Manganese and Zinc Spinel Ferrites Blended with Multi-Walled Carbon Nanotubes as Microwave Absorbing Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
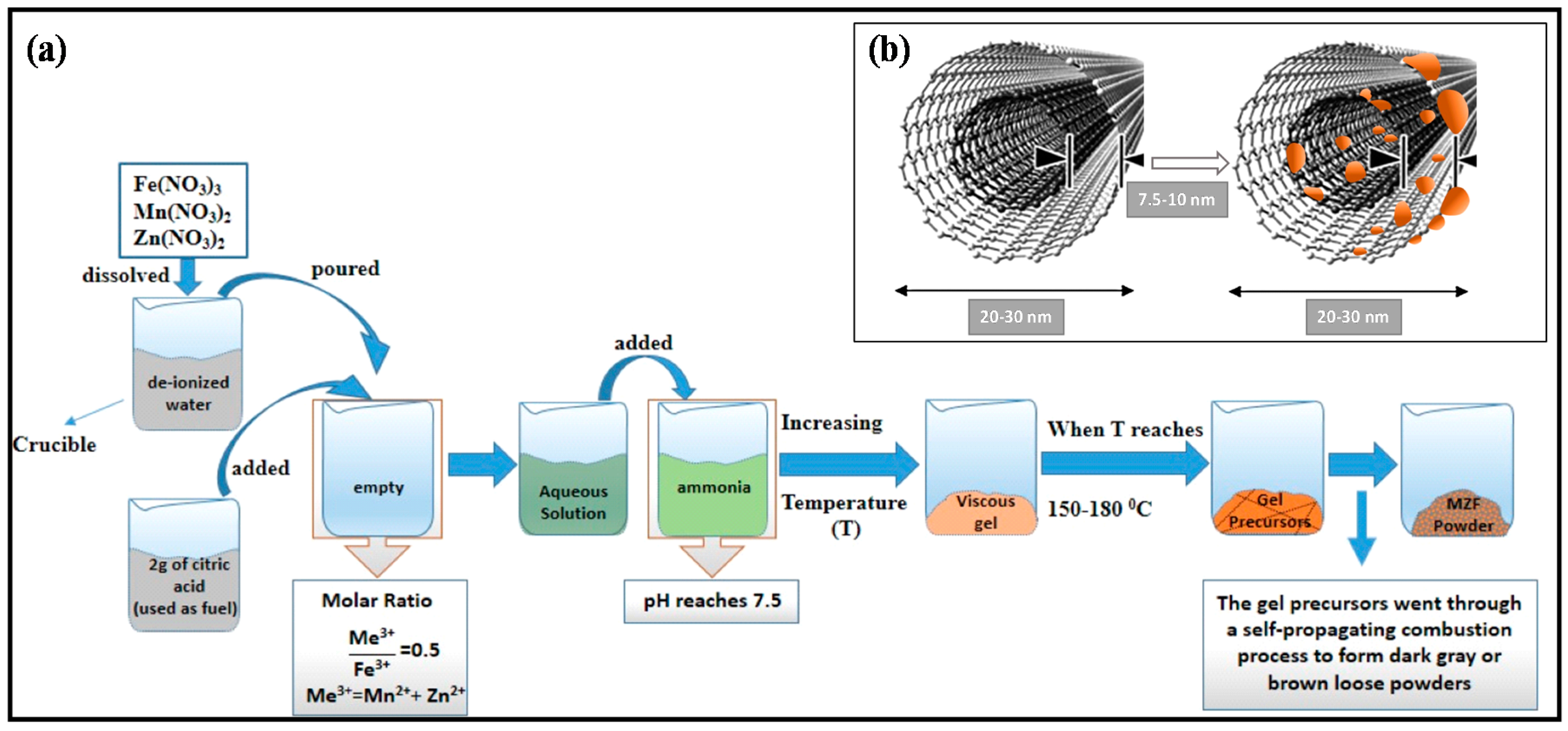
2.2. Preparation of Manganese and Zinc Ferrites (MZF)
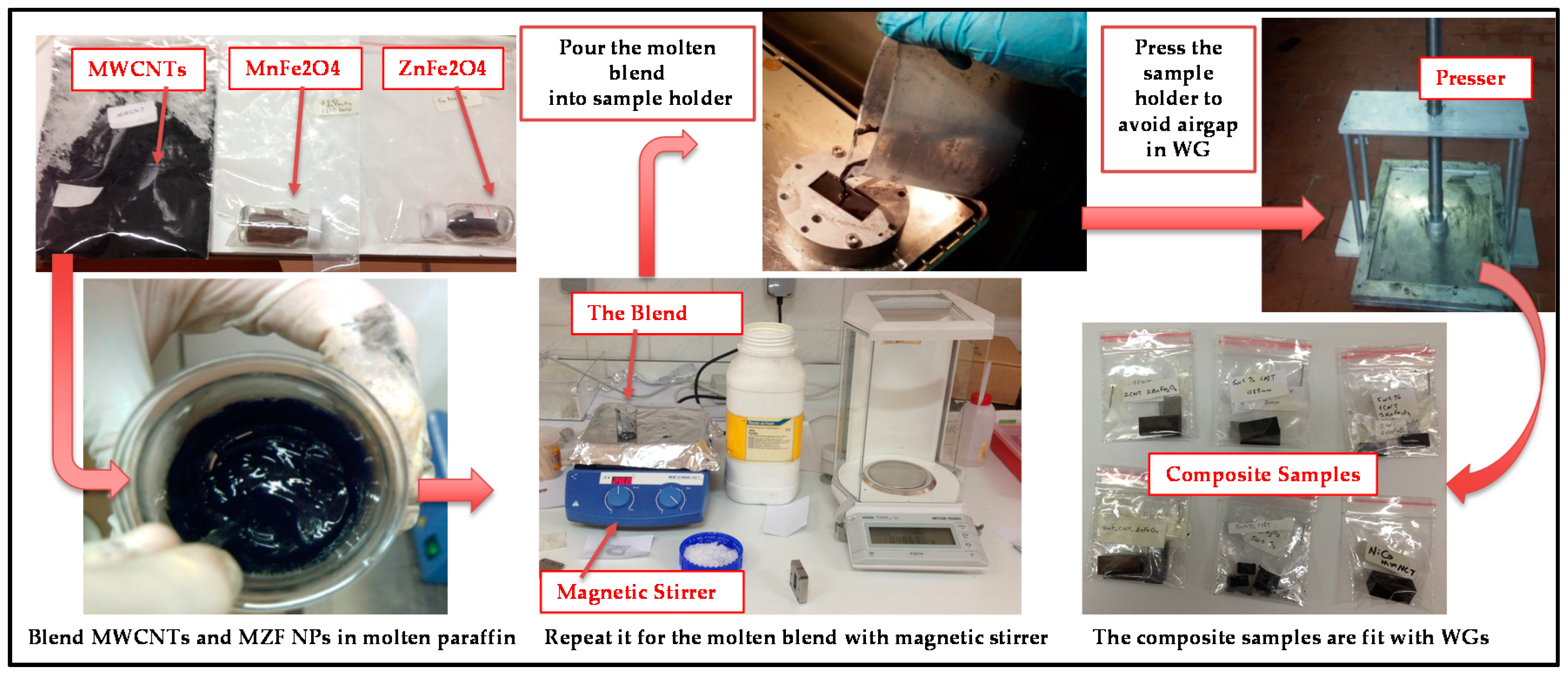
2.3. Preparation of Nanocomposite Samples
2.4. Instrumentation
3. Results and Discussion
3.1. Morphology and Crystal Structure Characterization
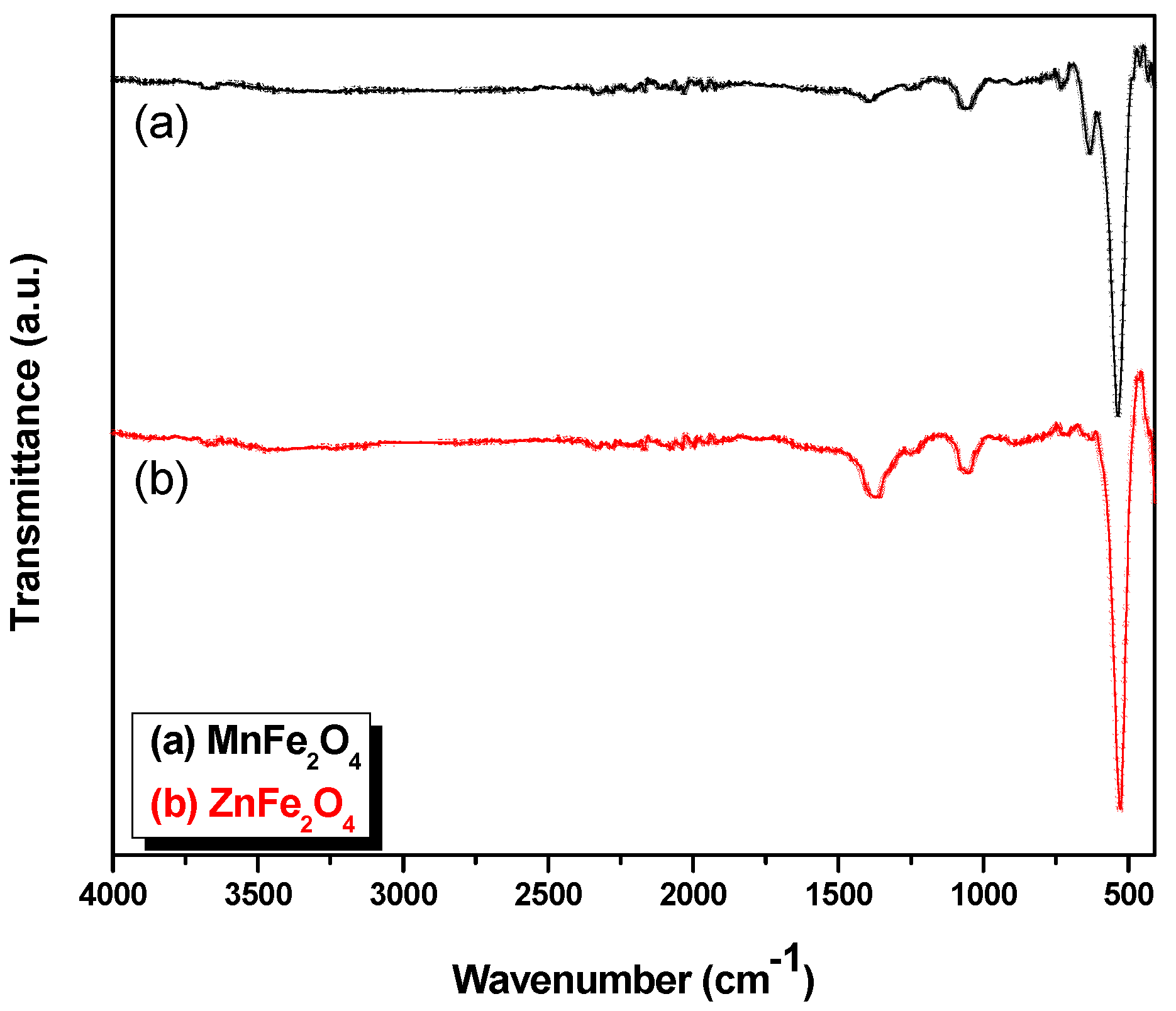
3.2. Fourier Transform Infrared (FT-IR) Analysis
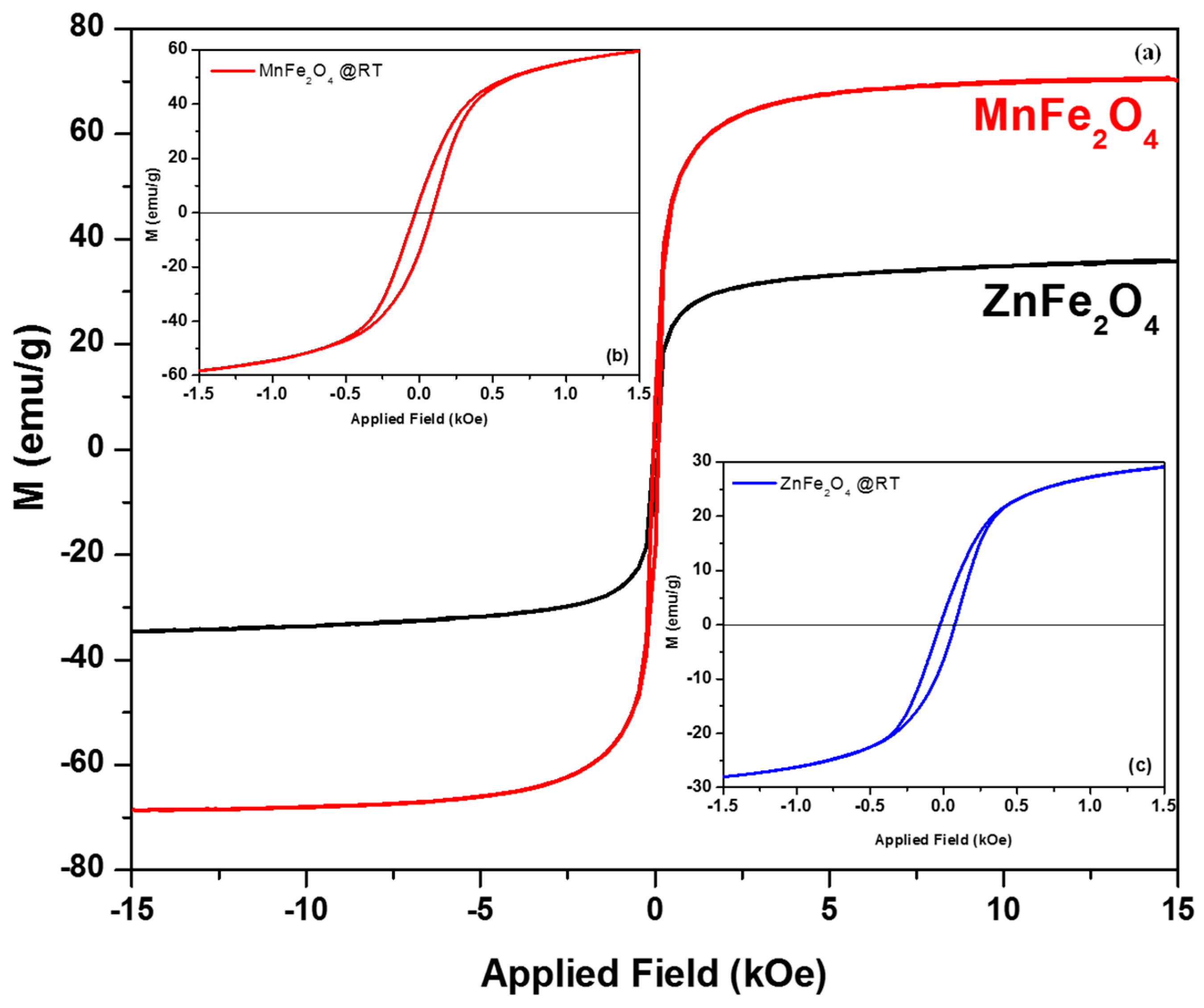
3.3. The Magnetization Measurements (VSM Analysis)
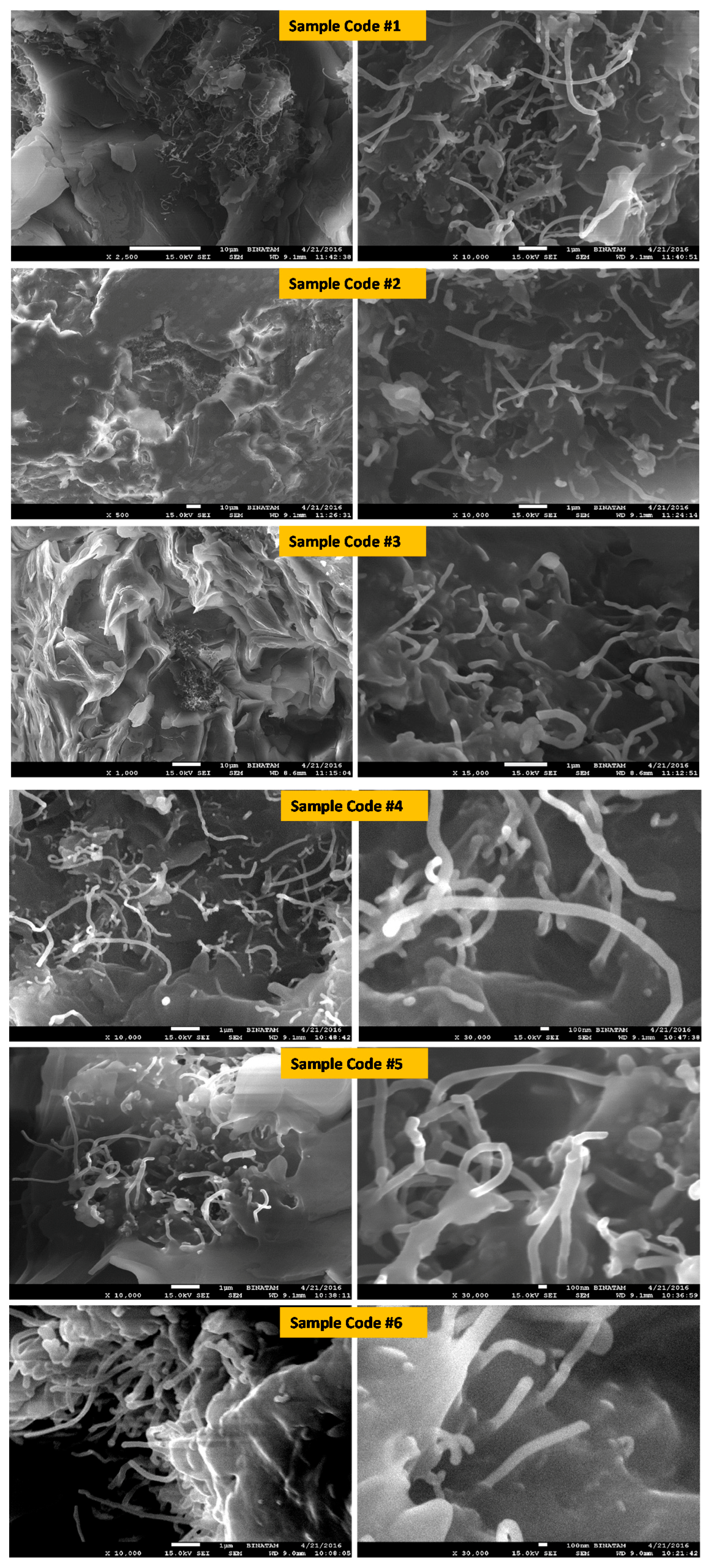
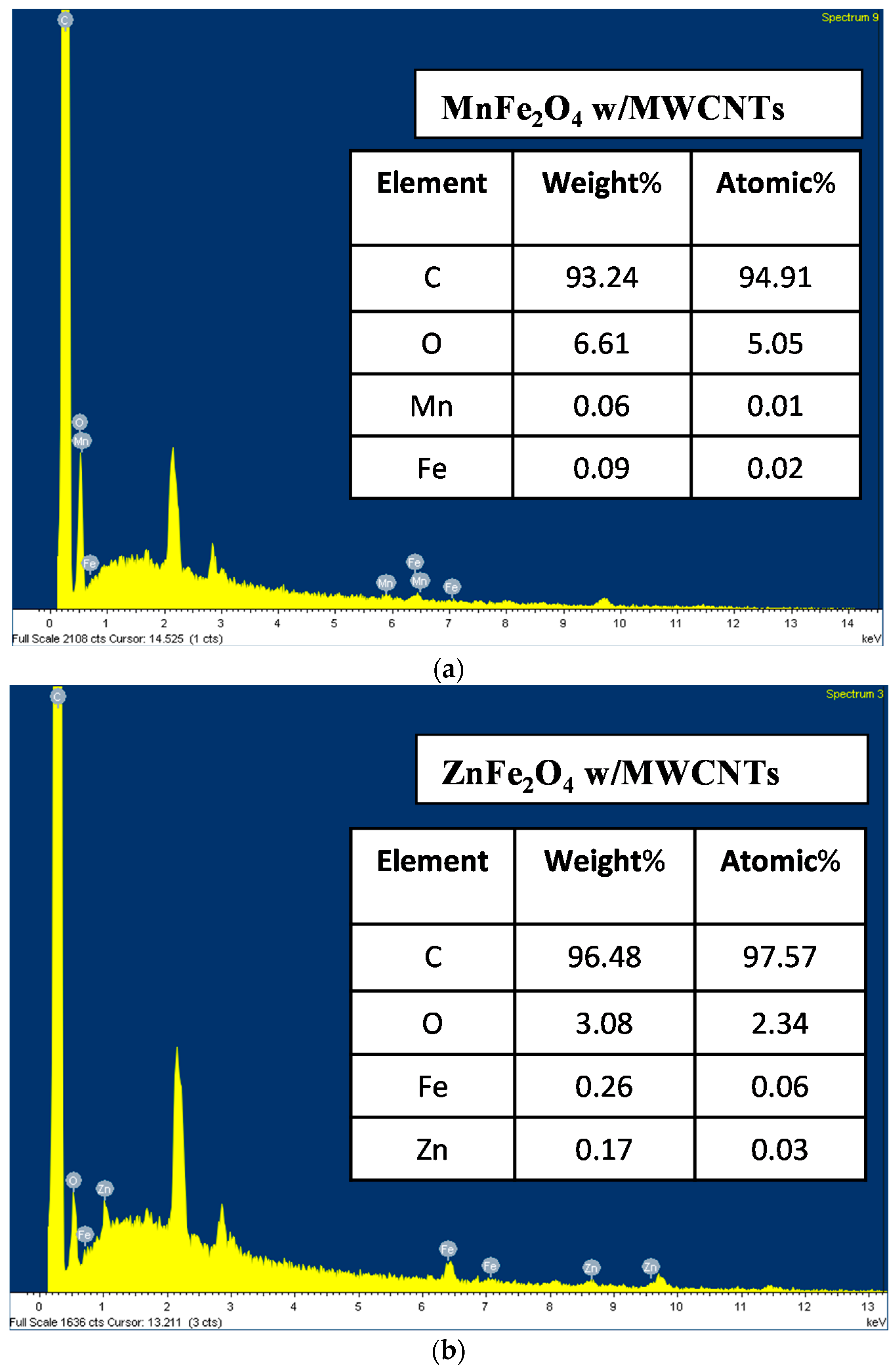
3.4. The Surface Morphology of the Samples (SEM) Analysis and the Energy Dispersive X-Ray (EDX)
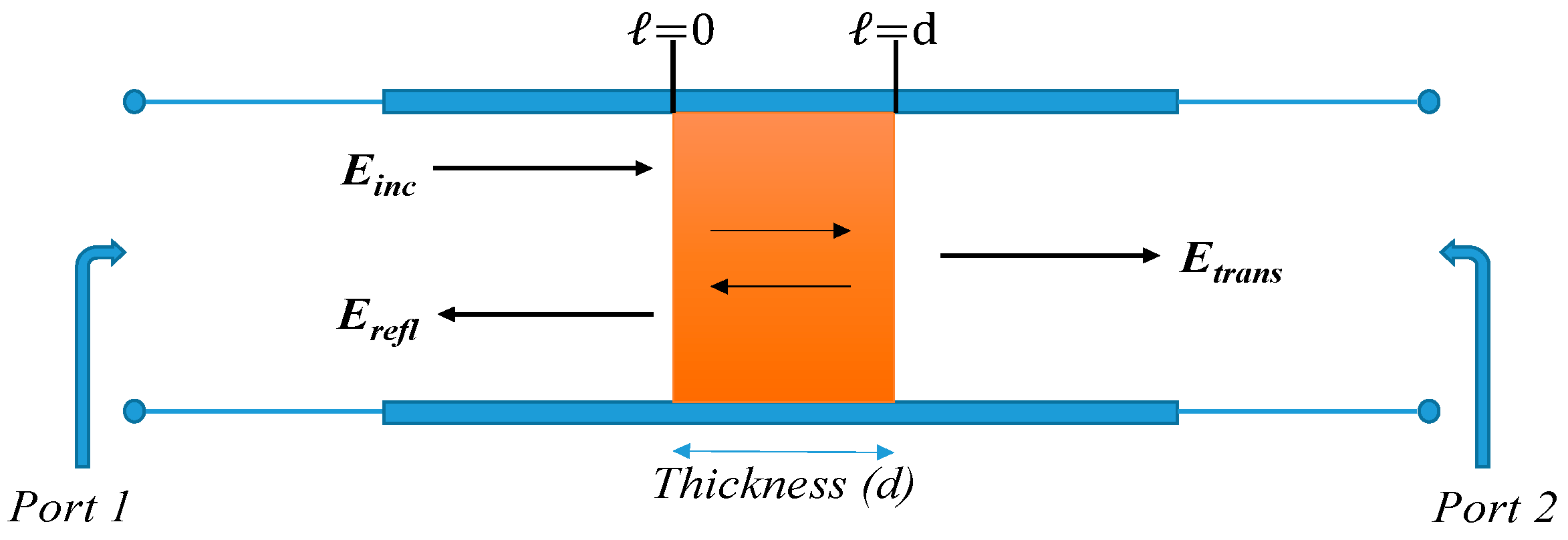
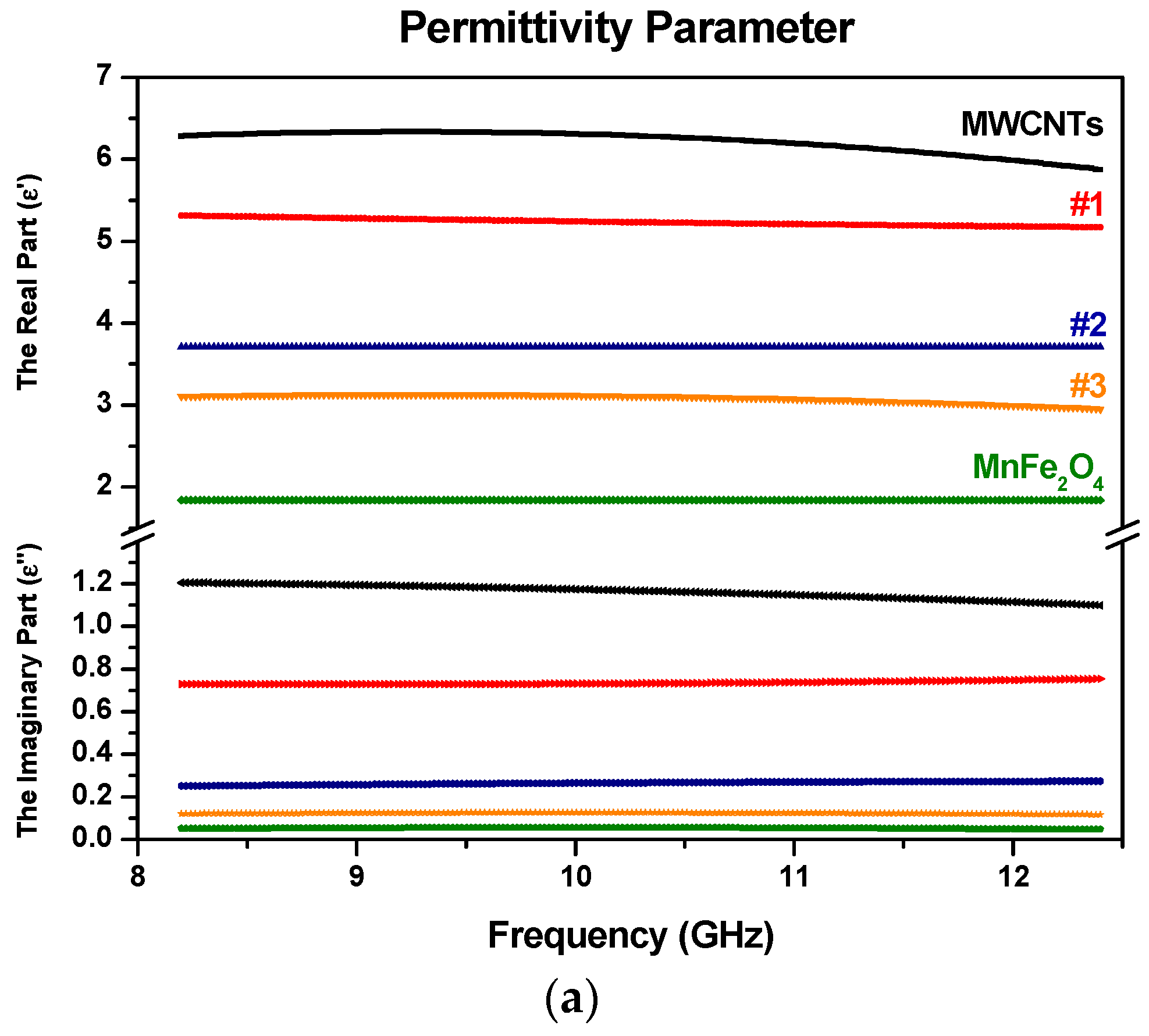
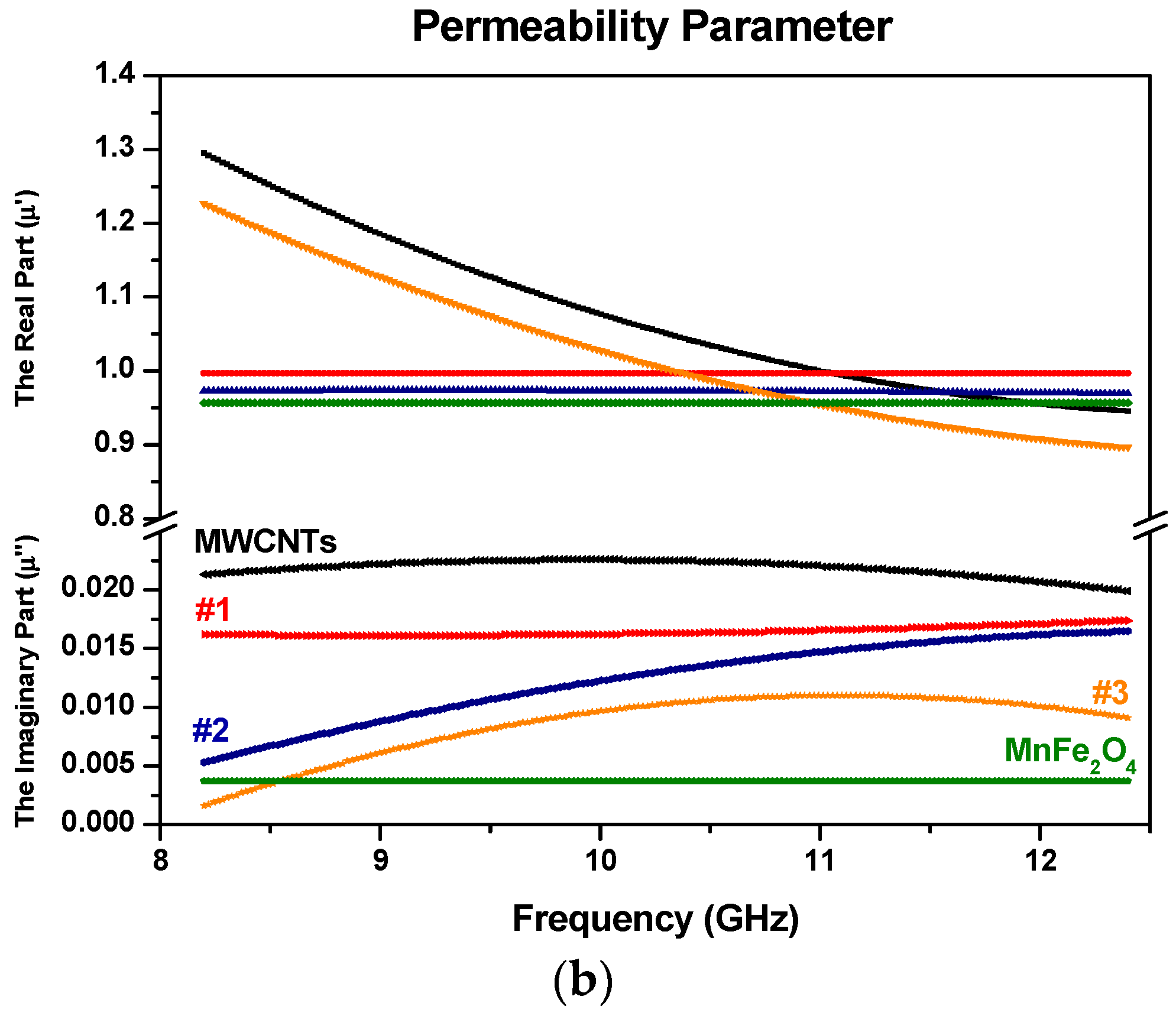
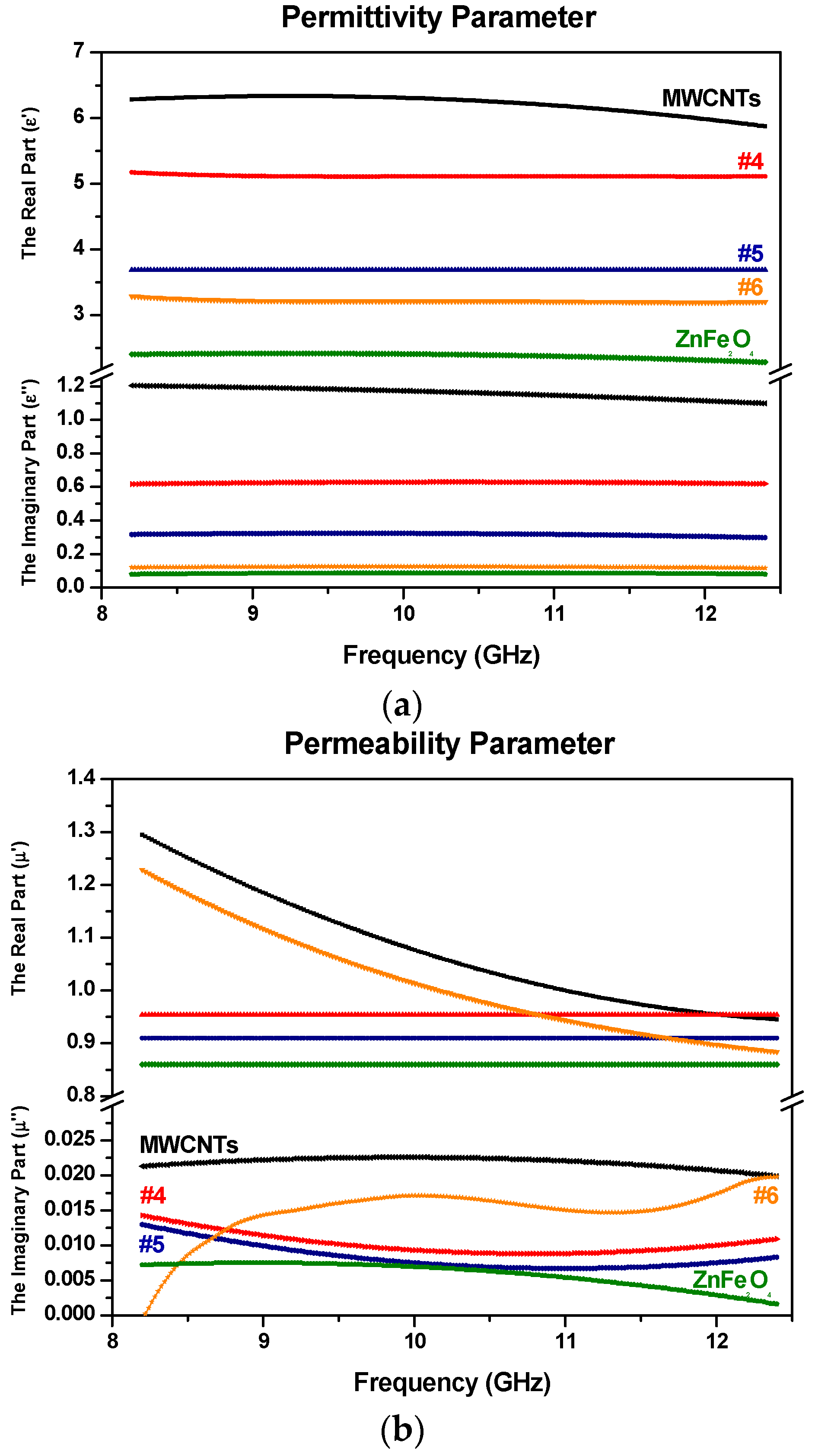
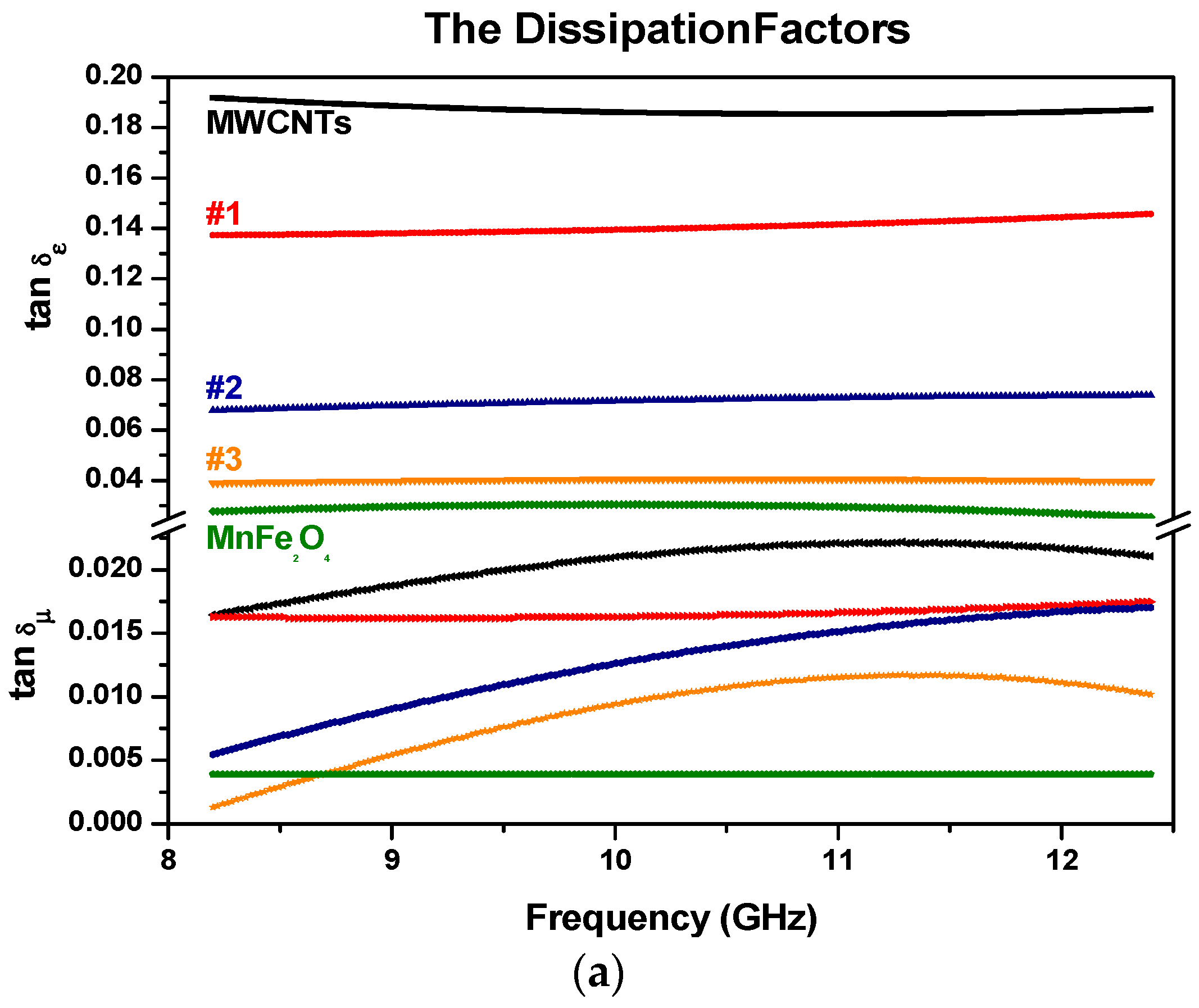
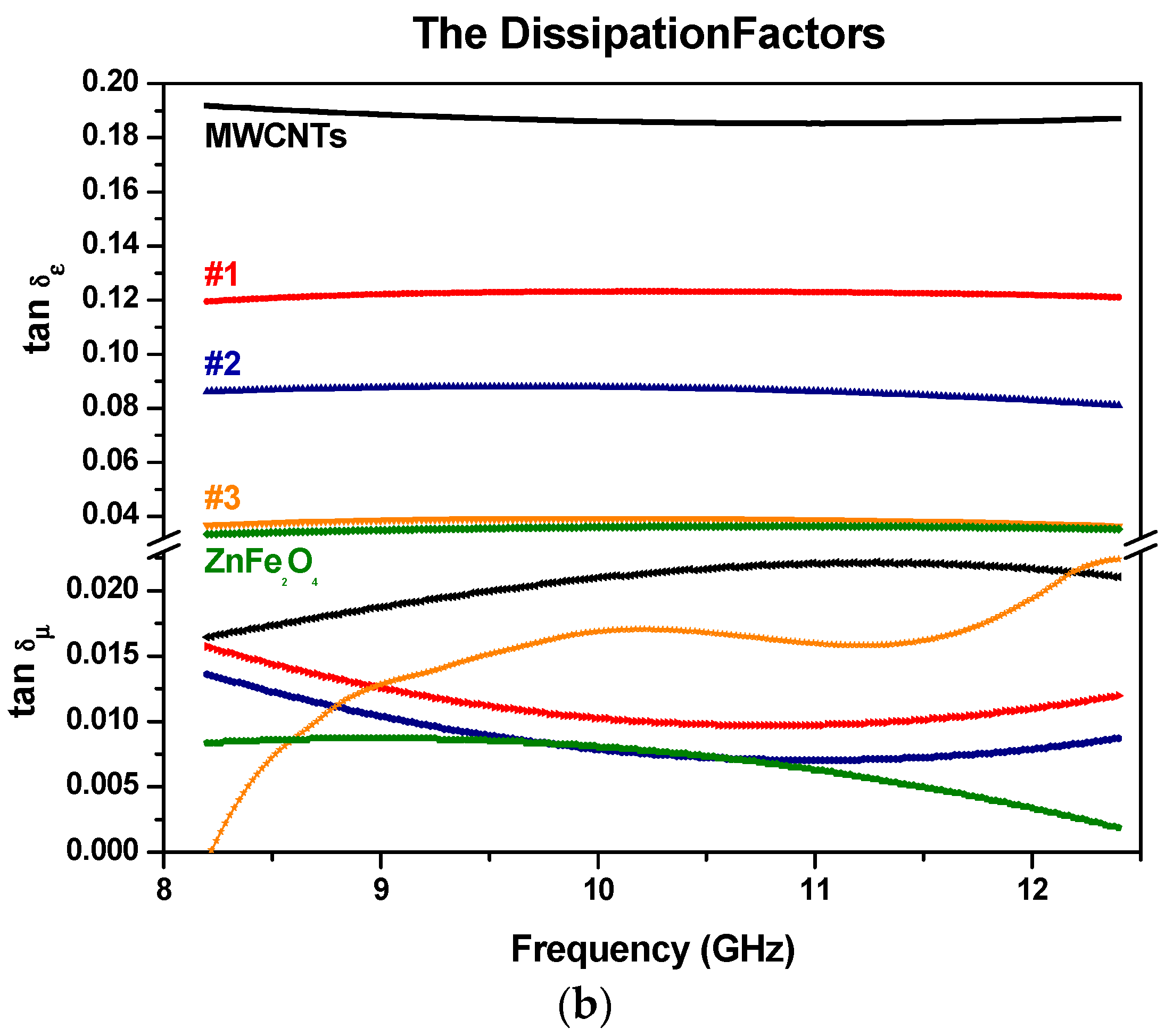
3.5. Electromagnetic Constitutive Parameters
3.6. Microwave Absorption Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rao, G.A.; Mahulikar, S.P. Integrated review of stealth technology and its role in airpower. Aeronaut. J. 2002, 106, 629–641. [Google Scholar]
- Barbarino, S.; Bilgen, O.; Ajaj, R.M.; Friswell, M.I.; Inman, D.J. A review of morphing aircraft. J. Intell. Mater. Syst. Struct. 2011, 22, 823–877. [Google Scholar] [CrossRef]
- Specialty Materials, Inc. Manufacturers of Boron and SCS Silicon Carbide Fibers and Boron Nanopowder. Available online: http://www.specmaterials.com/aerospaceapplications.htm (accessed on 12 December 2016).
- Wang, Z.; Wu, L.; Zhou, J.; Cai, W.; Shen, B.; Jiang, Z. Magnetite nanocrystals on multiwalled carbon nanotubes as a synergistic microwave absorber. J. Phys. Chem. C 2013, 117, 5446–5452. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Kompotiatis, L.; Kontogeorgakos, A.; Kordas, G. Microwave behavior of ferrites prepared via sol–gel method. J. Magn. Magn. Mater. 2002, 246, 360–365. [Google Scholar] [CrossRef]
- Lin, H.; Zhu, H.; Guo, H.; Yu, L. Investigation of the microwave-absorbing properties of Fe-filled carbon nanotubes. Mater. Lett. 2007, 61, 3547–3550. [Google Scholar] [CrossRef]
- Zou, T.; Li, H.; Zhao, N.; Shi, C. Electromagnetic and microwave absorbing properties of multi-walled carbon nanotubes filled with Ni nanowire. J. Alloy. Compd. 2010, 496, L22–L24. [Google Scholar] [CrossRef]
- Qing, Y.; Zhou, W.; Luo, F.; Zhu, D. Epoxy-silicone filled with multi-walled carbon nanotubes and carbonyl iron particles as a microwave absorber. Carbon 2010, 48, 4074–4080. [Google Scholar] [CrossRef]
- Qiu, J.; Qiu, T. Fabrication and microwave absorption properties of magnetite nanoparticle—Carbon nanotube-hollow carbon fiber composites. Carbon 2015, 81, 20–28. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, F.; Lin, J.; Yang, Z.; Zhang, M. Electromagnetic characteristics and microwave absorption properties of carbon-encapsulated cobalt nanoparticles in 2–18-GHz frequency range. Carbon 2014, 80, 103–111. [Google Scholar] [CrossRef]
- Lin, H.; Zhu, H.; Guo, H.; Yu, L. Microwave-absorbing properties of Co-filled carbon nanotubes. Mater. Res. Bull. 2008, 43, 2697–2702. [Google Scholar] [CrossRef]
- Shi, D.; He, P.; Zhao, P.; Guo, F.F.; Wang, F.; Huth, C.; Chaud, X.; Bud’ko, S.L.; Lian, J. Magnetic alignment of Ni/Co-coated carbon nanotubes in polystyrene composites. Compos. B Eng. 2011, 42, 1532–1538. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Chen, G.; Hua, S.; Ge, C.; Zhang, H.; Wu, R. Enhanced electromagnetic absorption properties of carbon nanotubes and zinc oxide whisker microwave absorber. J. Alloy. Compd. 2012, 514, 183–188. [Google Scholar] [CrossRef]
- Paton, K.R.; Windle, A.H. Efficient microwave energy absorption by carbon nanotubes. Carbon 2008, 46, 1935–1941. [Google Scholar] [CrossRef]
- Lu, M.; Cao, W.; Shi, H.; Fang, X.; Yang, J.; Hou, Z.; Jin, H.; Wang, W.; Yuan, J.; Cao, M. Multi-wall carbon nanotubes decorated with ZnO nanocrystals: Mild solution-process synthesis and highly efficient microwave absorption properties at elevated temperature. J. Mater. Chem. A 2014, 2, 10540–10547. [Google Scholar] [CrossRef]
- Teber, A.; Unver, I.; Kavas, H.; Aktas, B.; Bansal, R. Knitted radar absorbing materials (RAM) based on nickel–cobalt magnetic materials. J. Magn. Magn. Mater. 2016, 406, 228–232. [Google Scholar] [CrossRef]
- Khan, K. Microwave Absorption Properties of Radar Absorbing Nanosized Cobalt Ferrites for High Frequency Applications. J. Supercond. Nov. Magn. 2014, 27, 453–461. [Google Scholar] [CrossRef]
- Sunny, V.; Kurian, P.; Mohanan, P.; Joy, P.; Anantharaman, M. A flexible microwave absorber based on nickel ferrite nanocomposite. J. Alloy. Compd. 2010, 489, 297–303. [Google Scholar] [CrossRef]
- Xie, J.; Han, M.; Chen, L.; Kuang, R.; Deng, L. Microwave-absorbing properties of NiCoZn spinel ferrites. J. Magn. Magn. Mater. 2007, 314, 37–42. [Google Scholar] [CrossRef]
- Zhao, D.; Lv, Q.; Shen, Z. Fabrication and microwave absorbing properties of Ni–Zn spinel ferrites. J. Alloys Compd. 2009, 480, 634–638. [Google Scholar] [CrossRef]
- Gama, A.M.; Rezende, M.C.; Dantas, C.C. Dependence of microwave absorption properties on ferrite volume fraction in MnZn ferrite/rubber radar absorbing materials. J. Magn. Magn. Mater. 2011, 323, 2782–2785. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Asadnia, A. Synthesis, characterization, and microwave-absorbing properties of polypyrrole/MnFe2O4 nanocomposite. J. Nanomater. 2012, 2012, 3. [Google Scholar] [CrossRef]
- Sui, R.; Charpentier, P. Synthesis of metal oxide nanostructures by direct sol-gel chemistry in supercritical fluids. Chem. Rev. 2012, 112, 3057–3082. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Ong, C.; Neo, C.; Varadan, V.V.; Varadan, V.K. Microwave Electronics: Measurement and Materials Characterization; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- U.S. Research Nanomaterials, Inc. The Advanced Nanomaterials Provider. Available online: http://www.us-nano.com/inc/sdetail/228 (accessed on 12 December 2016).
- Debecker, D.P.; Hulea, V.; Mutin, P.H. Mesoporous mixed oxide catalysts via non-hydrolytic sol-gel: A Review. Appl. Catal. A Gen. 2013, 451, 192–206. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, H. Hybrid materials based on lanthanide organic complexes: A review. Chem. Soc. Rev. 2013, 382, 387–410. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.; Güner, S.; Bakis, Y.; Esir, S.; Baykal, A. Magnetic and Optical Properties of Mn1−xZnxFe2O4 Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2014, 24, 729–736. [Google Scholar] [CrossRef]
- Agilent Literature, Agilent Basics of Measuring the Dielectric Properties of Materials, Application Note. Available online: http://cp.literature.agilent.com/litweb/pdf/5989-2589EN.pdf (accessed on 25 December 2016).
- Micheli, D.; Pastore, R.; Giannini, G.; Vricella, A.; Marchetti, M. Low-cost low-observable satellites made of carbon nanostructured multilayers: Numerical investigation of scattering. In Proceedings of the 53rd Israel Annual Conference on Aerospace Sciences, Tel-Aviv & Haifa, Israel, 6–7 March 2013.
- Micheli, D.; Apollo, C.; Gradoni, G.; Marchetti, M.; Morles, R.B.; Pastore, R. Electromagnetic Characterization of Composite Materials and Microwave Absorbing Modeling. In Advances in Nanocomposites—Synthesis, Characterization and Industrial Applications; INTECH Open Access Publisher: Rijeka, Croatia, 2011; pp. 359–384. [Google Scholar]
- Micheli, D.; Apollo, C.; Pastore, R.; Morles, R.B.; Laurenzi, S.; Marchetto, M. Nanostructured composite materials for electromagnetic interference shielding applications. Acta Astronaut. 2011, 69, 747–757. [Google Scholar]
- Fang, G.; Chen, Z.; Li, H. Synthesis and properties of microencapsulated paraffin composites with SiO2 shell as thermal energy storage materials. Chem. Eng. J. 2010, 163, 154–159. [Google Scholar] [CrossRef]
- Cullity, B. Elements of X-ray Diffraction; Addison-Wesely Co., Reading: Boston, MA, USA, 1978. [Google Scholar]
- Gozuak, F.; Koseoglu, Y.; Baykal, A.; Kavas, H. Synthesis and characterization of CoxZn1-xFe2O4 magnetic nanoparticles via a PEG-assisted route. J. Magn. Magn. Mater. 2009, 321, 2170–2177. [Google Scholar] [CrossRef]
- Kanade, K.; Amalnerkar, D.; Potdar, H.; Kale, B. Nanocrystalline Mn–Zn–ferrite by novel oxalato-hydrazinated complex method. Mater. Chem. Phys. 2009, 117, 187–191. [Google Scholar] [CrossRef]
- Apte, S.; Naik, S.; Sonawane, R.; Kale, B.; Baeg, J. Synthesis of Nanosize-Necked Structure α- and γ-Fe2O3 and its Photocatalytic Activity. J. Am. Ceram. Soc. 2007, 90, 412–414. [Google Scholar] [CrossRef]
- Tang, Z.X.; Sorensen, C.M.; Klabunde, K.J.; Hadjipanayis, G.C. Size-dependent Curie temperature in nanoscale MnFe2O4 particles. Phys. Rev. Lett. 1991, 67, 3602. [Google Scholar] [CrossRef] [PubMed]
- Brahmachari, G.; Laskar, S.; Barik, P. Magnetically separable MnFe2O4 nano-material: An efficient and reusable heterogeneous catalyst for the synthesis of 2-substituted benzimidazoles and the extended synthesis of quinoxalines at room temperature under aerobic conditions. RSC Adv. 2013, 3, 14245–14253. [Google Scholar] [CrossRef]
- Clark, T.M.; Evans, B. Enhanced magnetization and cation distributions in nanocrystalline ZnFe2O4: A conversion electron Mossbauer spectroscopic investigation. IEEE Trans. Magn. 1997, 33, 3745–3747. [Google Scholar] [CrossRef]
- Jänis, A.; Olsson, R.T.; Savage, S.; Gedde, U.W.; Klement, U. Microwave absorbing properties of ferrite-based nanocomposites. In Proceedings of Behavior and Mechanics of Multifunctional and Composite Materials (SPIE 6526), San Diego, CA, USA, 12 April 2007. [CrossRef]
- Kong, I.; Ahmad, S.H.; Abdullah, M.H.; Hui, D.; Yusoff, A.N.; Puryanti, D. Magnetic and microwave absorbing properties of magnetite-thermoplastic natural rubber nanocomposites. J. Magn. Magn. Mater. 2010, 322, 3401–3409. [Google Scholar] [CrossRef]
- Yin, Y.; Zeng, M.; Liu, J.; Tang, W.; Dong, H.; Xia, R.; Yu, R. Enhanced high-frequency absorption of anisotropic Fe3O4/graphene nanocomposites. Nat. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Fu, Z.B.; Chen, H.B.; Zhong, M.L.; Wang, C.Y. Excellent electromagnetic absorption capability of Ni/carbon based conductive and magnetic foams synthesized via a green one-pot route. ACS Appl. Mater. Interfaces 2016, 8, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Li, J.; Zhang, X.; Ye, Q.; Xu, J.; Shen, X. Magnetic carbon nanofibers containing uniformly dispersed FE/Co/Ni nanoparticles as stable and high-performance electromagnetic wave absorbers. J. Mater. Chem. A 2014, 2, 16905–16914. [Google Scholar] [CrossRef]
- Liu, X.G.; Ou, Z.Q.; Geng, D.Y.; Han, Z.; Jiang, J.J.; Liu, W.; Zhang, Z.D. Influence of a graphite shell on the thermal and electromagnetic characteristics of FeNi nanoparticles. Carbon 2010, 48, 891–897. [Google Scholar] [CrossRef]
- Sozeri, H.; Mehmedi, Z.; Kavas, H.; Baykal, A. Magnetic and microwave properties of BaFe12O19 substituted with magnetic, non-magnetic and dielectric ions. Ceram. Int. 2015, 41, 9602–9609. [Google Scholar] [CrossRef]
- Naito, Y.; Suetake, K. Application of ferrite to electromagnetic wave absorber and its characteristics. IEEE Trans. Microw. Theory Tech. 1971, 19, 65–72. [Google Scholar] [CrossRef]

| MWCNT Dimensions | Bulk Density (g/cm3) | Specific Surface Area (m2/g) | Purity (wt %) | Aspect Ratio | Manufacturing Method |
|---|---|---|---|---|---|
| Inside Diameter 5–10 nm, Outside Diameter 20–30 nm (from HRTEM, Raman), length 10–30 µm (from TEM) | 0.28 | >110 (from BET) | >95 (carbon nanotubes from TGA and TEM) | 103 | Chemical Vapor Deposition (CVD) |
| >97 (carbon content) |
| Sample Code | Components |
|---|---|
| #1 | 75 wt % (MWCNTs) with 25 wt % (MnFe2O4) |
| #2 | 50 wt % (MWCNTs) with 50 wt % (MnFe2O4) |
| #3 | 25 wt % (MWCNTs) with 75 wt % (MnFe2O4) |
| #4 | 75 wt % (MWCNTs) with 25 wt % (ZnFe2O4) |
| #5 | 50 wt % (MWCNTs) with 50 wt % (ZnFe2O4) |
| #6 | 25 wt % (MWCNTs) with 75 wt % (ZnFe2O4) |
| Sample | Tetrahedral | Octahedral |
|---|---|---|
| MnFe2O4 | 433 | 535 |
| ZnFe2O4 | 430 | 528 |
| Sample | Ms | Hc | Mr | Mr/Ms |
|---|---|---|---|---|
| MnFe2O4 | 70.52 | 57 | 9.72 | 0.137 |
| ZnFe2O4 | 35.90 | 50 | 4.03 | 0.112 |
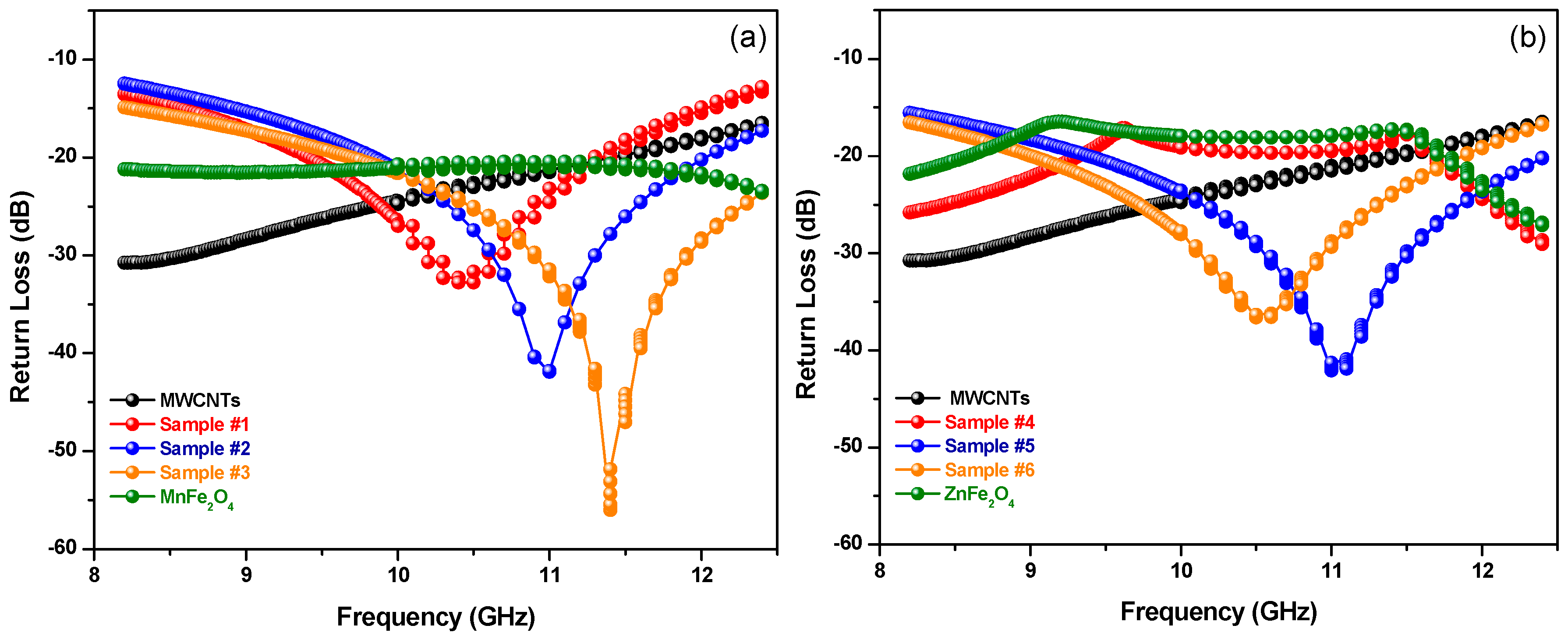
| Code | fr (GHz) | Return Loss (dB) | Bandwidth (GHz) (under −20 dB) |
|---|---|---|---|
| Sample #1 | 10.44 | −32.84 | 1.88 (9.42–11.3) |
| Sample #2 | 11.00 | −41.20 | 2.2 (9.84–12.04) |
| Sample #3 | 11.41 | −56.00 | 3.38 (9.7–13.08) |
| Sample #4 | - | - | - |
| Sample #5 | 11.05 | −42.06 | 3.34 (9.38–12.72) |
| Sample #6 | 10.55 | −36.72 | 2.8 (9.00–11.80) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teber, A.; Cil, K.; Yilmaz, T.; Eraslan, B.; Uysal, D.; Surucu, G.; Baykal, A.H.; Bansal, R. Manganese and Zinc Spinel Ferrites Blended with Multi-Walled Carbon Nanotubes as Microwave Absorbing Materials. Aerospace 2017, 4, 2. https://doi.org/10.3390/aerospace4010002
Teber A, Cil K, Yilmaz T, Eraslan B, Uysal D, Surucu G, Baykal AH, Bansal R. Manganese and Zinc Spinel Ferrites Blended with Multi-Walled Carbon Nanotubes as Microwave Absorbing Materials. Aerospace. 2017; 4(1):2. https://doi.org/10.3390/aerospace4010002
Chicago/Turabian StyleTeber, Ahmet, Kadir Cil, Turgut Yilmaz, Busra Eraslan, Dilara Uysal, Gokce Surucu, Abdul H. Baykal, and Rajeev Bansal. 2017. "Manganese and Zinc Spinel Ferrites Blended with Multi-Walled Carbon Nanotubes as Microwave Absorbing Materials" Aerospace 4, no. 1: 2. https://doi.org/10.3390/aerospace4010002