Modeling the Population Dynamics and Management of Italian Ryegrass under Two Climatic Scenarios in Brazil
Abstract
:1. Introduction
2. Results
2.1. Scenario 1 (Average Temperature 2007–2017)
2.2. Scenario 2 (2.5 °C Increase in Average Temperature)
3. Discussion
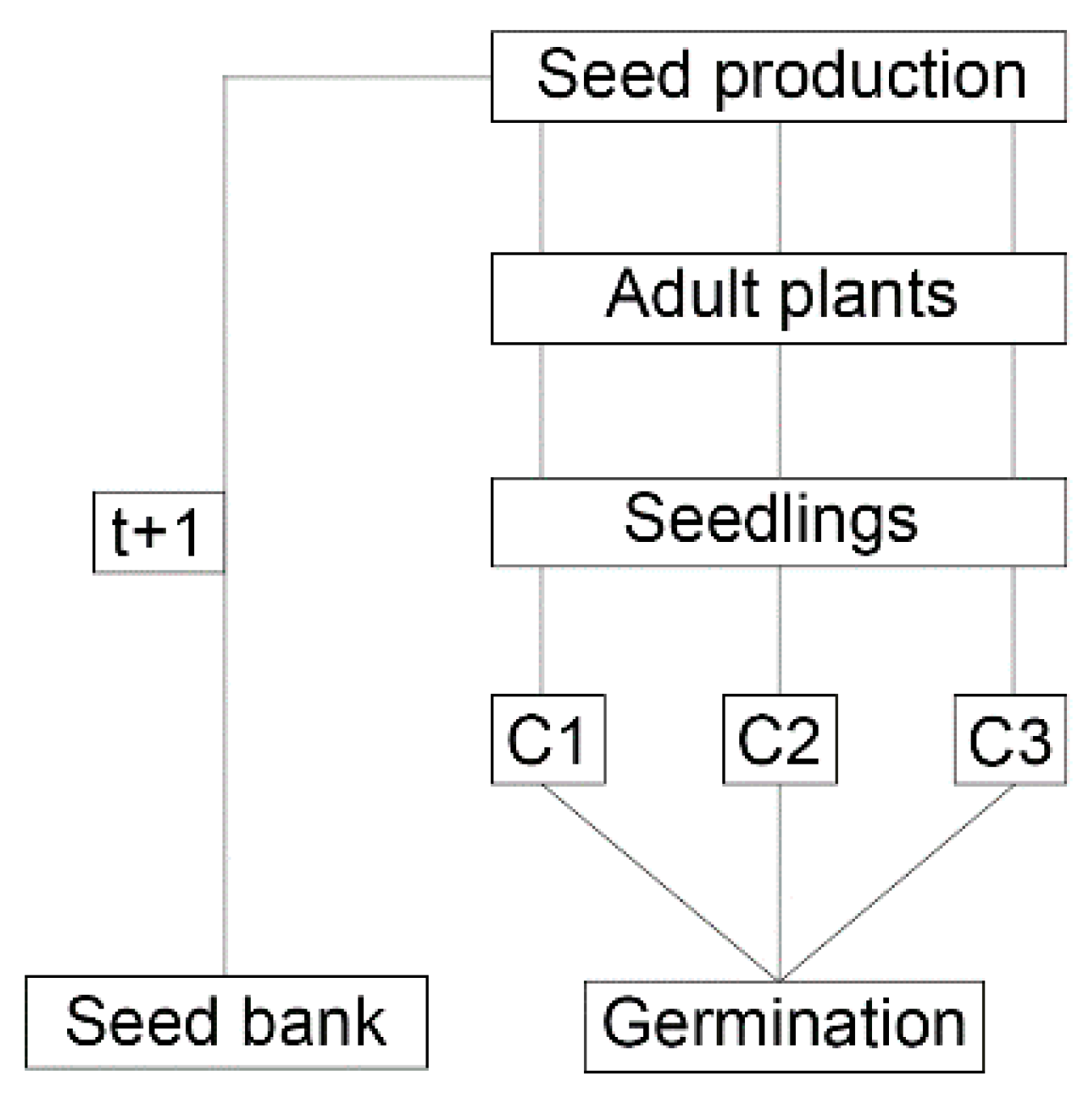
4. Materials and Methods
4.1. Plant Emergence
4.2. Seedling Survival
4.3. Seed Production
4.4. Seed Bank
4.5. Model Parameters
4.6. Assessing Management Strategies
Author Contributions
Funding
Conflicts of Interest
References
- U.S. National Plant Germplasm System. Available online: https://npgsweb.ars-grin.gov/gringlobal/taxonomydetail.aspx?id=22493 (accessed on 29 December 2019).
- Adegas, F.; Vargas, L.; Gazziero, D.; Karam, D. Impacto econômico da resistência de plantas daninhas a herbicidas no Brasil. Circular Técnica 2017, 132, 12. [Google Scholar]
- Vargas, L.; Roman, E.; Rizzardi, M.; Silva, V. Identificação de biótipos de azevém (Lolium multiflorum) resistentes ao herbicida glyphosate em pomares de maçã. Planta Daninha 2004, 22, 617–622. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Andujar, J.L. Weed Control Models. In Population Dynamics. Encyclopedia of Ecology; Jørgensen, S., Fath, B., Eds.; Elsevier: Oxford, MS, USA, 2008; Volume 5, pp. 3776–3780. [Google Scholar]
- Gonzalez-Andujar, J.L.; Fernández-Quintanilla, C. Modelling the population dynamics of annual ryegrass (Lolium rigidum) under various weed management systems. Crop Prot. 2004, 23, 723–729. [Google Scholar] [CrossRef] [Green Version]
- Zambrano-Navea, C.; Bastida, F.; Gonzalez-Andujar, J. A cohort-based stochastic model of the population dynamic and long-term management of Conyza bonariensis in fruiting tree crops. Crop Prot. 2016, 80, 15–20. [Google Scholar] [CrossRef]
- Gonzalez-Diaz, L.; Leguizamon, E.; Forcella, F.; Gonzalez-Andujar, J. Integration of emergence and population dynamic models for long term weed management using wild oat (Avena fatua L.) as an example. Span. J. Agric. 2007, 5, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Osipitan, O.; Dille, J.; Bagavathiannan, M.; Knezevic, S. Modeling Population Dynamics of Kochia (Bassia scoparia) in Response to Diverse Weed Control Options. Weed Sci. 2019, 57–67. [Google Scholar] [CrossRef]
- Neve, P.; Norsworthy, J.; Smith, K.; Zelaya, I. Modeling glyphosate resistance management strategies for Palmer amaranth (Amaranthus palmeri) in cotton. Weed Technol. 2011, 25, 335–343. [Google Scholar] [CrossRef]
- Bagavathiannan, M.; Norsworthy, J.; Smith, K.; Neve, P. Modeling the evolution of glyphosate resistance in barnyardgrass (Echinochloa crus-galli) in cotton-based production systems of the midsouthern United States. Weed Technol. 2013, 27, 475–487. [Google Scholar] [CrossRef]
- Vila-Aiub, M.; Goh, S.; Gaines, T.; Han, H.; Busi, R.; Yu, Q.; Powles, S. No fitness cost of glyphosate resistance endowed by massive EPSPS gene amplification in Amaranthus palmeri. Planta 2014, 239, 793–801. [Google Scholar] [CrossRef]
- Yanniccari, M.; Vila-Aiub, M.; Istilart, C.; Acciaresi, H.; Castro, A. Glyphosate resistance in perennial ryegrass (Lolium perenne L.) is associated with a fitness penalty. Weed Sci. 2016, 64, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Moreno, P.; Alcántara-de la Cruz, R.; Smeda, R.; De Prado, R. Differential resistance mechanisms to glyphosate result in fitness cost for Lolium perenne and L. multiflorum. Front. Plant Sci. 2017, 8, 1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmstorf, S.; Foster, G.; Cahill, N. Global temperature evolution: Recent trends and some pitfalls. Environ. Res. 2017, 12, 054001. [Google Scholar] [CrossRef]
- Wuebbles, D.; Fahey, D.; Hibbard, K.; DeAngelo, B.; Doherty, S.; Hayhoe, K.; Horton, R.; Kossin, J.; Taylor, P.; Waple, A.; et al. Climate Science Special Report: Fourth National Climate Assessment; Wuebbles, D., Fahey, D., Hibbard, K., Dokken, D., Stewart, B., Maycock, T., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2017; Volume 1, pp. 12–34.
- Varanasi, A.; Prasad, P.; Jugulam, M. Impact of climate change factors on weeds and herbicide efficacy. Adv. Agron. 2016, 135, 107–146. [Google Scholar]
- Kathiresan, R.; Gualbert, G. Impact of climate change on the invasive traits of weeds. Weed Biol. Manag. 2016, 16, 59–66. [Google Scholar] [CrossRef]
- Maia, F.; Maia, M.; Bekker, R.; Berton, R.; Caetano, L. Lolium multiflorum seeds in the soil: I. Soil seed bank dynamics in a no till system. Revista Brasileira de Sementes 2008, 30, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Nandula, V.K. Italian ryegrass (Lolium perenne ssp. multiflorum) and corn (Zea mays) competition. Am. J. Plant Sci. 2014, 5, 3914. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Andujar, J.L.; Fernández-Quintanilla, C. Modelling the population dynamics of Avena sterilis under dry-land cereal cropping systems. J. Appl. Ecol. 1991, 28, 16–27. [Google Scholar] [CrossRef]
- Primavesi, A.C.; Rodrigues, A.; Godoy, R. Recomendações técnicas para o cultivo de aveia; Comunicado Técnico nº 6; Embrapa Pecuaria Sudeste: Sao Carlos, Brazil, 2000; 39p. [Google Scholar]
- Ziska, L.H.; McConnell, L.L. Climate change, carbon dioxide, and pest biology: Monitor, mitigate, management. J. Agric. Food Chem. 2015, 64, 6–12. [Google Scholar] [CrossRef]
- Gregor, J. XXX.—Pollination and Seed Production in the Rye-Grasses (Lolium perenne and Lolium italicum). Earth Environ. Sci. Trans. R. Soc. Edinb. 1928, 55, 773–794. [Google Scholar] [CrossRef]
- Manalil, S.; Renton, M.; Diggle, A.; Busi, R.; Powles, S.B. Simulation modelling identifies polygenic basis of herbicide resistance in a weed population and predicts rapid evolution of herbicide resistance at low herbicide rates. Crop Prot. 2012, 40, 114–120. [Google Scholar] [CrossRef]
- Gonzalez-Andujar, J.L.; Fernandez-Quintanilla, C.; Bastida, F.; Calvo, R.; Izquierdo, J.; Lezaun, J.A. Assessment of a Decision Support System for Chemical Control of Annual Ryegrass (Lolium rigidum) in Winter Cereals. Weed Res. 2011, 51, 304–309. [Google Scholar] [CrossRef]
- Tribouillois, H.; Dürr, C.; Demilly, D.; Wagner, M.; Justes, E. Determination of germination response to temperature and water potential for a wide range of cover crop species and related functional groups. PLoS ONE 2016, 11, e0161185. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, J.; Bastida, F.; Lezaún, J.; Sánchez del Arco, M.; Gonzalez-Andujar, J. Development and evaluation of a model for predicting Lolium rigidum emergence in winter cereal crops in the Mediterranean area. Weed Res. 2013, 53, 269–278. [Google Scholar] [CrossRef]
- Andersen, L.; Breisinger, C.; Jemio, L.; Mason-D’Croz, D.; Ringler, C.; Robertson, R.; Wiebelt, M. Climate Change Impacts and Household Resilience: Prospects for, 2050 in Brazil, Mexico, and Peru; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2016; 94p. [Google Scholar]
- Cousens, R.; Mortimer, A.M. Dynamics of Weed Populations; Cambridge University Press: Cambridge, UK, 1995; p. 332. [Google Scholar]
- Pagnoncelli, F.; Trezzi, M.; Salomão, H.; Hartman, K.; Bortolanza, P.; Gonzalez-Andujar, J.L. Demography of Italian ryegrass (Lolium multiflorum) susceptible and resistant to glyphosate. Planta Daninha 2020. manuscript in preparation. [Google Scholar]
- Galvan, J.; Rizzardi, M.; Peruzzo, S.; Ovejero, R. Evolution of Ryegrass Seed Banks Depending on Soil Tillage and Crops. Planta Daninha 2015, 33, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Christoffoleti, P.; Trentin, R.; Tocchetto, S.; Marochi, A.; Galli, A.; López-Ovejero, R.; Nicolai, M. Alternative herbicides to manage Italian ryegrass (Lolium multiflorum Lam) resistant to glyphosate at different phenological stages. J. Environ. Sci. Health C. 2005, 40, 59–67. [Google Scholar] [CrossRef]
- Terrell, E.E. A Taxonomic Revision of the Genus Lolium; Technical Bulletin; US Department of Agriculture: Washington, DC, USA, 1968; p. 1392.


| Parameter | Susceptible | Resistant | Reference | |||
|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | |||
| Seed Bank | ||||||
| Seed Mortality | Sm | 0.49 | - | 0.49 | - | [31] |
| Emergence rate | e | 0.73 | 0.04 | 0.73 | 0.04 | [30] |
| Seedlings | ||||||
| Seedling Survival | sdlsi1 | 0.02 | 0.02 | 0.04 | 0.03 | [30] |
| Seedling Survival | sdlsi2 | 0.03 | 0.02 | 0.05 | 0.03 | [30] |
| Seedling Survival | sdlsi3 | 0.11 | 0.05 | 0.16 | 0.07 | [30] |
| Seed Production | ||||||
| Factor Reduction | fri1 | 0.07 | 0.07 | 0.02 | 0.06 | [30] |
| Factor Reduction | fri2 | 0 | - | 0 | - | [30] |
| Factor Reduction | fri3 | 0.46 | 0.1 | 0.34 | 0.12 | [30] |
| Max Seed Produced per Plant | f | 20300 | 1212 | 13830 | 1305 | [30] |
| Area to Produce f Seeds | b | 0.17 | 0.03 | 0.12 | 0.03 | [30] |
| Losses in Pollination | lp | 0.88 | 0.05 | 0.88 | 0.05 | [30] |
| Seed Losses (Standard Harvest) | sl | 0.19 | - | 0.19 | - | [5] |
| Management | ||||||
| Control rate (Post Emergence Early) | rcpostE | 0.98 | 0.005 | 0.98 | 0.005 | [32] |
| Control rate (Post Emergence Late) | rcpostL | 0.91 | 0.008 | 0.91 | 0.008 | [32] |
| Crop Rotation Wheat/Soybean | cr1 | 0.89 | 0.03 | 0.89 | 0.03 | [31] |
| Crop Rotation Oat/Soybean | cr2 | 0.48 | 0.13 | 0.48 | 0.13 | [31] |
| Crop Rotation Oat/Corn | cr3 | 0.89 | 0.03 | 0.89 | 0.03 | [31] |
| Management | Year 1 | Year 2 | Year 3 | |||
|---|---|---|---|---|---|---|
| Winter | Summer | Winter | Summer | Winter | Summer | |
| M1 | Null | |||||
| M2 | PostLC1 + PostEC2 | |||||
| M3 | PostLC1 + PostLC2 + PostEC3 | |||||
| M4 | PostLC2 + PostLC3 | |||||
| M5 | Wheat | Soybean | ||||
| M6 | Oat | Soybean | ||||
| M7 | Oat | Corn | ||||
| M8 | Wheat | Soybean | Oat | Corn | ||
| M9 | Oat | Soybean | Oat | Corn | ||
| M10 | Wheat | Soybean | Oat | Corn | Oat | Soybean |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D. B. Pagnoncelli, F., Jr.; Trezzi, M.M.; Gonzalez-Andujar, J.L. Modeling the Population Dynamics and Management of Italian Ryegrass under Two Climatic Scenarios in Brazil. Plants 2020, 9, 325. https://doi.org/10.3390/plants9030325
D. B. Pagnoncelli F Jr., Trezzi MM, Gonzalez-Andujar JL. Modeling the Population Dynamics and Management of Italian Ryegrass under Two Climatic Scenarios in Brazil. Plants. 2020; 9(3):325. https://doi.org/10.3390/plants9030325
Chicago/Turabian StyleD. B. Pagnoncelli, Fortunato, Jr., Michelangelo M. Trezzi, and Jose L. Gonzalez-Andujar. 2020. "Modeling the Population Dynamics and Management of Italian Ryegrass under Two Climatic Scenarios in Brazil" Plants 9, no. 3: 325. https://doi.org/10.3390/plants9030325




