New Adverse Drug Reaction Signals from 2017 to 2021—Genuine Alerts or False Alarms?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.1.1. Inclusion Criteria
- Adverse event study reporting on disproportionality statistics;
- Evaluating one named drug or a single class of drug;
- Comparing proportions of adverse event reports;
- Study aimed at generating signals for any adverse events with the drug;
- Analysis subsequently identified significant proportional increase in reports for one or more adverse events that the authors reported to be new or novel.
2.1.2. Exclusion Criteria
- Pre-specified or a priori hypothesis-testing study;
- Focused only on a specified single adverse effect, or adverse effects solely related to pre-specified organ systems;
- Vaccine studies.
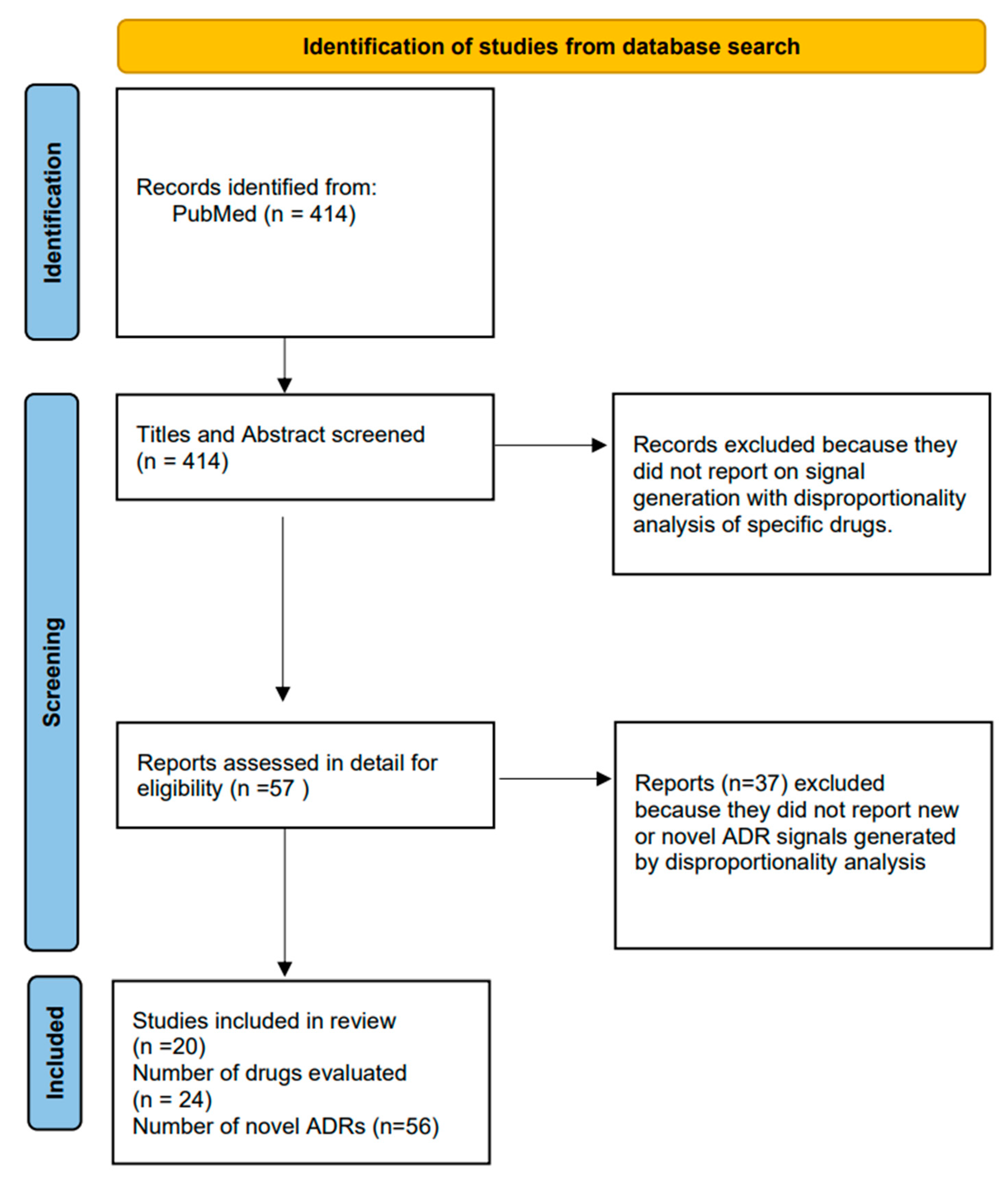
2.2. Screening Studies for Inclusion
2.3. Data Extraction
- PubMed using the adverse effect term and the name of the pharmacological compound or the class of the compounds;
- Google Scholar for citing articles related to the original proportionality analysis.
3. Results
3.1. Literature That Cited the Study Where an ADR Signal Had Been Newly Identified
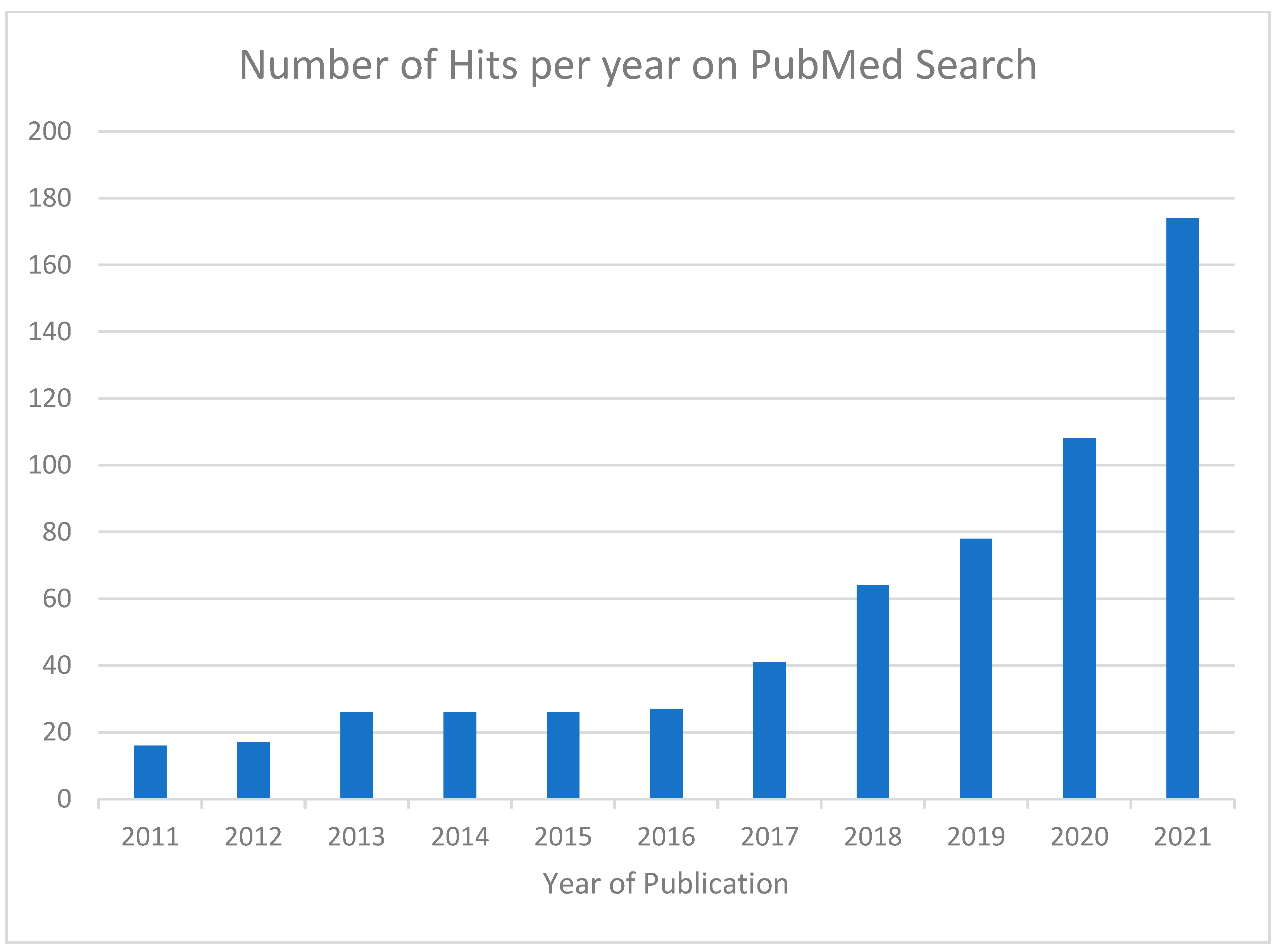
3.2. PubMed Search for Subsequent Evaluations of New ADR Signal
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hauben, M.; Madigan, D.; Gerrits, C.M.; Walsh, L.; Van Puijenbroek, E.P. The role of data mining in pharmacovigilance. Expert. Opin. Drug Saf. 2005, 4, 929–948. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.; Scosyrev, E.; Petrin, M.; Schmouder, R. Can Disproportionality Analysis of Post-marketing Case Reports be Used for Comparison of Drug Safety Profiles? Clin. Drug Investig. 2017, 37, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Trontell, A.E. How the US Food and Drug Administration defines and detects adverse drug events. Curr. Ther. Res. 2001, 62, 641–649. [Google Scholar] [CrossRef]
- Bihan, K.; Lebrun-Vignes, B.; Funck-Brentano, C.; Salem, J.E. Uses of pharmacovigilance databases: An overview. Therapie 2020, 75, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, L.; Sabatier, P.; Katsahian, S.; Jannot, A.S. Pharmacovigilance studies without a priori hypothesis: Systematic review highlights inappropriate multiple testing correction procedures. J. Clin. Epidemiol. 2023, 162, 127–134. [Google Scholar] [CrossRef]
- Raschi, E.; Salvo, F.; Khouri, C. Conceiving, conducting, reporting, interpreting, and publishing disproportionality analyses: A call to action. Br. J. Clin. Pharmacol. 2022, 88, 3535–3536. [Google Scholar] [CrossRef]
- Khouri, C.; Revol, B.; Lepelley, M.; Mouffak, A.; Bernardeau, C.; Salvo, F.; Pariente, A.; Roustit, M.; Cracowski, J.L. A meta-epidemiological study found lack of transparency and poor reporting of disproportionality analyses for signal detection in pharmacovigilance databases. J. Clin. Epidemiol. 2021, 139, 191–198. [Google Scholar] [CrossRef]
- Mouffak, A.; Lepelley, M.; Revol, B.; Bernardeau, C.; Salvo, F.; Pariente, A.; Roustit, M.; Cracowski, J.L.; Khouri, C. High prevalence of spin was found in pharmacovigilance studies using disproportionality analyses to detect safety signals: A meta-epidemiological study. J. Clin. Epidemiol. 2021, 138, 73–79. [Google Scholar] [CrossRef]
- Beau-Lejdstrom, R.; Crook, S.; Spanu, A.; Yu, T.; Puhan, M.A. Adverse Drug Reaction Risk Measures: A Comparison of Estimates from Drug Surveillance and Randomised Trials. Pharm. Med. 2019, 33, 331–339. [Google Scholar] [CrossRef]
- Maciá-Martínez, M.A.; de Abajo, F.J.; Roberts, G.; Slattery, J.; Thakrar, B.; Wisniewski, A.F. An Empirical Approach to Explore the Relationship Between Measures of Disproportionate Reporting and Relative Risks from Analytical Studies. Drug Saf. 2016, 39, 29–43. [Google Scholar] [CrossRef]
- Khouri, C.; Fusaroli, M.; Salvo, F.; Raschi, E. Transparency and robustness of safety signals. BMJ 2022, 379, o2588. [Google Scholar] [CrossRef]
- Loke, Y.K.; Price, D.; Derry, S.; Aronson, J.K. Case reports of suspected adverse drug reactions—Systematic literature survey of follow-up. BMJ 2006, 332, 335–339. [Google Scholar] [CrossRef]
- Bate, A.; Evans, S.J.W. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol. Drug Saf. 2009, 18, 427–436. [Google Scholar] [CrossRef]
- Evans, S.J.W.; Waller, P.C.; Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 2001, 10, 483–486. [Google Scholar] [CrossRef]
- Raschi, E.; Poluzzi, E.; Salvo, F.; Pariente, A.; De Ponti, F.; Marchesini, G.; Moretti, U. Pharmacovigilance of sodium-glucose co-transporter-2 inhibitors: What a clinician should know on disproportionality analysis of spontaneous reporting systems. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 533–542. [Google Scholar] [CrossRef]
- Choi, J.; Yoon, D.; Park, M.; Joung, K.I.; Shin, J.Y. Topiramate-related adverse events: Pattern and signals in the Korea Adverse Event Reporting System, 2010-2017. Medicine 2020, 99, e22669. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Choi, J.H.; Kim, M.G.; Rhie, S.J. Signal Detection of Adverse Drug Reactions of Cephalosporins Using Data from a National Pharmacovigilance Database. Pharmaceuticals 2021, 14, 425. [Google Scholar] [CrossRef]
- Cross, R.K.; Chiorean, M.; Vekeman, F.; Xiao, Y.; Wu, E.; Chao, J.; Wang, A.W. Assessment of the real-world safety profile of vedolizumab using the United States Food and Drug Administration adverse event reporting system. PLoS ONE 2019, 14, e0225572. [Google Scholar] [CrossRef] [PubMed]
- Gastaldon, C.; Raschi, E.; Kane, J.M.; Barbui, C.; Schoretsanitis, G. Post-Marketing Safety Concerns with Esketamine: A Disproportionality Analysis of Spontaneous Reports Submitted to the FDA Adverse Event Reporting System. Psychother. Psychosom. 2021, 90, 41–48. [Google Scholar] [CrossRef]
- Gatti, M.; Antonazzo, I.C.; Diemberger, I.; De Ponti, F.; Raschi, E. Adverse events with sacubitril/valsartan in the real world: Emerging signals to target preventive strategies from the FDA adverse event reporting system. Eur. J. Prev. Cardiol. 2021, 28, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Fusaroli, M.; Caraceni, P.; Poluzzi, E.; De Ponti, F.; Raschi, E. Serious adverse events with tocilizumab: Pharmacovigilance as an aid to prioritize monitoring in COVID-19. Br. J. Clin. Pharmacol. 2021, 87, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Fusaroli, M.; Raschi, E.; Moretti, U.; Poluzzi, E.; De Ponti, F. Serious adverse events with tedizolid and linezolid: Pharmacovigilance insights through the FDA adverse event reporting system. Expert. Opin. Drug Saf. 2021, 20, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Raschi, E.; De Ponti, F. Serious adverse events with novel beta-lactam/beta-lactamase inhibitor combinations: A large-scale pharmacovigilance analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1169–1176. [Google Scholar] [CrossRef]
- Ha, D.; Lee, S.E.; Song, I.; Lim, S.J.; Shin, J.Y. Comparison of signal detection of tumour necrosis factor-alpha inhibitors using the Korea Adverse Events Reporting System Database, 2005–2016. Clin. Rheumatol. 2020, 39, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.Y.; Cho, M.K.; Kim, S. Data mining for detecting signals of adverse drug reaction of doxycycline using the Korea adverse event reporting system database. J. Dermatol. Treat. 2022, 33, 2192–2197. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, A.; Noh, Y.; Jeon, H.L.; Choe, S.A.; Shin, J.Y. Signal detection of drospirenone-containing oral contraceptives: A disproportionality analysis using the Korea Adverse Event Reporting System Database, 2008–2017. BMJ Open 2021, 11, e045948. [Google Scholar] [CrossRef]
- Meirson, T.; Asher, N.; Bomze, D.; Markel, G. Safety of BRAF+MEK Inhibitor Combinations: Severe Adverse Event Evaluation. Cancers 2020, 12, 1650. [Google Scholar] [CrossRef]
- Omar, N.E.; Fahmy Soliman, A.I.; Eshra, M.; Saeed, T.; Hamad, A.; Abou-Ali, A. Postmarketing safety of anaplastic lymphoma kinase (ALK) inhibitors: An analysis of the FDA Adverse Event Reporting System (FAERS). ESMO Open 2021, 6, 100315. [Google Scholar] [CrossRef]
- Park, K.; Soukavong, M.; Kim, J.; Kwon, K.E.; Jin, X.M.; Lee, J.; Yang, B.R.; Park, B.J. Signal Detection of Imipenem Compared to Other Drugs from Korea Adverse Event Reporting System Database. Yonsei Med. J. 2017, 58, 564–569. [Google Scholar] [CrossRef]
- Peng, L.; Xiao, K.; Ottaviani, S.; Stebbing, J.; Wang, Y.J. A real-world disproportionality analysis of FDA Adverse Event Reporting System (FAERS) events for baricitinib. Expert. Opin. Drug Saf. 2020, 19, 1505–1511. [Google Scholar] [CrossRef]
- Seo, D.E.; Kim, S.; Park, B.J. Signals of Adverse Drug Reactions of Paliperidone Compared to Other Atypical Antipsychotics Using the Korean Adverse Event Reporting System Database. Clin. Drug Investig. 2020, 40, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Subeesh, V.; Singh, H.; Maheswari, E.; Beulah, E. Novel adverse events of vortioxetine: A disproportionality analysis in USFDA adverse event reporting system database. Asian J. Psychiatr. 2017, 30, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Yao, Y.; He, G.; Jia, Y.; Wang, K.; Chen, L. Systematic analysis of safety profile for darunavir and its boosted agents using data mining in the FDA Adverse Event Reporting System database. Sci. Rep. 2021, 11, 12438. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yin, N.; Zhao, Y.; Li, D.; Zhang, X.; Li, X.; Zhang, Y.; Faiola, F. Adverse Events During Pregnancy Associated With Entecavir and Adefovir: New Insights From a Real-World Analysis of Cases Reported to FDA Adverse Event Reporting System. Front. Pharmacol. 2021, 12, 772768. [Google Scholar] [CrossRef]
- Zhou, X.; Ye, X.; Guo, X.; Liu, D.; Xu, J.; Hu, F.; Zhai, Y.; Gao, Y.; Xu, X.; Dong, Z.; et al. Safety of SGLT2 Inhibitors: A Pharmacovigilance Study from 2013 to 2021 Based on FAERS. Front. Pharmacol. 2021, 12, 766125. [Google Scholar] [CrossRef]
| Key features |
|
| Limitations |
|
| Study ID | Number of Drugs with Signal | Compounds and Comparators | Number of New ADR Signals | Specific Significant New ADRs | Times Cited (Google Scholar) | Confirmation Studies (Google Scholar) | Confirmation Studies on Pubmed Search |
|---|---|---|---|---|---|---|---|
| Choi 2020 [16] | 1 | Topiramate vs. other antiepileptics | 1 | steatorrhoea | 4 | 0 | 0 |
| Choi 2021 [17] | 1 | Cefatrizine vs. other antibacterials | 2 | corneal oedema, corneal ulceration | 5 | 0 | 0 |
| Cross 2019 [18] | 1 | Vedolizumab vs. TNF antagonists | 2 | CNS haemorrhages and stroke | 15 | 0 | 0 |
| Gastaldon 2021 [19] | 1 | Esketamine vs. all other drugs | 1 | suicidal ideation | 61 | 0 | No confirmation of such a link in research conducted prior to or after this paper. |
| Gatti 2021 [22] | 1 | Tedizolid vs. linezolid | 1 | hepatic failure | 7 | 0 | 0 |
| Gatti 2021 [21] | 1 | Tocilizumab vs. all other drugs | 1 | acute pancreatitis | 42 | 0 | 0 |
| Gatti 2021 [20] | 1 | Sacubitril/ valsartan vs. other cardiovascular drugs | 4 | sudden cardiac death, nipple pain, hepatic cyst, pyoderma gangrenosum | 21 | 0 | At least 3 studies looking at efficacy at reducing sudden cardiac death, not as ADR. No studies on other ADRs. |
| Gatti 2021 [23] | 2 | Ceftolozane tazobactam, ceftazidime avibactam vs. all other drugs | 3 | agranulocytosis, pancytopaenia, acute pancreatitis | 7 | 0 | 0 |
| Ha 2020 [24] | 1 | Infliximab vs. all other drugs | 2 | palpitation, temperature sensation change | 6 | 0 | No confirmation studies for infliximab. |
| Heo 2021 [25] | 1 | Doxycycline vs. all other drugs | 8 | malaise, ileus, confusion, malignant neoplasm, ectopic pregnancy, ovarian hyperstimulation, vaginal haemorrhage, bone necrosis | 0 | 0 | 0 |
| Lee 2021 [26] | 1 | Drospirenone vs. other contraceptive pills | 3 | chest pain, dyspnoea, fatigue | 2 | 0 | 0 |
| Merrison 2020 [27] | 1 | Encorafenib vs. other agents in class | 2 | Guillain–Barre syndrome, seizures | 14 | 0 | 0 |
| Omar 2021 [28] | 4 | crizotinib, ceritinib, alectinib, brigatinib | 2 | pneumothorax, photosensitivity | 13 | 0 | 0 |
| Park 2017 [29] | 1 | Imipenem vs. all other drugs | 5 | cardiac arrest, cardiac failure, myocardial infarction, Parkinson’s syndrome, and prostate enlargement | 15 | 0 | 0 |
| Peng 2020 [30] | 1 | Baricitinib vs. all other drugs | 3 | pneumocystis pneumonia, nephritis | 0 | 0 | 0 |
| Seo 2020 [31] | 1 | Paliperidone vs. other atypical antipsychotics | 7 | seborrhoea, obesity, breast neoplasm, vaginitis, fibroid, gingivitis, intervertebral disc disorder | 2 | 0 | 0 |
| Subeesh 2017 [32] | 1 | Vortioxetine vs. all other drugs | 2 | weight loss, ketoacidosis | 12 | 1 (but weight loss trial preceded the FAERS signal) | Weight loss not confirmed in several other studies. |
| Tian 2021 [33] | 1 | Darunavir vs. all other drugs | 4 | neuropathy, diplopia, ptosis, ophthalmoplegia | 2 | 0 | 0 |
| Yang 2021 [34] | 1 | Entecavir/adefovir vs. other antivirals | 1 | spontaneous abortion | 4 | 0 | Retrospective cohort study reported no association with adverse birth outcomes. |
| Zhou 2021 [35] | 1 | Canagliflozin vs. all other drugs | 2 | cellulitis, osteomyelitis | 2 | Pooled analysis 2 RCTs—no increase in cellulitis | Inconsistent data on osteomyelitis. Non-significant in one meta-analysis and one observational study, but significant in another observational study (all studies preceded the disproportionality analysis). |
| Attribute | Key Considerations |
|---|---|
| Data quality | Conflicting data may occur because reports on the same case can be submitted by many different parties. The completeness and accuracy of the submitted information are variable, and sometimes not all the necessary information is provided in the report. |
| Causal relationship | Contents of submitted reports are based on reporter’s judgement and opinions. Cannot be certain that adverse event was definitely due to the drug. |
| Rates of occurrence | Duplicate reports can occur. No denominator data regarding number of users and therefore incidence rates cannot be calculated or compared. Number of submitted reports cannot be used to infer the true risk because many other external factors can affect reporting. |
| Factors that influence reporting | Media publicity, recently launched drug, striking features of adverse event influence reporting. |
| Types of adverse events recorded | Particularly useful for events with low background rates and which happen soon after using a new drug. Less helpful if event of interest has a high background rate, or has similar features to disease progression. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loke, Y.K.; Mattishent, K.; Navaneetharaja, N. New Adverse Drug Reaction Signals from 2017 to 2021—Genuine Alerts or False Alarms? Pharmacy 2024, 12, 33. https://doi.org/10.3390/pharmacy12010033
Loke YK, Mattishent K, Navaneetharaja N. New Adverse Drug Reaction Signals from 2017 to 2021—Genuine Alerts or False Alarms? Pharmacy. 2024; 12(1):33. https://doi.org/10.3390/pharmacy12010033
Chicago/Turabian StyleLoke, Yoon Kong, Katharina Mattishent, and Navena Navaneetharaja. 2024. "New Adverse Drug Reaction Signals from 2017 to 2021—Genuine Alerts or False Alarms?" Pharmacy 12, no. 1: 33. https://doi.org/10.3390/pharmacy12010033






