Development of a Prediction Model to Identify the Risk of Clostridioides difficile Infection in Hospitalized Patients Receiving at Least One Dose of Antibiotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
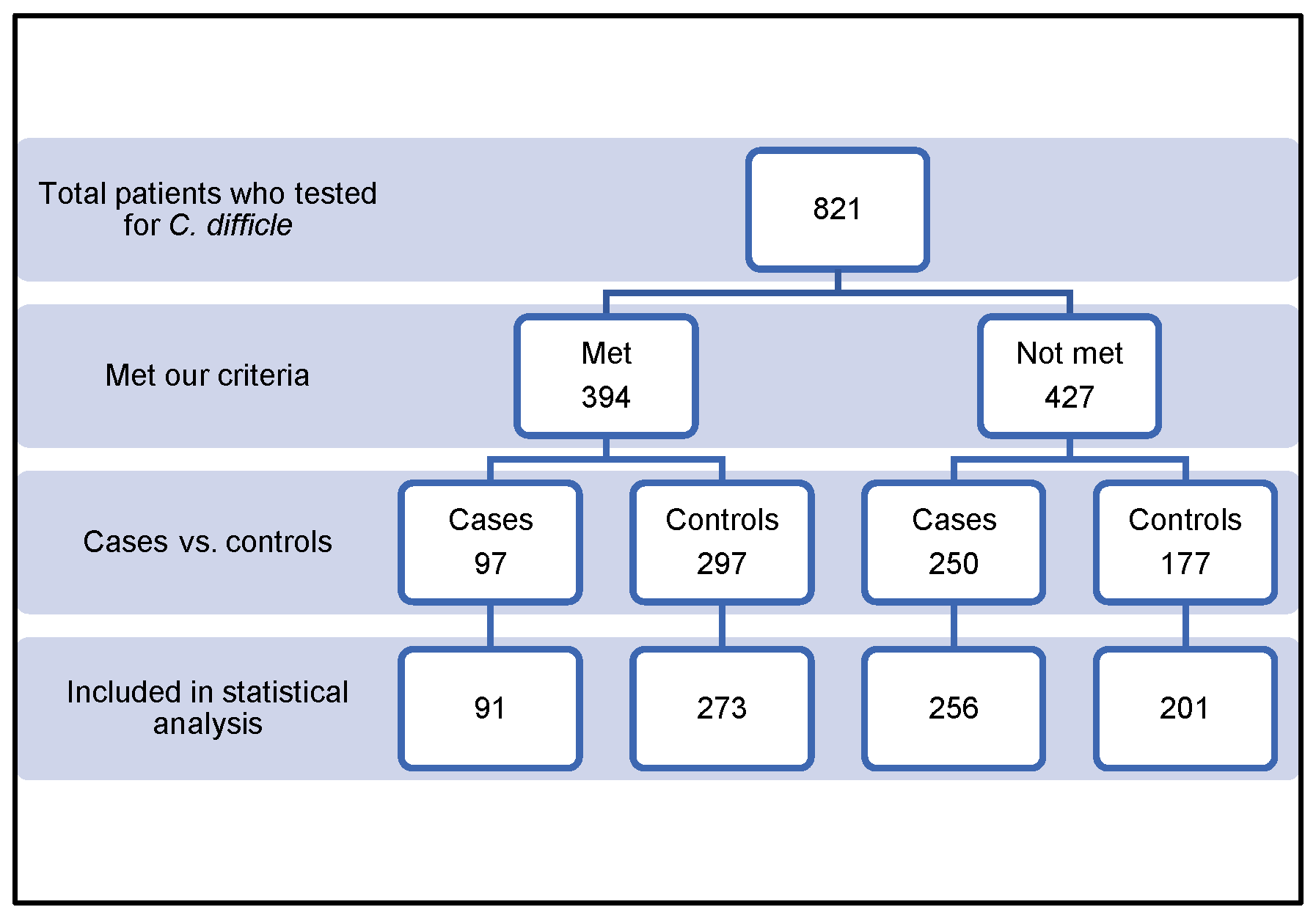
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Collection Procedures, Data Sources, and Measurements
2.5. Data Management and Analysis
2.6. Ethics Approval
3. Results
4. Discussion
4.1. Risk factors
4.1.1. Age
4.1.2. Kidney Dysfunction
4.1.3. Lymphoma/Leukemia
4.1.4. Solid Organ Transplant
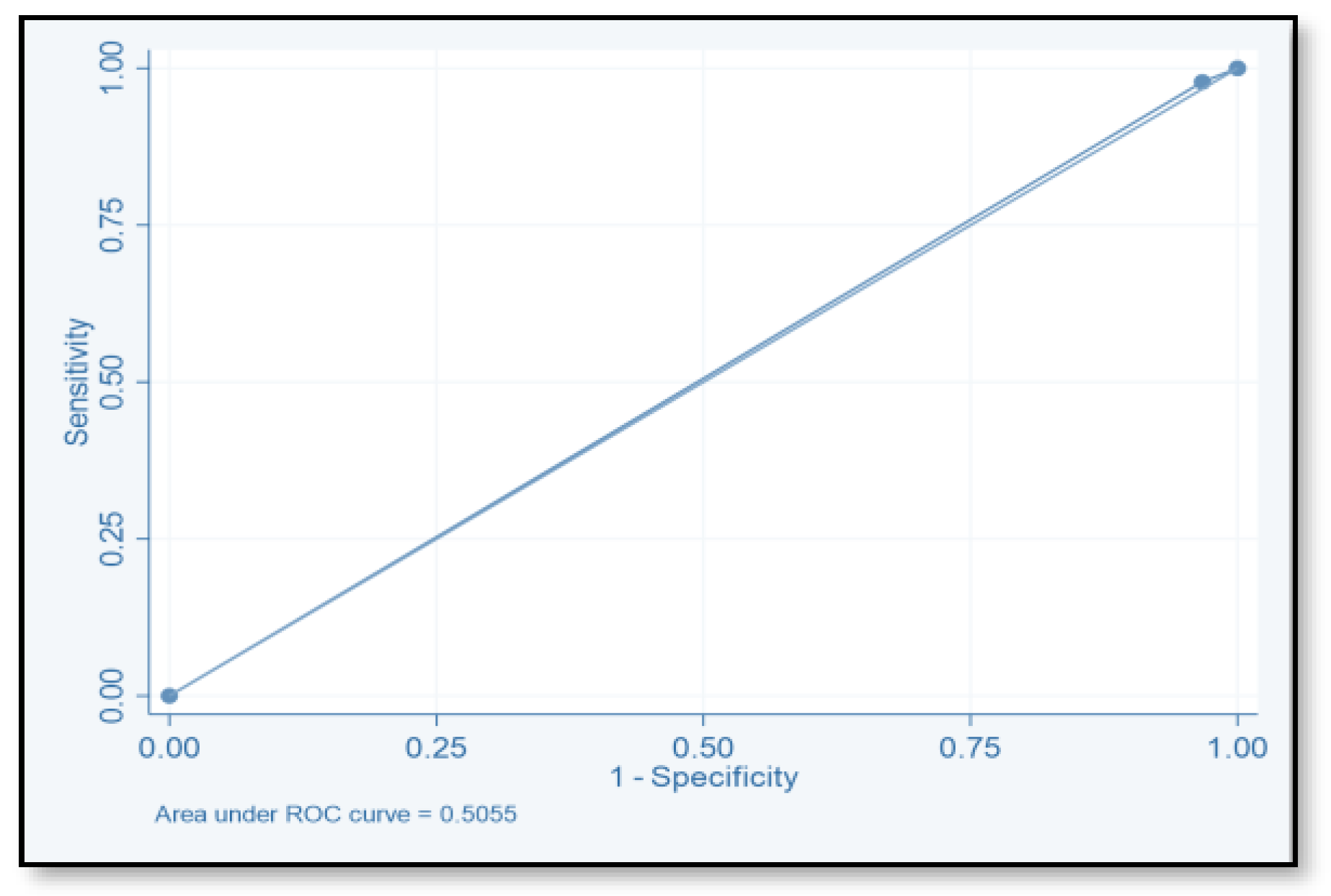
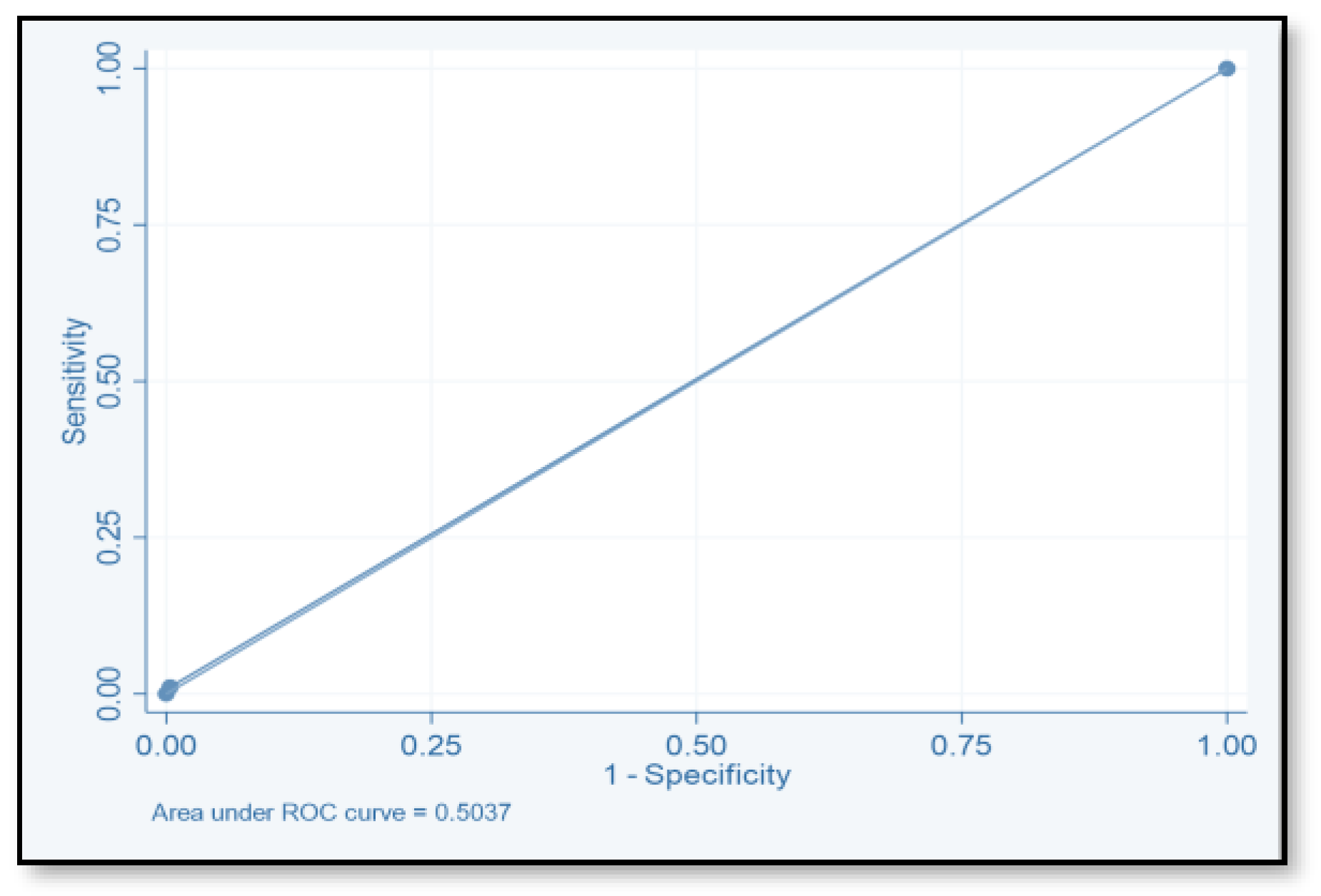
4.2. Risk Prediction Model
Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smits, W.K.; Lyras, D.; Lacy, D.B.; Wilcox, M.H.; Kuijper, E.J. Clostridium difficile infection. Nat. Rev. Dis. Primers 2016, 2, 16020. [Google Scholar] [CrossRef]
- George, R.H.; Symonds, J.M.; Dimock, F.; Brown, J.D.; Arabi, Y.; Shinagawa, N.; Keighley, M.R.; Alexander-Williams, J.; Burdon, D.W. Identification of Clostridium difficile as a cause of pseudomembranous colitis. Br. Med. J. 1978, 1, 695. [Google Scholar] [CrossRef]
- Sunenshine, R.H.; McDonald, L.C. Clostridium difficile-associated disease: New challenges from an established pathogen. Cleve. Clin. J. Med. 2006, 73, 187–197. [Google Scholar] [CrossRef]
- Oliveira, P.H.; Fang, G. Conserved DNA methyltransferases: A window into fundamental mechanisms of epigenetic regulation in bacteria. Trends Microbiol. 2021, 29, 28–40. [Google Scholar] [CrossRef]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, 987–994. [Google Scholar] [CrossRef]
- Donskey, C.J. Preventing transmission of Clostridium difficile: Is the answer blowing in the wind? Clin. Infect. Dis. 2010, 50, 1458–1461. [Google Scholar] [CrossRef]
- Longtin, Y.; Trottier, S.; Brochu, G.; Paquet-Bolduc, B.; Garenc, C.; Loungnarath, V.; Beaulieu, C.; Goulet, D.; Longtin, J. Impact of the type of diagnostic assay on Clostridium difficile infection and complication rates in a mandatory reporting program. Clin. Infect. Dis. 2013, 56, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Vaishnavi, C. Established and potential risk factors for Clostridum difficile infection. Ind. J. Med. Microbiol. 2009, 27, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Eze, P.; Balsells, E.; Kyaw, M.H.; Nair, H. Risk factors for Clostridium difficile infections—An overview of the evidence base and challenges in data synthesis. J. Glob. Health 2017, 7, 010417. [Google Scholar] [CrossRef] [PubMed]
- Durham, D.P.; Olsen, M.A.; Dubberke, E.R.; Galvani, A.P.; Townsend, J.P. Quantifying transmission of Clostridium difficile within and outside healthcare settings. Emerg. Infect. Dis. 2016, 22, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Tabak, Y.P.; Srinivasan, A.; Yu, K.C.; Kurtz, S.G.; Gupta, V.; Gelone, S.; Scoble, P.J.; McDonald, L.C. Hospital-level high-risk antibiotic use in relation to hospital-associated Clostridioides difficile infections: Retrospective analysis of 2016–2017 data from US hospitals. Infect. Control Hosp. Epidemiol. 2019, 40, 1229–1235. [Google Scholar] [CrossRef]
- Alzouby, S.; Baig, K.; Alrabiah, F.; Shibl, A.; Al-Nakhli, D.; Senok, A.C. Clostridioides difficile infection: Incidence and risk factors in a tertiary care facility in Riyadh, Saudi Arabia. J. Infect. Public Health 2020, 13, 1012–1017. [Google Scholar] [CrossRef]
- Oh, J.; Makar, M.; Fusco, C.; McCaffrey, R.; Rao, K.; Ryan, E.E.; Washer, L.; West, L.R.; Young, V.B.; Guttag, J.; et al. A generalizable, data-driven approach to predict daily risk of Clostridium difficile infection at two large academic health centers. Infect. Control Hosp. Epidemiol. 2018, 39, 425–433. [Google Scholar] [CrossRef]
- National Guard Health Affairs. Riyadh: KING Abdulaziz Medical City. 2018. Available online: https://ngha.med.sa/English/MedicalCities/AlRiyadh/Pages/default.aspx (accessed on 18 August 2018).
- van Werkhoven, C.H.; van der Tempel, J.; Jajou, R.; Thijsen, S.F.; Diepersloot, R.J.; Bonten, M.J.; Postma, D.F.; Oosterheert, J.J. Identification of patients at high risk for Clostridium difficile infection: Development and validation of a risk prediction model in hospitalized patients treated with antibiotics. Clin. Microbiol. Infect. 2015, 21, 786.e1–786.e8. [Google Scholar] [CrossRef]
- Davis, M.L.; Sparrow, H.G.; Ikwuagwu, J.O.; Musick, W.L.; Garey, K.W.; Perez, K.K. Multicentre derivation and validation of a simple predictive index for healthcare-associated Clostridium difficile infection. Clin. Microbiol. Infect. 2018, 24, 1190–1194. [Google Scholar] [CrossRef]
- Dubberke, E.R.; Yan, Y.; Reske, K.A.; Butler, A.M.; Doherty, J.; Pham, V.; Fraser, V.J. Development and validation of a Clostridium difficile infection risk prediction model. Infect. Control Hosp. Epidemiol. 2011, 32, 360–366. [Google Scholar] [CrossRef]
- Garey, K.W.; Dao-Tran, T.K.; Jiang, Z.D.; Price, M.P.; Gentry, L.O.; Dupont, H.L. A clinical risk index for Clostridium difficile infection in hospitalised patients receiving broad-spectrum antibiotics. J. Hosp. Infect. 2008, 70, 142–147. [Google Scholar] [CrossRef]
- Tilton, C.S.; Johnson, S.W. Development of a risk prediction model for hospital-onset Clostridium difficile infection in patients receiving systemic antibiotics. Am. J. Infect. Control 2019, 47, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.C.; Wieczorkiewicz, J.T.; Tuazon, J. Evaluation of advanced age as a risk factor for severe Clostridium difficile infection. J. Clin. Gerontol. Geriatr. 2016, 7, 12–16. [Google Scholar] [CrossRef]
- Henrich, T.J.; Krakower, D.; Bitton, A.; Yokoe, D.S. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg. Infect. Dis. 2009, 15, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Andersen, K.; Stecher, B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013, 83, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Betjes, M.G. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 2013, 9, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Aronsson, B.; Barany, P.; Nord, C.E.; Nyström, B.; Stenvinkel, P. Clostridium difficile-associated diarrhoea in uremic patients. Eur. J. Clin. Microbiol. 1987, 6, 352–356. [Google Scholar] [CrossRef] [PubMed]
- McConnell, J.B.; Stewart, W.K.; Thjodleifsson, B.; Wormsley, K.G. Gastric function in chronic renal failure: Effects of maintenance haemodialysis. Lancet 1975, 2, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Keddis, M.T.; Khanna, S.; Noheria, A.; Baddour, L.M.; Pardi, D.S.; Qian, Q. Clostridium difficile infection in patients with chronic kidney disease. Mayo Clin. Proc. 2012, 87, 1046–1053. [Google Scholar] [CrossRef]
- Kim, S.C.; Seo, M.Y.; Lee, J.Y.; Kim, K.T.; Cho, E.; Kim, M.G.; Jo, S.K.; Cho, W.Y.; Kim, H.K. Advanced chronic kidney disease: A strong risk factor for Clostridium difficile infection. Korean J. Intern. Med. 2016, 31, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Selvey, L.A.; Slimings, C.; Joske, D.J.; Riley, T.V. Clostridium difficile infections amongst patients with haematological malignancies: A data linkage study. PLoS ONE 2016, 11, e0157839. [Google Scholar] [CrossRef]
- Bal, A.; Morris, A.; Naeem, M.A.; Thomas, T.; Keng, M.K. Clostridium difficile infection in acute myeloid leukemia patients. J. Clin. Oncol. 2017, 35, e18503. [Google Scholar] [CrossRef]
- Vehreschild, M.J.; Weitershagen, D.; Biehl, L.M.; Tacke, D.; Waldschmidt, D.; Töx, U.; Wisplinghoff, H.; Bergwelt-Baildon, M.V.; Cornely, O.A.; Vehreschild, J.J. Clostridium difficile infection in patients with acute myelogenous leukemia and in patients undergoing allogeneic stem cell transplantation: Epidemiology and risk factor analysis. Biol. Blood Marrow Transplant. 2014, 20, 823–828. [Google Scholar] [CrossRef]
- Shah, K.; Curtin, B.F.; Chu, C.; Hwang, D.; Flasar, M.H.; von Rosenvinge, E. Characteristics of Clostridium difficile infection in patients hospitalized with myelodysplastic syndrome or acute myelogenous leukemia. World J. Clin. Oncol. 2017, 8, 398–404. [Google Scholar] [CrossRef]
- Gorschlüter, M.; Marklein, G.; Höfling, K.; Clarenbach, R.; Baumgartner, S.; Hahn, C.; Ziske, C.; Mey, U.; Heller, R.; Eis-Hübinger, A.M.; et al. Abdominal infections in patients with acute leukaemia: A prospective study applying ultrasonography and microbiology. Br. J. Haematol. 2002, 117, 351–358. [Google Scholar] [CrossRef]
- Leung, S.; Metzger, B.S.; Currie, B.P. Incidence of Clostridium difficile infection in patients with acute leukemia and lymphoma after allogeneic hematopoietic stem cell transplantation. Infect. Control Hosp. Epidemiol. 2010, 31, 313–315. [Google Scholar] [CrossRef]
- Willems, L.; Porcher, R.; Lafaurie, M.; Casin, I.; Robin, M.; Xhaard, A.; Andreoli, A.L.; Rodriguez-Otero, P.; Dhedin, N.; Socié, G.; et al. Clostridium difficile infection after allogeneic hematopoietic stem cell transplantation: Incidence, risk factors, and outcome. Biol. Blood Marrow Transplant. 2012, 18, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.; Weinberg, A.; Rana, M.; Patel, G.; Huprikar, S. The epidemiology and clinical features of Clostridium difficile infection in liver transplant recipients. Transplantation 2016, 100, 1939–1943. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.D.; Treadway, S.B.; Hanna, D.B.; Huff, C.A.; Neofytos, D.; Carroll, K.C.; Marr, K.A. Epidemiology and outcomes of Clostridium difficile infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 2012, 54, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.; Zacharioudakis, I.M.; Zervou, F.N.; Ziakas, P.D.; Mylonakis, E. Prevalence of Clostridium difficile infection among solid organ transplant recipients: A meta-analysis of published studies. PLoS ONE 2015, 10, e0124483. [Google Scholar] [CrossRef] [PubMed]
- Tsapepas, D.S.; Martin, S.T.; Miao, J.; Shah, S.A.; Scheffert, J.; Fester, K.; Ma, K.; Lat, A.; Egan, R.; McKeen, J.T. Clostridium difficile infection, a descriptive analysis of solid organ transplant recipients at a single center. Diagn. Microbiol. Infect. Dis. 2015, 81, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H.B.; Mehta, V.; Girman, C.J.; Adhikari, D.; Johnson, M.L. Regression coefficient-based scoring system should be used to assign weights to the risk index. J. Clin. Epidemiol. 2016, 79, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.; Khan, D.; Anthony, D.; Paton, J. Waterlow score to predict patients at risk of developing Clostridium difficile-associated disease. J. Hosp. Infect. 2009, 71, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Castro, I.; Tasias, M.; Calabuig, E.; Salavert, M. Doctor, my patient has CDI and should continue to receive antibiotics. The (unresolved) risk of recurrent CDI. Rev. Esp. Quimioter. 2019, 32, 47–54. [Google Scholar]
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin. Infect. Dis. 2021, 73, e1029–e1044. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Khanafer, N.; Daneman, N.; Fisman, D.N. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob. Agents Chemother. 2013, 57, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Zacharioudakis, I.M.; Zervou, F.N.; Shehadeh, F.; Mylona, E.K.; Mylonakis, E. Association of community factors with hospital-onset clostridioides (Clostridium) difficile infection: A population based U.S.-wide study. EClinicalMedicine 2019, 8, 12–19. [Google Scholar] [CrossRef] [PubMed]
| Reason | No. |
|---|---|
| Age < 18 years | 210 |
| Incomplete data | 16 |
| No previous admission | 130 |
| No antibiotics were administered | 57 |
| Hospitalization < 48 h | 10 |
| Pregnant woman | 2 |
| Recurrent Clostridioides difficile infection | 2 |
| Not matched | 30 |
| Characteristics | Chi-Square Analysis | Conditional Logistic Regression | ||||||
|---|---|---|---|---|---|---|---|---|
| Controls n (%) 273 (75.0) | Cases n (%) 91 (25.0) | Total n (%) | p-Value | OR (95% CI) | p-Value | |||
| Age groups | <70 | 166 (60.8) | 43 (47.3) | 209 (57.3) | 0.024 | 1 (matched) | 1.000 | |
| ≥70 | 107 (39.2) | 48(52.8) | 155 (42.7) | |||||
| Age (years) mean ± SD | 61 ± 18.5 | 67.5 ± 19.6 | ||||||
| Age mean cases + control | 62.76 ± 18.5 (18.96) | |||||||
| Sex | Male | 132 (48.4) | 44 (48.4) | 176 (48.4) | 1.000 | 1 (matched) | 1.000 | |
| Female | 141 (51.6) | 47 (51.6) | 188 (51.6) | |||||
| Mean body mass index (kg/m2) | 26.50 ± 7.08 | 25.01 ± 7.4 | – | 0.087 | 0.97 (0.93–1.01) | 0.070 | ||
| Creatinine clearance | 62.2 ± 43.0 | 53.6 ± 37.5 | – | 0.088 | 0.992 (0.94–1.01) | 0.039 | ||
| Length of stay | Total (days) | 38.21 ± 61.15 (1–402) | 48.07 ± 69.9 (3–373) | – | 0.200 | 1.01 (0.94–1.01) | ||
| Before CDI test | 17.1 ± 32.6 | 18.9 ± 26.4 | – | 0.636 | 1.01 (0.99–1.01) | 0.636 | ||
| After CDI test | 21.4 ± 38.3 | 30.1 ± 59.9 | – | 0.107 | 1.01 (0.99–1.01) | 0.121 | ||
| Characteristics | Chi-Square Analysis | Conditional Logistic Regression | ||||
|---|---|---|---|---|---|---|
| Controls n (%) 273 (75.0) | Cases n (%) 91 (25.0) | Total n (%) | p-Value | OR (95% CI) | p-Value | |
| Diabetes mellitus | 144 (52.7) | 49 (53.8) | 193 (53.0) | 0.856 | 1.06 (0.62–1.79) | 0.839 |
| Hypertension | 155 (56.8) | 59 (64.8) | 214 (58.8) | 0.176 | 1.06 (0.88–2.78) | 0.125 |
| Dyslipidemia | 61 (22.4) | 19 (20.9) | 80 (22.0) | 0.758 | 0.9 (0.49–1.66) | 0.739 |
| Ulcerative colitis/Crohn disease | 10 (3.7) | 1 (1.1) | 11 (3.0) | 0.304 | 0.27 (0.03–2.22) | 0.223 |
| Chronic kidney disease | 67 (24.5) | 36 (39.6) | 103 (28.3) | 0.006 | 2.09 (1.24–3.51) | 0.006 |
| Liver disease | 29 (10.6) | 16 (17.6) | 45 (12.4) | 0.081 | 1.79 (0.922–3.5) | 0.085 |
| Solid organ transplantation | 11 (4.0) | 8 (8.8) | 19 (5.2) | 0.077 | 2.26 (0.89–15.77) | 0.088 |
| Gastrointestinal disease | 15 (5.5) | 7 (7.7) | 22 (6.0) | 0.446 | 1.42 (0.67–3.53) | 0.455 |
| Solid cancer or malignancy | 40 (14.7) | 18 (19.8) | 58 (15.9) | 0.247 | 1.42 (0.78–2.6) | 0.255 |
| Lymphoma or leukemia | 9 (3.3) | 7 (7.7) | 16 (4.4) | 0.076 | 2.45 (0.88–6.81) | 0.086 |
| Congestive heart disease | 83 (30.4) | 37 (40.7) | 120 (33.0) | 0.071 | 1.66 (0.98–2.82) | 0.059 |
| Chronic obstructive pulmonary disease | 7 (2.6) | 5 (5.5) | 12 (3.3) | 0.175 | 2.53 (0.7–9.11) | 0.156 |
| Nasogastric tube feeding | 17 (6.2) | 6 (6.6) | 23 (6.3) | 0.901 | 1.06(0.4–2.87) | 0.898 |
| Characteristics | Chi-Square Analysis | Conditional Logistic Regression | ||||
|---|---|---|---|---|---|---|
| Controls n (%) 273 (75.0) | Cases n (%) 91 (25.0) | Total n (%) | p-Value | OR (95% CI) | p-Value | |
| Gastrointestinal | 33 (12.1) | 9 (9.9) | 42 (11.5) | 0.570 | 0.79 (0.36–1.75) | 0.564 |
| Cardiovascular | 19 (7.0) | 8 (8.8) | 27 (7.4) | 0.564 | 0.127 (0.55–2.99) | 0.570 |
| Urology | 2 (0.7) | 1 (1.1) | 3 (0.8) | 1.000 | 1.73 (0.09–30.8) | 0.708 |
| General | 4 (1.5) | 2 (2.2) | 6 (1.6) | 0.642 | 1.5 (0.27–8.19) | 0.640 |
| Orthopedic | 9 (3.3) | 3 (3.3) | 12 (3.3) | 1.000 | 1 (0.261–3.84) | 1 |
| Total no. | 67 | 23 | 90 | – | – | – |
| Characteristics | Chi-Square Analysis | Conditional Logistic Regression | ||||
|---|---|---|---|---|---|---|
| Controls n (%) 273 (75.0) | Cases n (%) 91 (25.0) | Total n (%) | p-Value | OR (95% CI) | p-Value | |
| Proton pump inhibitors | 249 (91.2) | 85 (93.4) | 334 (91.8) | 0.509 | 1.27 (0.53–3.09) | 0.593 |
| Ranitidine | 68 (24.9) | 18 (19.8) | 86 (23.6) | 0.319 | 0.74 (0.41–1.34) | 0.321 |
| Statin | 125 (45.8) | 43 (47.3) | 168 (46.2) | 0.808 | 1.07 (0.64–1.8) | 0.792 |
| Immune suppressant | 70 (25.7) | 27 (29.7) | 97 (26.7) | 0.463 | 1.22 (0.72–2.08) | 0.449 |
| Predictor Variables | Model Parameters | p-Value | |
|---|---|---|---|
| Beta | OR (95% CI) | ||
| Age ≥ 70 years | 0.6446045 | 1.90 (1.04–3.45) | 0.034 |
| Body mass index | −0.027 | 0.97 (0.94–1.01) | |
| Creatinine clearance | 0.0047384 | 1.01 (1.00–1.04) | 0.323 |
| Total length of stay total | −0.0018063 | 1.00 (0.99–1.01) | 0.672 |
| Length of stay after CDI test | 0.0073645 | 1.01 (0.99–1.02) | 0.214 |
| Hypertension | 0.271428 | 1.31 (0.70–2.50) | 0.402 |
| Chronic kidney disease | 0.7432566 | 2.10 (1.02–4.31) | 0.043 |
| Liver disease | 0.4167027 | 1.51 (0.72–3.21) | 0.276 |
| Solid organ transplantation | 1.251179 | 3.49 (1.20–10.12) | 0.021 |
| Lymphoma or leukemia | 1.316815 | 3.73 (1.24–11.22) | 0.019 |
| Congestive heart disease | 0.1407125 | 1.15 (0.62–2.12) | 0.653 |
| Chronic obstructive pulmonary disease | 0.4731865 | 1.605 (0.472–5.45) | 0.449 |
| Predictor Variables | Model Parameters | |||
|---|---|---|---|---|
| Beta | OR (95% CI) | No. of Points | Score | |
| Age ≥ 70 years | 0.6446045 | 1.9 (1.04–3.4) | 1.9 | 2 |
| Chronic kidney disease | 0.7432566 | 2.1 (1.02–4.3) | 2.3 | 2 |
| Solid organ transplantation | 1.251179 | 3.5 (1.20–10.1) | 3.8 | 4 |
| Lymphoma or leukemia | 1.316815 | 3.7 (1.24–11.2) | 4.1 | 4 |
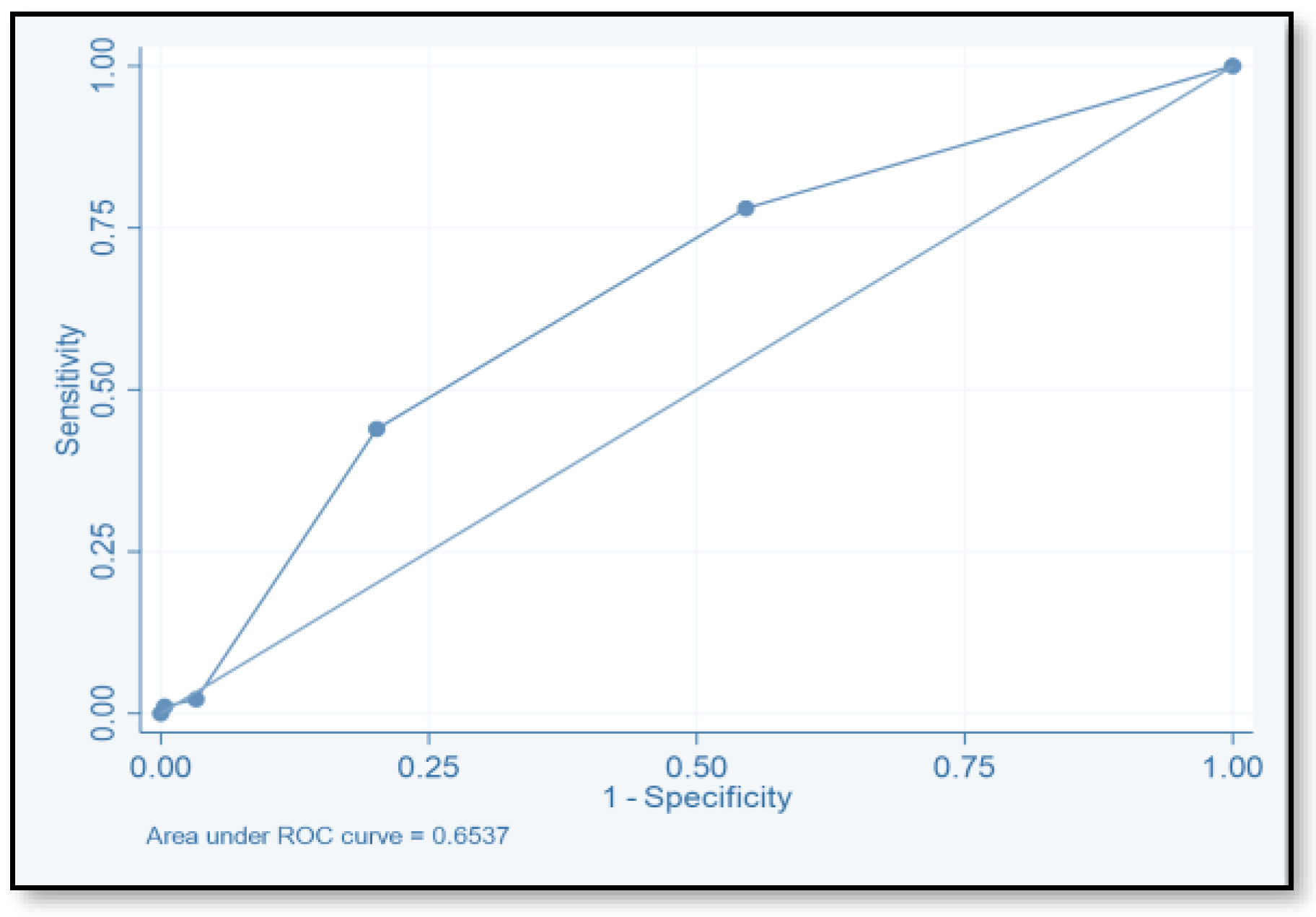
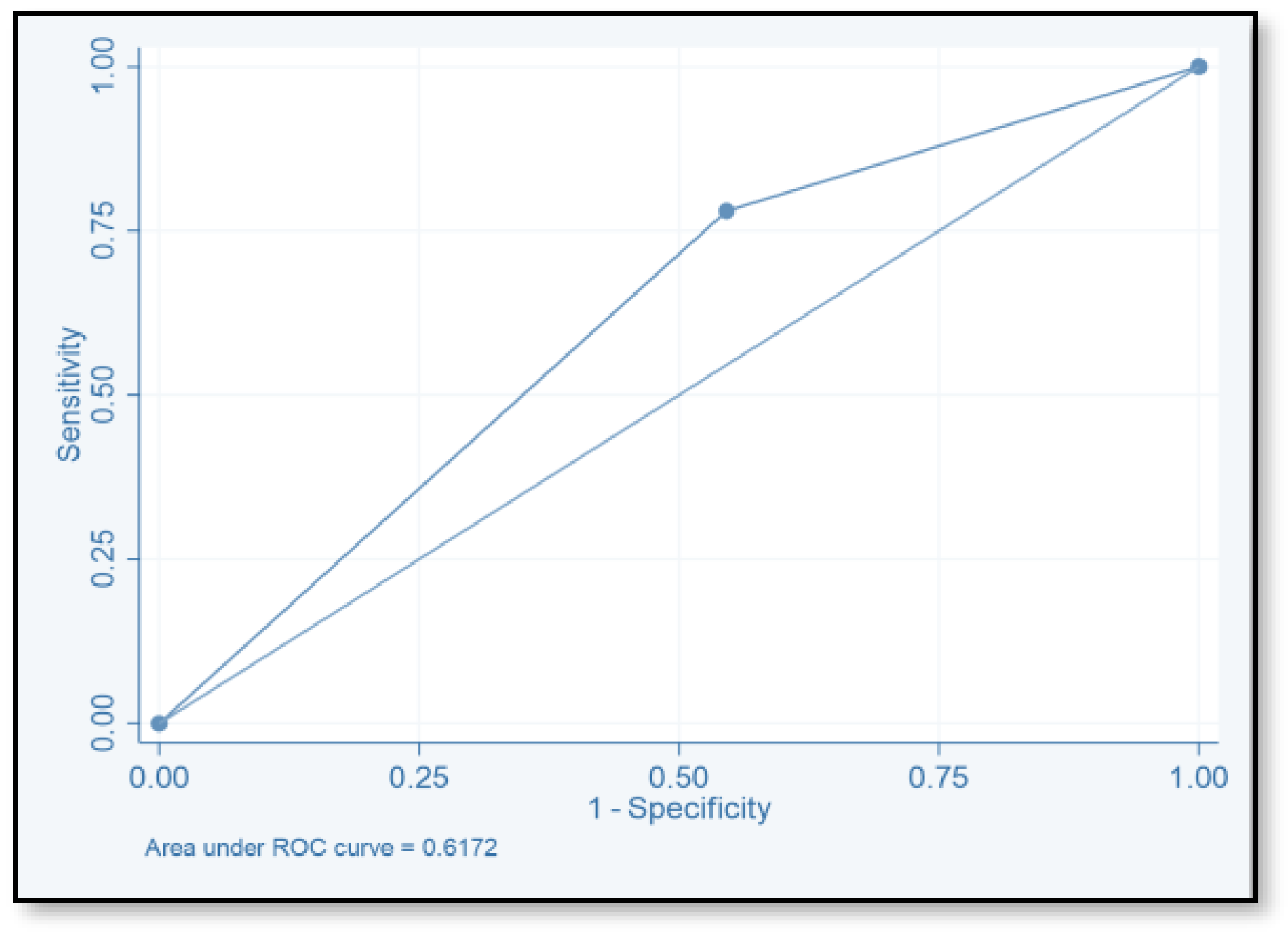
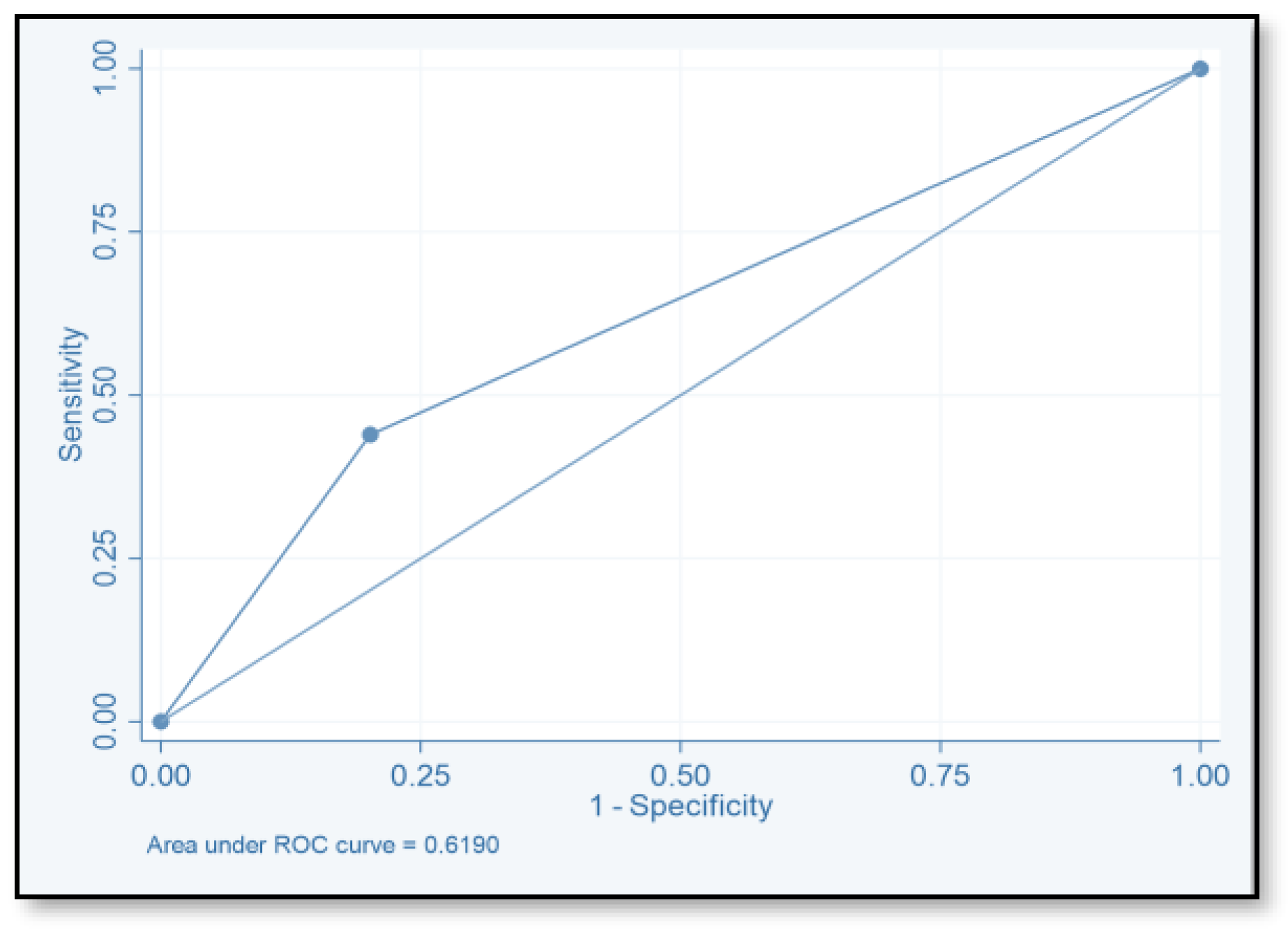
| Score | TP | FP | TN | FN | SE (%) | SP (%) | PPV | NPV | ACC |
|---|---|---|---|---|---|---|---|---|---|
| ≥2 | 71 | 149 | 124 | 20 | 78.02 | 45.4 | 32.3 | 86.1 | 78.02 |
| ≥4 | 40 | 55 | 518 | 51 | 44 | 79.9 | 42.1 | 81.04 | 44 |
| ≥6 | 2 | 264 | 9 | 89 | 2.2 | 96.7 | 18.2 | 0.7 | 2.2 |
| ≥8 | 1 | 1 | 272 | 90 | 1.1 | 99.6 | 50 | 75.1 | 1.1 |
| ≥10 | 0 | 0 | 273 | 91 | 1.1 | 99.6 | |||
| ≥12 | 0 | 0 | 273 | 91 | 1.1 | 99.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamri, A.; Bin Abbas, A.; Al Hassan, E.; Almogbel, Y. Development of a Prediction Model to Identify the Risk of Clostridioides difficile Infection in Hospitalized Patients Receiving at Least One Dose of Antibiotics. Pharmacy 2024, 12, 37. https://doi.org/10.3390/pharmacy12010037
Alamri A, Bin Abbas A, Al Hassan E, Almogbel Y. Development of a Prediction Model to Identify the Risk of Clostridioides difficile Infection in Hospitalized Patients Receiving at Least One Dose of Antibiotics. Pharmacy. 2024; 12(1):37. https://doi.org/10.3390/pharmacy12010037
Chicago/Turabian StyleAlamri, Abdulrahman, AlHanoof Bin Abbas, Ekram Al Hassan, and Yasser Almogbel. 2024. "Development of a Prediction Model to Identify the Risk of Clostridioides difficile Infection in Hospitalized Patients Receiving at Least One Dose of Antibiotics" Pharmacy 12, no. 1: 37. https://doi.org/10.3390/pharmacy12010037






