The Chloroplast Ribonucleoprotein CP33B Quantitatively Binds the psbA mRNA
Abstract
:1. Introduction
2. Results
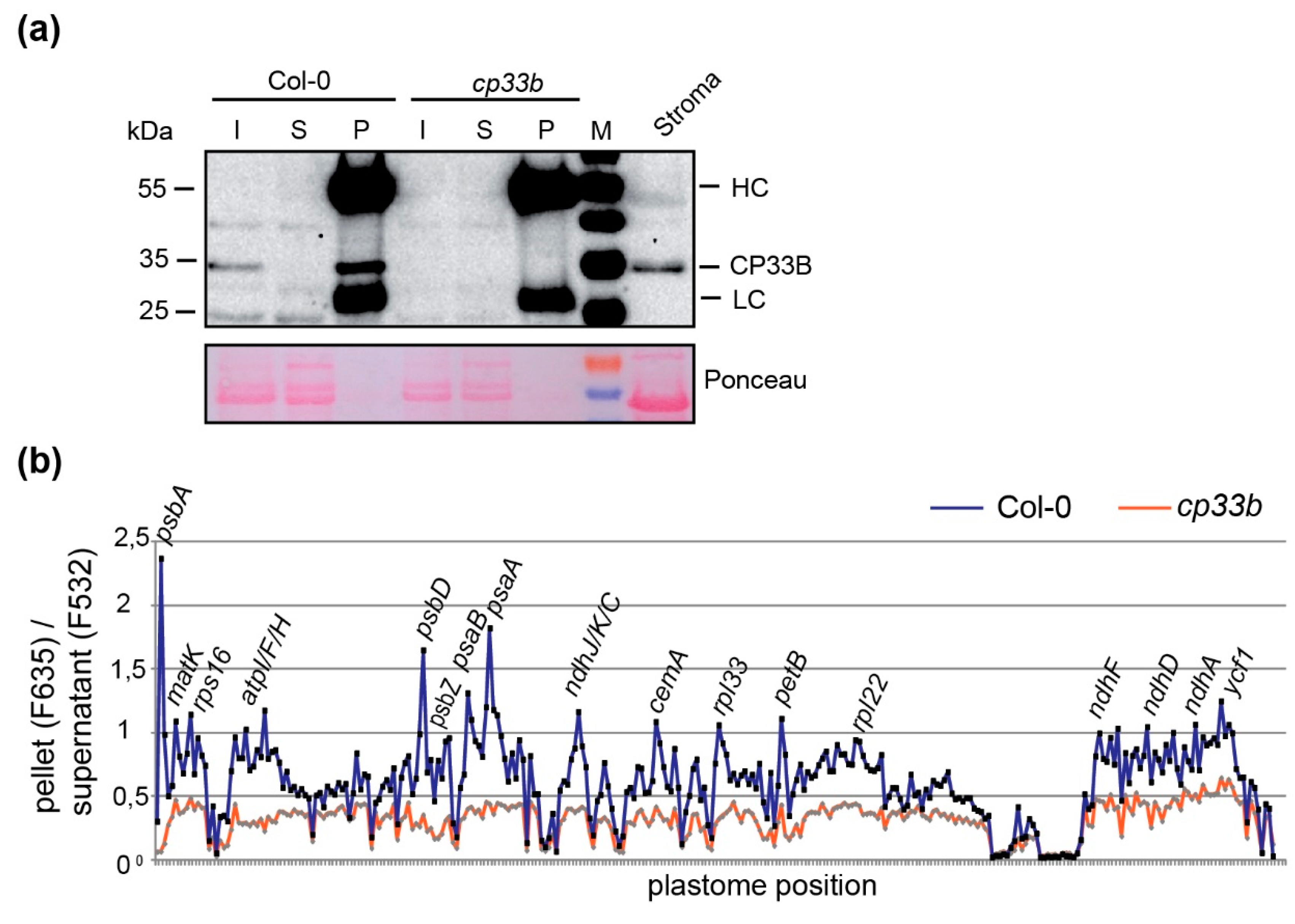
2.1. CP33B Localizes to the Chloroplast
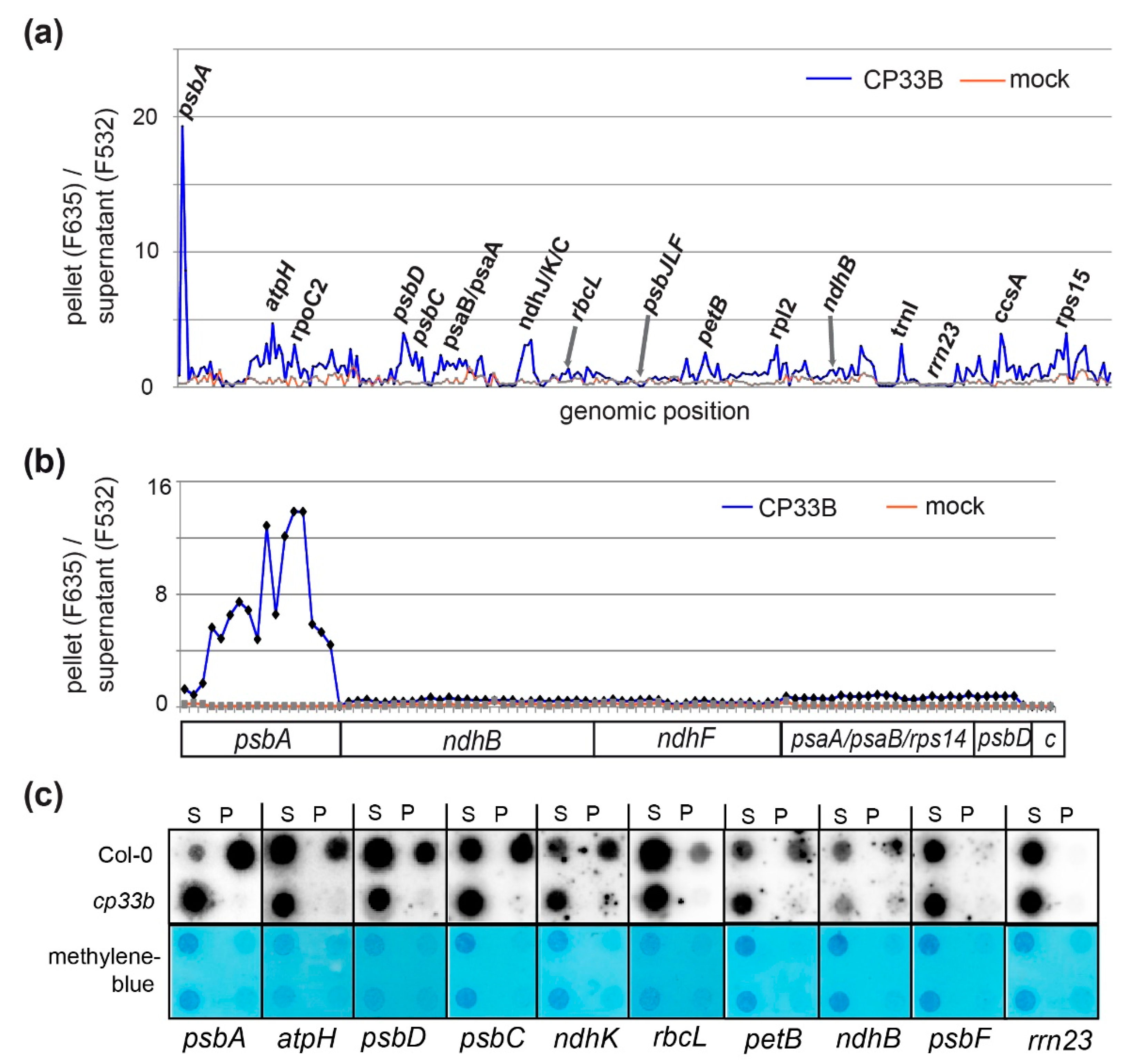
2.2. CP33B Has a Preference for the psbA mRNA Over Other Chloroplast Transcripts
2.3. CP33B Quantitatively Co-immunoprecipitates psbA mRNAs
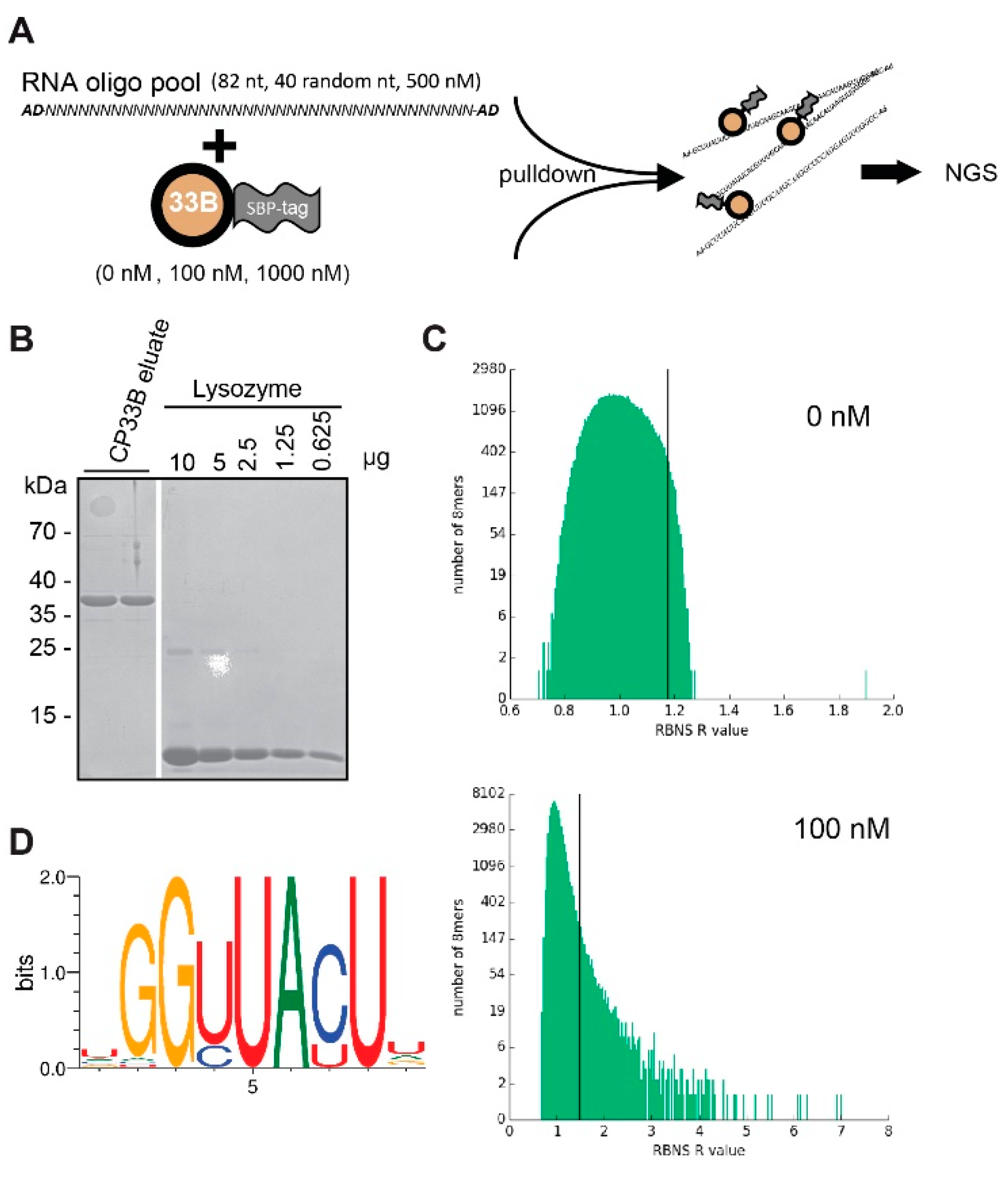
2.4. CP33B Prefers an RNA Sequence Motif in Vitro
2.5. Membrane-bound CP33B is Also Associated with Chloroplast mRNAs
3. Discussion
3.1. CP33B is a Global RBP With a Clear Preference for and Ability to Sequester the psbA mRNA Pool
3.2. cpRNPs Interact With Their Target Transcripts via Multiple Binding Sites
4. Materials and Methods
4.1. Plant Material
4.2. Localization by Fluorescence Microscopy
| Oligonucleotide | Sequence |
| 33B.fw.xhoI.TP | ATACTCGAGATGGCGGTTTTGGAAGC |
| 33B.rev.ncoI.cDNA | ATACCATGGCCACAATGTTTTCTTCG |
4.3. Dot-Blot Analysis
| Oligo Name | Sequence | Target Gene |
| CK_atpH5‘_for | GATTTAGATAGGGATTCGATTAG | atpH |
| CK_atpH5‘_T7_rev | GTAATACGACTCACTATAGGGTCCAGGTCCAATAGAAGCAAG | atpH |
| Ck_psbD_for | CTATTTCGCTTTAGGGGGTTGG | psbD |
| Ck_psbD_T7_rev | TAATACGACTCACTATAGGGCAGCAATGGGACCAGAGAATGC | psbD |
| CK_rbcL_for | GCAGCATTCCGAGTAACTCC | rbcL |
| CK_rbcL_T7 | GTAATACGACTCACTATAGGGCCACGTAGACATTCATAAACTGC | rbcL |
| CK-petB-for | CGTCCAACCGTTACTGAAGC | petB |
| CK-petB-T7-rev | GTAATACGACTCACTATAGGGAATAGCGTCAGGTACACC | petB |
| CK-psbF-for | GTCTGGAAGCACAGGAGAACG | psbF |
| CK-psbF-T7-rev | GTAATACGACTCACTATAGGGCAAAACGGCCTGTTATTAATGG | psbF |
| ndhBex1.rp | CCGATGGAGAGAAGAACCTATG | ndhB |
| ndhBex1.T7 | GTAATCGACTCACTATAGGGTAGCCAAGAGAAACCATGAACC | ndhB |
| ndhK.rp | CTATGGCCGCTTCTTTATGG | ndhK |
| ndhK.T7 | GTAATCGACTCACTATAGGGATTTCCAATATGCGGACTGC | ndhK |
| psbA.rp | CATTCATTGCTGCTCCTCCAG | psbA |
| psbA.t7neu | GTAATCGACTCACTATAGGGATTCCTAGAGGCATACCATCAG | psbA |
| psbC.rp | AGTGGCCCATTTTGTACCTG | psbC |
| psbC.T7 | GTAATCGACTCACTATAGGGCCCCCAAAGGGAGATTTTAG | psbC |
| rrn23.rp | CCTAGATGGCGAGAGTCCAG | rrn23 |
| rrn23.T7 | GTAATCGACTCACTATAGGGAAGACTCGCTTTCGCTACG | rrn23 |
4.4. Immunoblots and Antibody Production
4.5. RIP-chip Analysis
4.6. RBNS Analysis
4.6.1. Cloning of GST:SBP:CP33B
| Oligonucleotide | Sequence |
| CP33BXhoIrev | AATTCTCGAGTCACTCCACAATGTTTTCTTCGG |
| MfeICP33Bfor | AATCAATTGGTCTCCGTACTCTGTTCCGTC |
4.6.2. RNA Oligo Production
| Oligonucleotide | Sequence |
| RBNS T7 Template | CCTTGACACCCGAGAATTCCA -(N)40- GATCGTCGGACTGTAGAACTCCCTATAGTGAGTCGTATTA |
| RNA PCR (RP1) | AATGATACGGCGACCACCGAGATCTACACGTTCAGAGTTCTACAGTCCGACGATC |
| RT primer | GCCTTGGCACCCGAGAATTCCA |
| T7 Promoter Oligo | TAATACGACTCACTATAGGG |
4.6.3. RBNS Assay
| Sample | NEXTflex Barcode Primer Sequence |
| Input | 5 ′-CAAGCAGAAGACGGCATACGAGAT- CGTGATGTGACTGGAGTTCAGACGT- GTGCTCTTCCGATC-s-T-3 ′ |
| 0 protein sample | 5 ′- CAAGCAGAAGACGGCATACGAGA- TGTAGCCGTGACTGGAGTTCAGACGT- GTGCTCTTCCGATC-s-T- |
| 100 nM CP33B | 5 ′-CAAGCAGAAGACGGCATACGAGA- TCTGATCGTGACTGGAGTTCAGACGT- GTGCTCTTCCGATC-s-T-3 ′ |
| 1000 nM CP33B | 5 ′-CAAGCAGAAGACGGCATACGAGAT- AAGCTAGTGACTGGAGTTCAGACGT- |
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barkan, A.; Small, I. Pentatricopeptide repeat proteins in plants. Annu. Rev. Plant Biol. 2014, 65, 415–442. [Google Scholar] [CrossRef] [PubMed]
- Ruwe, H.; Kupsch, C.; Teubner, M.; Schmitz-Linneweber, C. The RNA-recognition motif in chloroplasts. J. Plant Physiol. 2011, 168, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Sugiura, M. Nucleic acid-binding specificities of tobacco chloroplast ribonucleoproteins. Nucleic Acids Res. 1991, 19, 2893–2896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.Q.; Sugiura, M. Three distinct ribonucleoproteins from tobacco chloroplasts: Each contains a unique amino terminal acidic domain and two ribonucleoprotein consensus motifs. EMBO J. 1990, 9, 3059–3066. [Google Scholar] [CrossRef]
- Ye, L.; Sugiura, M. Domains required for nucleic acid binding activities in chloroplast ribonucleoproteins. Nucleic Acids Res. 1992, 20, 6275–6279. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Ohta, M.; Sugiura, M.; Sugita, M. Chloroplast ribonucleoproteins are associated with both mRNAs and intron-containing precursor tRNAs. FEBS Lett. 1999, 460, 437–441. [Google Scholar] [CrossRef] [Green Version]
- Kupsch, C.; Ruwe, H.; Gusewski, S.; Tillich, M.; Small, I.; Schmitz-Linneweber, C. Arabidopsis chloroplast RNA binding proteins CP31A and CP29A associate with large transcript pools and confer cold stress tolerance by influencing multiple chloroplast RNA processing steps. Plant Cell 2012, 10, 4266–4280. [Google Scholar] [CrossRef] [Green Version]
- Teubner, M.; Fuss, J.; Kuhn, K.; Krause, K.; Schmitz-Linneweber, C. The RNA recognition motif protein c CP33A is a global ligand of chloroplast mRNAs and is essential for plastid biogenesis and plant development. Plant J. 2017, 89, 472–485. [Google Scholar] [CrossRef] [Green Version]
- Watkins, K.P.; Williams-Carrier, R.; Chotewutmontri, P.; Friso, G.; Teubner, M.; Belcher, S.; Ruwe, H.; Schmitz-Linneweber, C.; van Wijk, K.J.; Barkan, A. Exploring the proteome associated with the mRNA encoding the D1 reaction center protein of photosystem II in plant chloroplasts. Plant J. 2019. [Google Scholar] [CrossRef]
- Nakamura, T.; Ohta, M.; Sugiura, M.; Sugita, M. Chloroplast ribonucleoproteins function as a stabilizing factor of ribosome-free mRNAs in the stroma. J. Biol. Chem. 2001, 276, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Lisitsky, I.; Liveanu, V.; Schuster, G. RNA-binding characteristics of a ribonucleoprotein from spinach chloroplast. Plant Physiol. 1995, 107, 933–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raab, S.; Toth, Z.; de Groot, C.; Stamminger, T.; Hoth, S. Aba-responsive RNA-binding proteins are involved in chloroplast and stromule function in Arabidopsis seedlings. Planta 2006, 224, 900–914. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Lee, K.; Gu, L.; Kim, J.I.; Kang, H. Functional characterization of a plastid-specific ribosomal protein PSRP2 in Arabidopsis thaliana under abiotic stress conditions. Plant Physiol. Biochem. 2013, 73, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Guo, M.; Jeong, B.R.; Tian, F.; Elthon, T.E.; Cerny, R.L.; Staiger, D.; Alfano, J.R. A type III effector ADP-ribosylates RNA-binding proteins and quells plant immunity. Nature 2007, 447, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Schuster, G.; Gruissem, W. Chloroplast mRNA 3’ end processing requires a nuclear-encoded RNA-binding protein. EMBO J. 1991, 10, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Sugiura, M. Involvement of a site-specific trans-acting factor and a common RNA-binding protein in the editing of chloroplast mRNAs: Development of a chloroplast in vitro RNA editing system. EMBO J. 2001, 20, 1144–1152. [Google Scholar] [CrossRef] [Green Version]
- Tillich, M.; Hardel, S.L.; Kupsch, C.; Armbruster, U.; Delannoy, E.; Gualberto, J.M.; Lehwark, P.; Leister, D.; Small, I.D.; Schmitz-Linneweber, C. Chloroplast ribonucleoprotein CP31A is required for editing and stability of specific chloroplast mRNAs. Proc. Natl. Acad. Sci. USA 2009, 106, 6002–6007. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Suarez, M.; Castro-Sanchez, A.; Toledo-Ortiz, G.; Gonzalez de la Vara, L.E.; Garcia, E.; Loza-Tavera, H. Protein phosphorylation regulates in vitro spinach chloroplast petD mRNA 3’-untranslated region stability, processing, and degradation. Biochimie 2013, 95, 400–409. [Google Scholar] [CrossRef]
- Tillich, M.; Beick, S.; Schmitz-Linneweber, C. Chloroplast RNA-binding proteins: Repair and regulation of chloroplast transcripts. RNA Biol. 2010, 7, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Zoschke, R.; Bock, R. Chloroplast translation: Structural and functional organization, operational control and regulation. Plant Cell 2018, 30, 745–770. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zerges, W. Translational regulation in chloroplasts for development and homeostasis. Biochim. Biophys. Acta 2015, 1847, 809–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickelsen, J.; Bohne, A.; Westhoff, P. Chloroplast gene expression—Translation. In Plastid Biology; Theg, S., Wollman, F., Eds.; Springer: New York, NY, USA, 2014; pp. 49–78. [Google Scholar]
- Chotewutmontri, P.; Barkan, A. Multilevel effects of light on ribosome dynamics in chloroplasts program genome-wide and psbA-specific changes in translation. PLoS Genet. 2018, 14, e1007555. [Google Scholar] [CrossRef] [PubMed]
- Tyystjarvi, E.; Aro, E.M. The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc. Natl. Acad. Sci. USA 1996, 93, 2213–2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundby, C.; McCaffery, S.; Anderson, J.M. Turnover of the photosystem II D1 protein in higher plants under photoinhibitory and nonphotoinhibitory irradiance. J. Biol. Chem. 1993, 268, 25476–25482. [Google Scholar] [PubMed]
- Nishiyama, Y.; Murata, N. Revised scheme for the mechanism of photoinhibition and its application to enhance the abiotic stress tolerance of the photosynthetic machinery. Appl. Microbiol. Biotechnol. 2014, 98, 8777–8796. [Google Scholar] [CrossRef]
- Schult, K.; Meierhoff, K.; Paradies, S.; Toller, T.; Wolff, P.; Westhoff, P. The nuclear-encoded factor HCF173 is involved in the initiation of translation of the psbA mRNA in Arabidopsis thaliana. Plant Cell 2007, 19, 1329–1346. [Google Scholar] [CrossRef] [Green Version]
- Williams-Carrier, R.; Brewster, C.; Belcher, S.; Rojas, M.; Chotewutmontri, P.; Ljungdahl, S.; Barkan, A. The arabidopsis pentatricopeptide repeat protein LPE1 and its maize ortholog are required for translation of the chloroplast psbJ RNA. Plant J. 2019, 99, 56–66. [Google Scholar] [CrossRef]
- McDermott, J.J.; Watkins, K.P.; Williams-Carrier, R.; Barkan, A. Ribonucleoprotein capture by in vivo expression of a designer pentatricopeptide repeat protein in Arabidopsis. Plant Cell 2019, 31, 1723–1733. [Google Scholar] [CrossRef] [Green Version]
- Danon, A.; Mayfield, S.P. Light regulated translational activators: Identification of chloroplast gene specific mRNA binding proteins. EMBO J. 1991, 10, 3993–4001. [Google Scholar] [CrossRef]
- Alexander, C.; Faber, N.; Klaff, P. Characterization of protein-binding to the spinach chloroplast psbA mRNA 5’ untranslated region. Nucleic Acids Res. 1998, 26, 2265–2272. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Danon, A.; Christopher, D.A. RNA binding-proteins interact specifically with the Arabidopsis chloroplast psbA mRNA 5’ untranslated region in a redox-dependent manner. Plant Cell Physiol. 2001, 42, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Ossenbuhl, F.; Hartmann, K.; Nickelsen, J. A chloroplast RNA binding protein from stromal thylakoid membranes specifically binds to the 5’ untranslated region of the psbA mRNA. Eur. J. Biochem. 2002, 269, 3912–3919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohne, A.V.; Schwarz, C.; Schottkowski, M.; Lidschreiber, M.; Piotrowski, M.; Zerges, W.; Nickelsen, J. Reciprocal regulation of protein synthesis and carbon metabolism for thylakoid membrane biogenesis. PLoS Biol. 2013, 11, e1001482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Link, S.; Engelmann, K.; Meierhoff, K.; Westhoff, P. The atypical short-chain dehydrogenases HCF173 and HCF244 are jointly involved in translational initiation of the psbA mRNA of Arabidopsis. Plant Physiol. 2012, 160, 2202–2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majeran, W.; Friso, G.; Asakura, Y.; Qu, X.; Huang, M.; Ponnala, L.; Watkins, K.P.; Barkan, A.; van Wijk, K.J. Nucleoid-enriched proteomes in developing plastids and chloroplasts from maize leaves: A new conceptual framework for nucleoid functions. Plant Physiol. 2011, 158, 156–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terasawa, K.; Sato, N. Visualization of plastid nucleoids in situ using the PEND-GFP fusion protein. Plant Cell Physiol. 2005, 46, 649–660. [Google Scholar] [CrossRef]
- Schmitz-Linneweber, C.; Williams-Carrier, R.; Barkan, A. RNA immunoprecipitation and microarray analysis show a chloroplast pentatricopeptide repeat protein to be associated with the 5’ region of mRNAs whose translation it activates. Plant Cell 2005, 17, 2791–2804. [Google Scholar] [CrossRef] [Green Version]
- Zoschke, R.; Nakamura, M.; Liere, K.; Sugiura, M.; Börner, T.; Schmitz-Linneweber, C. An organellar maturase associates with multiple group II introns. Proc. Natl. Acad. Sci. USA 2010, 107, 3245–3250. [Google Scholar] [CrossRef] [Green Version]
- Lambert, N.; Robertson, A.; Jangi, M.; McGeary, S.; Sharp, P.A.; Burge, C.B. RNA bind-n-seq: Quantitative assessment of the sequence and structural binding specificity of RNA binding proteins. Mol. Cell 2014, 54, 887–900. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, D.; Freese, P.; Alexis, M.S.; Su, A.; Hochman, M.; Palden, T.; Bazile, C.; Lambert, N.J.; Van Nostrand, E.L.; Pratt, G.A.; et al. Sequence, structure, and context preferences of human RNA binding proteins. Mol. Cell 2018, 70, 854–867. [Google Scholar] [CrossRef] [Green Version]
- Campbell, Z.T.; Bhimsaria, D.; Valley, C.T.; Rodriguez-Martinez, J.A.; Menichelli, E.; Williamson, J.R.; Ansari, A.Z.; Wickens, M. Cooperativity in RNA-protein interactions: Global analysis of RNA binding specificity. Cell Rep. 2012, 1, 570–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jean-Philippe, J.; Paz, S.; Caputi, M. Hnrnp a1: The swiss army knife of gene expression. Int. J. Mol. Sci. 2013, 14, 18999–19024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreyfuss, G.; Kim, V.N.; Kataoka, N. Messenger-RNA-binding proteins and the messages they carry. Nat. Rev. Mol. Cell Biol. 2002, 3, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Scherly, D.; Boelens, W.; Dathan, N.A.; van Venrooij, W.J.; Mattaj, I.W. Major determinants of the specificity of interaction between small nuclear ribonucleoproteins U1A and U2B’’ and their cognate RNAs. Nature 1990, 345, 502–506. [Google Scholar] [CrossRef]
- Zoschke, R.; Barkan, A. Genome-wide analysis of thylakoid-bound ribosomes in maize reveals principles of cotranslational targeting to the thylakoid membrane. Proc. Natl. Acad. Sci. USA 2015, 112, E1678–E1687. [Google Scholar] [CrossRef] [Green Version]
- Schmitz-Linneweber, C.; Williams-Carrier, R.; Williams, P.; Kroeger, T.; Vichas, A.; Barkan, A. A pentatricopeptide repeat protein binds to and facilitates the trans-splicing of the maize chloroplast rps12 pre-mRNA. Plant Cell 2006, 18, 2650–2663. [Google Scholar] [CrossRef] [Green Version]
- Pfalz, J.; Bayraktar, O.A.; Prikryl, J.; Barkan, A. Site-specific binding of a PPR protein defines and stabilizes 5’ and 3’ mRNA termini in chloroplasts. EMBO J. 2009, 28, 2042–2052. [Google Scholar] [CrossRef]
- Fuss, J.; Liegmann, O.; Krause, K.; Rensing, S.A. Green targeting predictor and ambiguous targeting predictor 2: The pitfalls of plant protein targeting prediction and of transient protein expression in heterologous systems. New Phytol. 2013, 200, 1022–1033. [Google Scholar] [CrossRef]
- Lambert, N.J.; Robertson, A.D.; Burge, C.B. RNA bind-n-seq: Measuring the binding affinity landscape of RNA-binding proteins. Methods Enzymol. 2015, 558, 465–493. [Google Scholar]
- Github. Available online: https://github.com/cburgelab/RBNS_pipeline; (accessed on 16 March 2020).


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teubner, M.; Lenzen, B.; Espenberger, L.B.; Fuss, J.; Nickelsen, J.; Krause, K.; Ruwe, H.; Schmitz-Linneweber, C. The Chloroplast Ribonucleoprotein CP33B Quantitatively Binds the psbA mRNA. Plants 2020, 9, 367. https://doi.org/10.3390/plants9030367
Teubner M, Lenzen B, Espenberger LB, Fuss J, Nickelsen J, Krause K, Ruwe H, Schmitz-Linneweber C. The Chloroplast Ribonucleoprotein CP33B Quantitatively Binds the psbA mRNA. Plants. 2020; 9(3):367. https://doi.org/10.3390/plants9030367
Chicago/Turabian StyleTeubner, Marlene, Benjamin Lenzen, Lucas Bernal Espenberger, Janina Fuss, Jörg Nickelsen, Kirsten Krause, Hannes Ruwe, and Christian Schmitz-Linneweber. 2020. "The Chloroplast Ribonucleoprotein CP33B Quantitatively Binds the psbA mRNA" Plants 9, no. 3: 367. https://doi.org/10.3390/plants9030367




