physiol. 2000, 124, 767–779. [Google Scholar] [CrossRef] [Green Version]
- Hirose, N.; Takei, K.; Kuroha, T.; Kamada-Nobusada, T.; Hayashi, H.; Sakakibara, H. Regulation of cytokinin biosynthesis, compartmentalization and translocation. J. Exp. Bot. 2008, 59, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Bacher, N.; Tiefenthaler, M.; Sturm, S.; Stuppner, H.; Ausserlechner, M.J.; Kofler, R.; Konwalinka, G. Oxindole alkaloids from Uncaria tomentosa induce apoptosis in proliferating, G0/G1-arrested and bcl-2-expressing acute lymphoblastic leukaemia cells. Br. J. Haematol. 2006, 132, 615–622. [Google Scholar] [CrossRef]
- Abo-Salem, H.M.; Nassrallah, A.; Soliman, A.A.; Ebied, M.S.; Elawady, M.E.; Abdelhamid, S.A.; El-Sawy, E.R.; Al-Sheikh, Y.; Aboul-Soud, M.A. Synthesis and Bioactivity Assessment of Novel Spiro Pyrazole-Oxindole Congeners Exhibiting Potent and Selective in vitro Anticancer Effects. Molecules 2020, 25, 1124. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, P.-C.; Lee, W.-J.; Yang, S.-F.; Tan, P.; Chen, H.-Y.; Lee, L.-M.; Chang, J.-L.; Lai, G.-M.; Chow, J.-M.; Chien, M.-H. Nobiletin suppresses the proliferation and induces apoptosis involving MAPKs and caspase-8/-9/-3 signals in human acute myeloid leukemia cells. Tumor Boil. 2014, 35, 11903–11911. [Google Scholar] [CrossRef]
- Şen, A.; Atmaca, P.; Terzioglu, G.; Arslan, S. Anticarcinogenic effect and carcinogenic potential of the dietary phenolic acid: O-coumaric acid—PubMed. Nat. Prod. Commun. 2013, 8, 1269–1274. [Google Scholar]
- Abaza, M.S.I.; Al-Attiyah, R.; Bhardwaj, R.; Abbadi, G.; Koyippally, M.; Afzal, M. Syringic acid from Tamarix aucheriana possesses antimitogenic and chemo-sensitizing activities in human colorectal cancer cells. Pharm. Biol. 2013, 51, 1110–1124. [Google Scholar] [CrossRef] [Green Version]
- Gheena, S.; Ezhilarasan, D. Syringic acid triggers reactive oxygen species-mediated cytotoxicity in HepG2 cells. Hum. Exp. Toxicol. 2019, 38, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.S.; Fornace, A.J., Jr. Role of p53 family members in apoptosis. J. Cell. Physiol. 2000, 182. [Google Scholar] [CrossRef]
- Hastak, K.; Agarwal, M.K.; Mukhtar, H.; Agarwal, M.L. Ablation of either p21 or Bax prevents p53-dependent apoptosis induced by green tea polyphenol epigallocatechin-3-gallate. FASEB J. 2005, 19, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mayo, L.D.; Donner, D.B. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. USA 2001, 98, 11598–11603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlachojannis, J.; Magora, F.; Chrubasik, S. Willow Species and Aspirin: Different Mechanism of Actions. Phytother. Res. 2011, 25, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Sabaa, M.; Elfayoumi, H.M.; Elshazly, S.; Youns, M.; Barakat, W. Anticancer activity of salicin and fenofibrate. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 1061–1071. [Google Scholar] [CrossRef]
- Hamadou, M.H.; Kerkatou, M.; Gatto, P.; Pancher, M.; Bisio, A.; Inga, A.; Menad, A.; Benayache, S.; Benayache, F.; Ameddah, S. Apigenin rich-Limonium duriusculum (de Girard) Kuntze promotes apoptosis in HCT116 cancer cells. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Maeda, Y.; Takahashi, H.; Nakai, N.; Yanagita, T.; Ando, N.; Okubo, T.; Saito, K.; Shiga, K.; Hirokawa, T.; Hara, M.; et al. Apigenin induces apoptosis by suppressing Bcl-xl and Mcl-1 simultaneously via signal transducer and activator of transcription 3 signaling in colon cancer. Int. J. Oncol. 2018, 52, 1661–1673. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Lin, C.-H.; Lin, J.-T.; Cheng, Y.-F.; Chen, H.-M.; Kao, S.-H. Purine analogue ENERGI-F706 induces apoptosis of 786-O renal carcinoma cells via 5′-adenosine monophosphate-activated protein kinase activation. Mol. Med. Rep. 2015, 12, 4566–4571. [Google Scholar] [CrossRef] [Green Version]
- Xia, B.; Wang, J. Effects of Adenosine on Apoptosis of Ovarian Cancer A2780 Cells via ROS and Caspase Pathways. Onco Targets Ther. 2019, 12, 9473–9480. [Google Scholar] [CrossRef] [Green Version]
- Su, W.-W.; Huang, J.-Y.; Chen, H.-M.; Lin, J.-T.; Kao, S.-H. Adenine inhibits growth of hepatocellular carcinoma cells via AMPK-mediated S phase arrest and apoptotic cascade. Int. J. Med. Sci. 2020, 17, 678–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, Y.; Hori, Y.; Sakai, S.; Honma, Y. Control of differentiation and apoptosis of human myeloid leukemia cells by cytokinins and cytokinin nucleosides, plant redifferentiation-inducing hormones. Cell Growth Differ. 2002, 13, 19–26. [Google Scholar] [PubMed]
- Wawrzyniak, D.; Rolle, K.; Barciszewski, J. Biological activity of N6-furfuryladenosine. Postępy Biochem. 2019, 65, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Müller, W.E.; Eckert, G.P. Cytoprotective effects of olive mill wastewater extract and its main constituent hydroxytyrosol in PC12 cells. Pharmacol. Res. 2010, 62, 322–327. [Google Scholar] [CrossRef]
- Merchant, K.; Kumi-Diaka, J.; Rathinavelu, A.; Esiobu, N.; Zoeller, R.; Hartmann, J.; Johnson, M. Molecular basis of the anti-cancer effects of genistein isoflavone in LNCaP prostate cancer cells. Funct. Foods Heal. Dis. 2011, 1, 91. [Google Scholar] [CrossRef]
- Lee, J.; Park, A.; Son, H.; Lim, H.-J.; Rha, Y.-A.; Kang, H. Spirulina Extract Enhanced a Protective Effect in Type 1 Diabetes by Anti-Apoptosis and Anti-ROS Production. Nutrients 2017, 9, 1363. [Google Scholar] [CrossRef] [Green Version]
- Ferhi, S.; Santaniello, S.; Zerizer, S.; Cruciani, S.; Fadda, A.; Sanna, D.; Dore, A.; Maioli, M.; D’Hallewin, G. Total Phenols from Grape Leaves Counteract Cell Proliferation and Modulate Apoptosis-Related Gene Expression in MCF-7 and HepG2 Human Cancer Cell Lines. Molecules 2019, 24, 612. [Google Scholar] [CrossRef] [Green Version]
- Ďuračková, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 8408, 459–469. [Google Scholar]
- Abramovič, H. Antioxidant Properties of Hydroxycinnamic Acid Derivatives. Coffee Health Dis. Prev. 2015, 843–852. [Google Scholar] [CrossRef]
- Guo, F.; Wu, R.; Xu, J. Salicin prevents TNF-α-induced cellular senescence in human umbilical vein endothelial cells (HUVECs). Artif. Cells Nanomed. Biotechnol. 2019, 47, 2618–2623. [Google Scholar] [CrossRef]








| Fraction/Extract | MCF-7 | HCT-116 | HeLa | HepG2 |
|---|---|---|---|---|
| F1 | NE | NE | NE | NE |
| F2 | 128.1 | 151.49 | 141.55 | 136.74 |
| F3 | 111.74 | 195.56 | 156.23 | 172.39 |
| E1 | NE * | NE | NE | NE |
| E2 | NE | NE | NE | NE |
| E3 | NE | NE | NE | NE |
| E4 | NE | NE | NE | NE |
| Name | Molecular Formula | Molecular Weight | [M + H]+ | Rt (min) | Area F2 | Area F3 |
|---|---|---|---|---|---|---|
| Catechol | C6H6O2 | 110.0363 | 111.0441 | 15.63 | 5.69 × 106 | 1.68 × 107 |
| 2-Aminopurine | C5H5N5 | 135.0541 | 136.0619 | 17.17 | 4.57 × 106 | 1.78 × 107 |
| Vanillin | C8H8O3 | 152.0469 | 153.0547 | 18.00 | 1.04 × 107 | 1.34 × 107 |
| Coumaric Acid | C9H8O3 | 164.0471 | 165.0549 | 16.36 | 6.51 × 106 | 4.70 × 107 |
| Syringic Acid | C9H10O5 | 198.0501 | 199.0579 | 10.90 | NF | 6.90 × 106 |
| Trans-Zeatin | C10H13N5O | 219.1104 | 220.1182 | 7.30 | NF | 4.40 × 106 |
| Adenosine | C10H13N5O4 | 267.0964 | 268.1042 | 4.30 | 5.45 × 106 | 3.47 × 107 |
| Apigenin | C15H10O5 | 270.0523 | 271.0602 | 22.17 | 1.27 × 107 | 5.60 × 107 |
| Salicin | C13H18O7 | 286.1048 | 287.1126 | 20.01 | NF | 3.67 × 106 |
| Olomoucine | C15H18N6O | 298.1539 | 299.1617 | 23.66 | 9.27 × 106 | NF |
| Isopentenyladenosine | C15H21N5O4 | 298.1540 | 299.1619 | 23.65 | NF | 2.13 × 107 |
| Isorhamnetin | C16H12O7 | 316.0584 | 317.0656 | 20.27 | 2.95 × 106 | 2.95 × 106 |
| Tangeritin | C20H20O7 | 372.1209 | 373.1282 | 23.02 | 3.00 × 106 | 4.05 × 106 |
| Quercetin | C21H20O11 | 448.1006 | 449.1084 | 19.65 | NF | 2.83 × 108 |
| isoquercetin | C21H20O12 | 464.0955 | 465.1028 | 18.88 | 1.49 × 107 | 1.66 × 107 |
| Rutin | C27H30O16 | 610.1534 | 611.1612 | 18.30 | 1.08 × 106 | 1.90 × 107 |
| Name | Molecular Formula | Molecular Weight | [M − H]− | Rt (min) | Area F2 | Area F3 |
|---|---|---|---|---|---|---|
| Catechol | C6H6O2 | 110.0373 | 109.0295 | 8.88 | NF | 1.24 × 109 |
| 6-Methoxypurine | C6H6N4O | 150.0547 | 149.0469 | 1.88 | NF | 2.67 × 106 |
| 2,6-Diaminopurine | C5H6N6 | 150.0659 | 149.0581 | 21.82 | NF | 2.31 × 107 |
| Coumaric Acid | C9H8O3 | 164.0479 | 163.0401 | 12.9 | NF | 4.52 × 108 |
| Gallic Acid | C7H6O5 | 170.022 | 169.0142 | 2.12 | NF | 1.76 × 107 |
| Syringic Acid | C9H10O5 | 198.0533 | 197.0455 | 10.82 | 2.51 × 106 | 6.91 × 106 |
| 6-Anilinopurine | C11H9N5 | 211.0863 | 210.0785 | 12.64 | NF | 3.67 × 106 |
| Kinetin | C10H9N5O | 215.0812 | 214.0734 | 2.28 | NF | 5.40 × 105 |
| Trans-Zeatin | C10H13N5O | 219.1125 | 218.1047 | 2.33 | 1.50 × 106 | 6.94 × 106 |
| Benzyladenine | C12H11N5 | 225.102 | 224.0942 | 19.95 | NF | 1.44 × 106 |
| Adenosine | C10H13N5O4 | 267.0973 | 266.0895 | 7.85 | NF | 9.08 × 105 |
| Apigenin | C15H10O5 | 270.0533 | 269.0455 | 22.33 | 1.84 × 107 | 6.60 × 107 |
| Salicin | C13H18O7 | 286.1058 | 285.098 | 10.57 | 1.23 × 108 | 1.68 × 108 |
| Olomoucine | C15H18N6O | 298.1547 | 297.1469 | 17.35 | 4.33 × 105 | NF |
| Geniposidic Acid | C16H22O10 | 374.1213 | 373.114 | 18.36 | 5.38 × 106 | 5.88 × 106 |
| 3,5,6,7,8,3′,4′-Heptemthoxyflavone | C22H24O9 | 432.1419 | 431.1348 | 20.87 | 3.95 × 106 | 5.21 × 106 |
| Quercetin | C21H20O11 | 448.1011 | 447.0933 | 19.72 | 2.16 × 107 | 7.85 × 108 |
| Rutin | C27H30O16 | 610.1539 | 609.1461 | 18.5 | 4.78 × 106 | 4.49 × 107 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboul-Soud, M.A.M.; Ashour, A.E.; Challis, J.K.; Ahmed, A.F.; Kumar, A.; Nassrallah, A.; Alahmari, T.A.; Saquib, Q.; Siddiqui, M.A.; Al-Sheikh, Y.; et al. Biochemical and Molecular Investigation of In Vitro Antioxidant and Anticancer Activity Spectrum of Crude Extracts of Willow Leaves Salix safsaf. Plants 2020, 9, 1295. https://doi.org/10.3390/plants9101295
Aboul-Soud MAM, Ashour AE, Challis JK, Ahmed AF, Kumar A, Nassrallah A, Alahmari TA, Saquib Q, Siddiqui MA, Al-Sheikh Y, et al. Biochemical and Molecular Investigation of In Vitro Antioxidant and Anticancer Activity Spectrum of Crude Extracts of Willow Leaves Salix safsaf. Plants. 2020; 9(10):1295. https://doi.org/10.3390/plants9101295
Chicago/Turabian StyleAboul-Soud, Mourad A. M., Abdelkader E. Ashour, Jonathan K. Challis, Atallah F. Ahmed, Ashok Kumar, Amr Nassrallah, Tariq A. Alahmari, Quaiser Saquib, Maqsood A. Siddiqui, Yazeed Al-Sheikh, and et al. 2020. "Biochemical and Molecular Investigation of In Vitro Antioxidant and Anticancer Activity Spectrum of Crude Extracts of Willow Leaves Salix safsaf" Plants 9, no. 10: 1295. https://doi.org/10.3390/plants9101295