Barriers and Facilitators of Partner Treatment of Chlamydia: A Qualitative Investigation with Prescribers and Community Pharmacists
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Design
2.3. Interview Design
2.4. Pilot
2.5. Participant Recruitment
2.6. Inclusion Criteria
- Prescribers
- Were registered and practicing as either a GP, sexual health physician, or NP in the Perth metropolitan region;
- Had treated at least one index patient diagnosed with chlamydia in the past three months; and
- Were able to speak and read English
- Pharmacists
- Were registered as a pharmacist in the Perth metropolitan region;
- Worked a minimum of 20 h per week in a community pharmacy; and
- Were able to speak and read English
2.7. Exclusion Criteria
- Prescribers
- Worked in a clinic exclusively treating groups at high risk of complicated chlamydial infections and/or other concurrent STIs (e.g., men who have sex with men or Aboriginal and Torres Strait Islander people)
- Pharmacists
- Exclusively worked in a hospital pharmacy
2.8. Data Collection
- Audio recordings (audiotaped on two separate devices)
- Researcher field notes
- Participant demographics form
2.9. Data Entry and Analysis
- Immersion in data: reading and re-reading transcripts.
- Developing a first impression: highlighting key words and phrases that captured major thoughts.
- Developing a coding scheme: labelling similar codes that accurately depict the data. A code was a raw data point that was grouped with similar data points to form a theme.
- Identification of themes: establishing themes and super-themes based on relationships between categories. Theming was done using pen and paper, and similar barriers and facilitators were grouped together. A theme name was created for each group.
- Cross-checking between researchers: checking raw data and ensuring accurate representations of results. Where discrepancies existed, the raw data were examined to view responses in context and a consensus was reached.
2.10. Definitions
3. Results
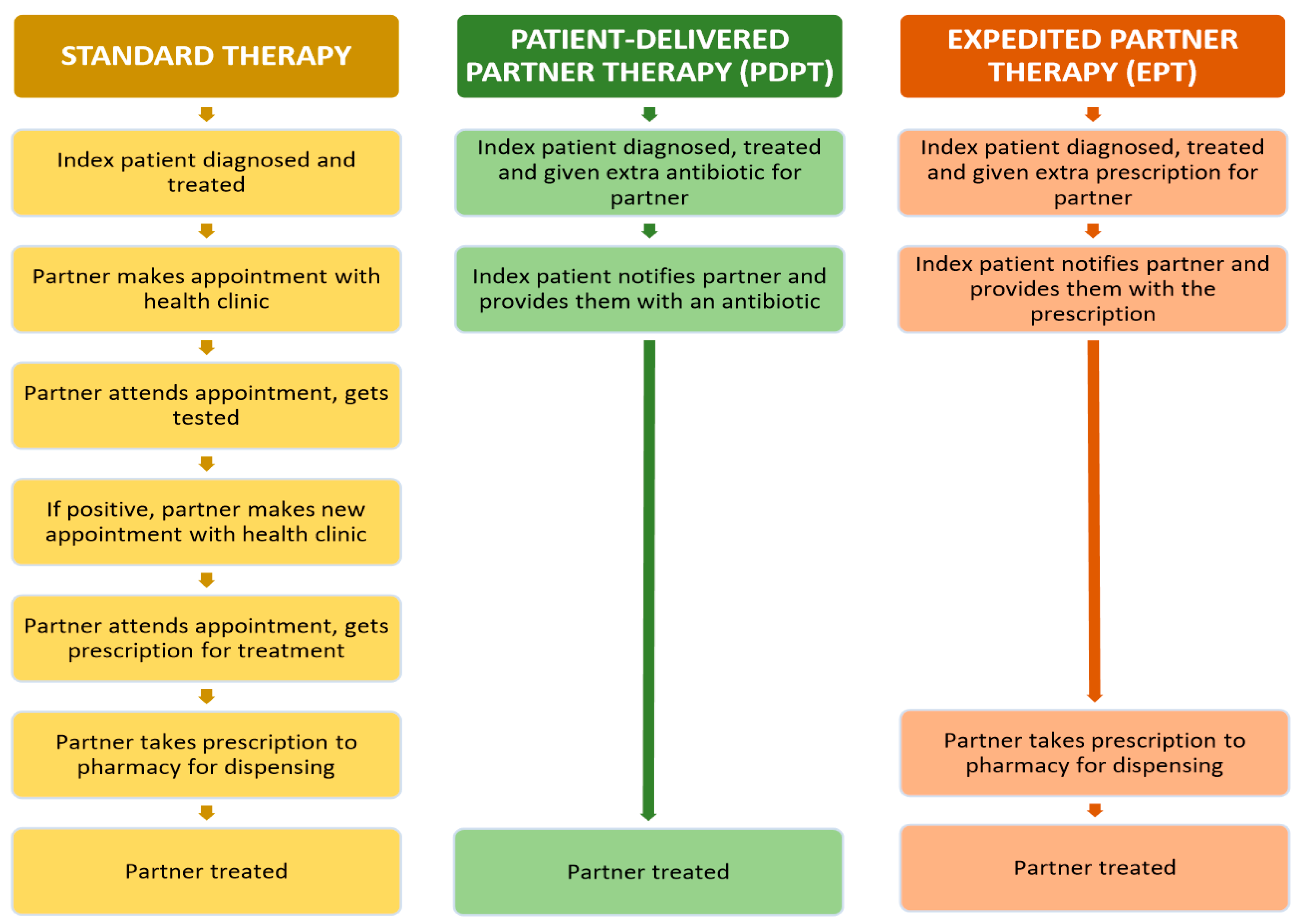
3.1. Standard Partner Therapy
“I’d say probably 90% or more [of partners] agree to just have the treatment that [testing] day, because it saves them coming back for a second visit”—Prescriber 2
“We’re not legally supposed to provide a script for azithromycin for the partner…or give the patient a dose of azithromycin to take home for their partner…but occasionally we do do that here”—Prescriber 6
“Usually we would offer the partner treatment, regardless of whether their test is positive or negative, because we work on giving people empirical treatment”—Prescriber 8
3.2. Accelerated Partner Therapy
3.3. Pharmacists’ Role and Pharmacy Services
“Having a pharmacist dispense the morning after pill is no different from them dispensing azithromycin as a stat dose for somebody with suspected chlamydia”—Prescriber 5
“Giving information about the disease state or how it is spread…encourage people to have check-ups with their doctor…We can also talk to them [about] how to take medication…following up on partners.”—Pharmacist 2
“To help provide medications, to counsel patients…I think preventive health as well, so like public health and public awareness”—Pharmacist 4
“It’s benefit and risk at the end of it…If you use [azithromycin] and you don’t actually have [chlamydia], you wouldn’t really have any significant risk.”—Pharmacist 6
4. Discussion
4.1. Standard Partner Therapy
4.2. Accelerated Partner Therapy (APT)
4.2.1. Prescriber Issues
4.2.2. Pharmacy Issues
4.2.3. Medication Issues
4.2.4. Process Issues
4.3. Role of the Pharmacist
4.4. Strengths and Limitations
4.5. Future Direction
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Australian Government Department of Health. National Notifiable Diseases Surveillance Systems. Available online: http://www9.health.gov.au/cda/source/rpt_2.cfm (accessed on 11 May 2018).
- Lallemand, A.; Bremer, V.; Jansen, K.; Nielsen, S.; Münstermann, D.; Lucht, A.; Tiemann, C. Prevalence of chlamydia trachomatis infection in women, heterosexual men and MSM visiting HIV counselling institutions in North Rhine-Westphalia, Germany-should chlamydia testing be scaled up? BMC Infect. Dis. 2016, 16, 610. [Google Scholar] [CrossRef] [PubMed]
- Akande, V.; Turner, C.; Horner, P.; Horne, A.; Pacey, A.; Society, B.F. Impact of chlamydia trachomatis in the reproductive setting: British fertility society guidelines for practice. Hum. Fertil. 2010, 13, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, J.A.; Kissinger, P.; Calvet, H.; Whittington, W.L.; Ransom, R.L.; Sternberg, M.R.; Berman, S.M.; Kent, C.K.; Martin, D.H.; Oh, M.K. Patient-delivered partner treatment with azithromycin to prevent repeated chlamydia trachomatis infection among women: A randomized, controlled trial. Sex. Transm. Dis. 2003, 30, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, C.L.; Gottlieb, S.L.; Taylor, B.D.; Low, N.; Xu, F.; Ness, R.B. Risk of sequelae after chlamydia trachomatis genital infection in women. J. Infect. Dis. 2010, 201, S134–S155. [Google Scholar] [CrossRef] [PubMed]
- Batteiger, B.E.; Tu, W.; Ofner, S.; Van Der Pol, B.; Stothard, D.R.; Orr, D.P.; Katz, B.P.; Fortenberry, J.D. Repeated chlamydia trachomatis genital infections in adolescent women. J. Infect. Dis. 2010, 201, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Shain, R.N.; Perdue, S.T.; Piper, J.M.; Holden, A.E.; Champion, J.D.; Newton, E.R.; Korte, J.E. Behaviors changed by intervention are associated with reduced STD recurrence: The importance of context in measurement. Sex. Transm. Dis. 2002, 29, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Pavlin, N.L.; Parker, R.; Fairley, C.K.; Gunn, J.M.; Hocking, J. Take the sex out of STI screening! Views of young women on implementing chlamydia screening in general practice. BMC Infect. Dis. 2008, 8, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Walker, S.; Fairley, C.K.; Bilardi, J.; Chen, M.Y.; Bradshaw, C.S.; Urban, E.; Pirotta, M.; Birden, H.; Donovan, B. What do young women think about having a chlamydia test? Views of women who tested positive compared with women who tested negative. Sex. Health 2013, 10, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.A.; Jaccard, J.; Millstein, S.G.; Viadro, C.I.; Eaton, J.L.; Miller, W.C. Young adults’ attitudes, beliefs, and feelings about testing for curable STDs outside of clinic settings. J. Adolesc. Health 2004, 34, 266–269. [Google Scholar] [CrossRef]
- Tilson, E.C.; Sanchez, V.; Ford, C.L.; Smurzynski, M.; Leone, P.A.; Fox, K.K.; Irwin, K.; Miller, W.C. Barriers to asymptomatic screening and other STD services for adolescents and young adults: Focus group discussions. BMC Public Health 2004, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, S.B.; Smith, M.C.; Lawton, B.A. “If everyone does it, it’s not a big deal.” Young people talk about chlamydia testing. N. Z. Med. J. 2008, 121, 33–42. [Google Scholar] [PubMed]
- Hogan, A.H.; Howell-Jones, R.S.; Pottinger, E.; Wallace, L.M.; McNulty, C.A. “… They should be offering it”: A qualitative study to investigate young peoples’ attitudes towards chlamydia screening in GP surgeries. BMC Public Health 2010, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Balfe, M.; Brugha, R.; O’Connell, E.; McGee, H.; O’Donovan, D.; Vaughan, D. Why don’t young women go for chlamydia testing? A qualitative study employing Goffman’s stigma framework. Health Risk Soc. 2010, 12, 131–148. [Google Scholar] [CrossRef]
- Chacko, M.R.; von Sternberg, K.; Velasquez, M.M.; Wiemann, C.M.; Smith, P.B.; DiClemente, R. Young women’s perspective of the pros and cons to seeking screening for chlamydia and gonorrhea: An exploratory study. J. Pediatr. Adolesc. Gynecol. 2008, 21, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Estcourt, C.; Sutcliffe, L.; Cassell, J.; Mercer, C.H.; Copas, A.; James, L.; Low, N.; Horner, P.; Clarke, M.; Symonds, M. Can we improve partner notification rates through expedited partner therapy in the UK? Findings from an exploratory trial of Accelerated Partner Therapy (APT). Sex. Transm. Infect. 2012, 88, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Department of Health (Northern Territory). Patient Delivered Partner Therapy Guidelines. Available online: http://www.health.nt.gov.au/library/scripts/objectifyMedia.aspx?file=pdf/94/33.pdf&siteID=1&str_title=Patient%20Delivered%20Partner%20Therapy%20Guidelines.pdf (accessed on 11 April 2016).
- Kissinger, P.; Brown, R.; Reed, K.; Salifou, J.; Drake, A.; Farley, T.A.; Martin, D.H. Effectiveness of patient delivered partner medication for preventing recurrent chlamydia trachomatis. Sex. Transm. Infect. 1998, 74, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S. Patient-delivered partner therapy for chlamydia—A realistic public health measure in the UK. BJOG 2009, 116, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.; Glasier, A.; Muir, A.; Scott, G.; Johnstone, A.; Quarrell, H.; Oroz, C.; McIntyre, M.; Miranda, D.; Todd, G. Expedited partner therapy for chlamydia trachomatis at the community pharmacy. BJOG 2010, 117, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Kerani, R.P.; Fleming, M.; Golden, M.R. Acceptability and intention to seek medical care after hypothetical receipt of patient-delivered partner therapy or electronic partner notification postcards among men who have sex with men: The partner’s perspective. Sex. Transm. Dis. 2013, 40, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Shivasankar, S.; Challenor, R. Patient-delivered partner therapy in the UK: What do the professionals think? Int. J. STD AIDS 2008, 19, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Victorian State Government. Patient Delivered Partner Therapy Clinical Guidelines. Available online: https://www2.health.vic.gov.au/about/publications/policiesandguidelines/pdpt-clinical-guidelines (accessed on 11 April 2016).
- Healthdirect. Australia’s Healthcare System. Available online: https://www.healthdirect.gov.au/australias-healthcare-system (accessed on 21 November 2017).
- InterNations. Moving to Perth. Available online: https://www.internations.org/perth-expats/guide/moving-to-perth-15654 (accessed on 1 October 2017).
- Wakerman, J.; Humphreys, J.S.; Wells, R.; Kuipers, P.; Entwistle, P.; Jones, J. Primary health care delivery models in rural and remote Australia—A systematic review. BMC Health Serv. Res. 2008, 8, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, D.R. A general inductive approach for analyzing qualitative evaluation data. Am. J. Eval. 2006, 27, 237–246. [Google Scholar] [CrossRef]
- Ritchie, J.; Spencer, L. Qualitative data analysis for applied policy research. In Analyzing Qualitative Data; Bryman, A., Burgess, R.G., Eds.; Routledge: London, UK, 1994; pp. 173–194. [Google Scholar]
- McNulty, C.A.; Freeman, E.; Bowen, J.; Shefras, J.; Fenton, K.A. Barriers to opportunistic chlamydia testing in primary care. Br. J. Gen. Pract. 2004, 54, 508–514. [Google Scholar] [PubMed]
- Lorch, R.; Hocking, J.; Temple-Smith, M.; Law, M.; Yeung, A.; Wood, A.; Vaisey, A.; Donovan, B.; Fairley, C.K.; Kaldor, J. The chlamydia knowledge, awareness and testing practices of Australian general practitioners and practice nurses: Survey findings from the Australian Chlamydia Control Effectiveness Pilot (ACCEPt). BMC Fam. Pract. 2013, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Lorch, R.; Hocking, J.; Guy, R.; Vaisey, A.; Wood, A.; Lewis, D.; Temple-Smith, M. Practice nurse chlamydia testing in australian general practice: A qualitative study of benefits, barriers and facilitators. BMC Fam. Pract. 2015, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Pavlin, N.L.; Parker, R.M.; Piggin, A.K.; Hopkins, C.A.; Temple-Smith, M.J.; Fairley, C.K.; Tomnay, J.E.; Bowden, F.J.; Russell, D.B.; Hocking, J.S. Better than nothing? Patient-delivered partner therapy and partner notification for chlamydia: The views of Australian general practitioners. BMC Infect. Dis. 2010, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Hocking, J.S.; Parker, R.M.; Pavlin, N.; Fairley, C.K.; Gunn, J.M. What needs to change to increase chlamydia screening in general practice in Australia? The views of general practitioners. BMC Public Health 2008, 8, 425. [Google Scholar] [CrossRef] [PubMed]
- Coyne, K.M.; Cohen, C.; Smith, N.; Mandalia, S.; Barton, S. Patient-delivered partner medication in the UK: An unlawful but popular choice. Int. J. STD AIDS 2007, 18, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Introcaso, C.E.; Rogers, M.E.; Abbott, S.A.; Gorwitz, R.J.; Markowitz, L.E.; Schillinger, J.A. Expedited partner therapy in federally qualified health centers—New York City, 2012. Sex. Transm. Dis. 2013, 40, 881–885. [Google Scholar] [CrossRef] [PubMed]
- McNutt, L.-A.; Davis, C.F.; Bednarczyk, R.A.; Fischer, A.; Zeolla, M.; Coles, B.F. Alternative approaches to partner notification, diagnosis, and treatment: Pharmacists’ perspectives on proposed patient delivered partner therapy in New York State, 2007. Sex. Transm. Dis. 2009, 36, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Mooney-Somers, J.; Micallef, J.; Bateson, D.; Harvey, C.; Van Gemert, C.; Lewis, L.; Kaldor, J.; Guy, R. Patient Delivered Partner Therapy for Chlamydia: Support and Concern among Doctors and Nurses Working in Australian Family Planning Clinics. In Proceedings of the Sexual Health Conference, Darwin, Australia, 21–23 October 2013; Available online: https://ses.library.usyd.edu.au/handle/2123/11976 (accessed on 16 June 2016).
- Temkin, E.; Klassen, A.C.; Mmari, K.; Gillespie, D.G. A qualitative study of patients’ use of expedited partner therapy. Sex. Transm. Dis. 2011, 38, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Queddeng, K.; Chaar, B.; Williams, K. Emergency contraception in Australian community pharmacies: A simulated patient study. Contraception 2011, 83, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Higgins, S.J.; Hattingh, H.L. Requests for emergency contraception in community pharmacy: An evaluation of services provided to mystery patients. Res. Soc. Adm. Pharm. 2013, 9, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Hussainy, S.Y.; Stewart, K.; Chapman, C.B.; Taft, A.J.; Amir, L.H.; Hobbs, M.K.; Shelley, J.M.; Smith, A.M. Provision of the emergency contraceptive pill without prescription: Attitudes and practices of pharmacists in Australia. Contraception 2011, 83, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Golden, M.R.; Whittington, W.L.; Handsfield, H.H.; Hughes, J.P.; Stamm, W.E.; Hogben, M.; Clark, A.; Malinski, C.; Helmers, J.R.; Thomas, K.K. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N. Engl. J. Med. 2005, 352, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.; Glasier, A.; Scott, G.; Young, H.; Melvin, L.; Johnstone, A.; Elton, R. Novel interventions to reduce re-infection in women with chlamydia: A randomized controlled trial. Hum. Reprod. 2009, 24, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Fortenberry, J.D.; Juliar, B.E.; Tu, W.; Orr, D.P.; Batteiger, B.E. The prevalence of chlamydia, gonorrhea, and trichomonas in sexual partnerships: Implications for partner notification and treatment. Sex. Transm. Dis. 2005, 32, 260. [Google Scholar] [CrossRef] [PubMed]
- Bixby Center for Global Reproductive Health. Does Emergency Contraception Promote Sexual Risk-Taking? Available online: https://bixbycenter.ucsf.edu/sites/bixbycenter.ucsf.edu/files/DoesECPromoteSexRiskTaking_2008.pdf (accessed on 12 October 2017).
- Taylor, K.L.; Clifford, R.M.; Marshall, L. Acceptance of a chlamydia screening program in community pharmacies. J. Pharm. Pract. Res. 2007, 37, 287–291. [Google Scholar] [CrossRef]
- Gudka, S.; Marshall, L.; Creagh, A.; Clifford, R.M. To develop and measure the effectiveness and acceptability of a pharmacy-based chlamydia screening intervention in Australia. BMJ Open 2013, 3, e003338. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.; Melvin, L.; Glasier, A.; Scott, G.; Johnstone, A.; Young, H. Willingness of gynaecologists, doctors in family planning, GPs, practice nurses and pharmacists to adopt novel interventions for treating sexual partners of women with chlamydia. BJOG 2007, 114, 1516–1521. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, E.A.; Marx, J.; Terry, M.A.; Stall, R.; Flatt, J.; Borrero, S.; Miller, E. Perspectives on expedited partner therapy for chlamydia: A survey of health care providers. Int. J. STD AIDS 2016, 27, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, E.A.; Marx, J.; Terry, M.A.; Stall, R.; Pallatino, C.; Miller, E. Healthcare providers’ perspectives on expedited partner therapy for chlamydia: A qualitative study. Sex. Transm. Infect. 2015, 91, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, T.; Sutcliffe, L.; Estcourt, C. Is accelerated partner therapy partner notification for sexually transmissible infections acceptable and feasible in general practice? Sex. Health 2011, 8, 17–22. [Google Scholar] [CrossRef] [PubMed]
| Prescribers (n = 11) | Pharmacists (n = 12) | |
|---|---|---|
| Median age in years (range) | 45 (31–69) | 30.5 (27–52) |
| Median years of experience in current role (range) | 14 (1–30) | 8 (5–18) |
| Gender F (%) | 9 (82%) | 8 (67%) |
| Job title * (n) | ||
| Community pharmacist | N/A | 12 |
| General practitioner | 4 | N/A |
| Medical director | 1 | N/A |
| Medical officer | 1 | N/A |
| Nurse practitioner | 2 | N/A |
| Public health physician | 2 | N/A |
| Sexual health physician | 5 | N/A |
| Median hours worked per week (range) | (not measured) | 38 (20–60) |
| Standard Partner Therapy | Key Illustrative Quote | |
|---|---|---|
| Barriers | Sexual partner does not seek testing | “For casual contacts I think we probably don’t have a very good hit rate because it’s hard for them to get partners in…and it is time consuming for the contacts to come and get tested.”—Prescriber 1 “But, you know, we don’t live in an ideal world, and often people will not present for screening or for testing”—Prescriber 9 |
| Delay to testing | “There may be a delay, so [the index patient] may get re-infected. There may be a delay in getting appointments, they might not understand the importance of it.”—Prescriber 7 | |
| Facilitators | Able to test for other sites of infection | “There’s advantages of [the sexual partner] coming in to get tested because you can erase a dramatic infection—for example, rectal infection and that is treated differently”—Prescriber 1 |
| Able to assess sexual partner | “We’re able to see them and get a history and assess for any risks outside of chlamydia. See what their medication tolerance and compliance would be.”—Prescriber 3 | |
| Able to provide education | “We’ll educate them on what the treatment is, what the treatment options are.”—Prescriber 4 | |
| Prescriber Issues | Key Illustrative Quote | |
|---|---|---|
| Barriers | Legal professional responsibility | “It’s a safe drug but it’s my name on the box”—Prescriber 7 |
Prescribing for an unseen partner
| “My biggest worry is that if we don’t [test and educate partner] then we’re not actually giving people information that they might need about protecting themselves”—Prescriber 6 | |
| Lack of remuneration for service | “The reality is unfortunately that unless there’s a Medicare billing item attached to it, it can be a barrier for [prescribers] to do it.”—Prescriber 10 | |
| Suggested Facilitators | Discreet prescription annotation to indicate need for extended consultation | “There could be some sort of standard note that we attach [to the prescription] to say that this person hasn’t been seen, could you screen for allergies … I’d feel very comfortable doing that”—Prescriber 6 “If [the prescription] is annotated in quite a subtle way, but quite clear to the health professional that would be adequate. So it’s not obvious to anyone”—Pharmacist 12 |
| Pharmacist training | “It is a field that pharmacists can be educated on so it’s not something that we just can’t do”—Pharmacist 8 | |
| Medicare subsidisation | “To have some sort of Medicare billable item would be good because a lot of [prescriber] work goes unbilled”—Prescriber 10 | |
| Pharmacy Issues | ||
| Barriers | Lack of pharmacy staff | “The consult room is often there but it’s hard to use…you often don’t have time to do that if you’re the only pharmacist working”—Pharmacist 3 |
| Lack of privacy in pharmacy | “[Pharmacists] ask at the top of their voice what you’re here for, have you taken this before, which is all part of their job but then sometimes it’s quite personal information that they ask”—Prescriber 6 | |
| Suggested Facilitators | Offer of a confidential consultation | “[In the consultation room] we often spend much more than five minutes with clients who are interested in knowing more about their medications, knowing more of the treatment options”—Pharmacist 8 |
| Financial remuneration | “If it’s more of an extended consultation I think that could possibly come under Medicare payment for a service if it was something that when you sat down, you had to explain what chlamydia is, what the treatment is about … I think it’s fair to offer remuneration for that”—Pharmacist 2 | |
| Medication Issues | ||
| Barriers | Potential for adverse drug reactions | “There is the safety aspect obviously because you don’t know what that person’s medical history is, you don’t know what allergies they have, what other medication they’re on … you don’t want to influence somebody in another way without even knowing them”—Prescriber 5 |
| Potential for antibiotic resistance | “[The] concerns are mainly [that] you don’t actually know if the partner actually has the STD, so resistance comes to mind”—Pharmacist 3 | |
| Suggested Facilitators | Prescriber-led telephone consultation with partner prior to writing prescription | “It might be worthwhile if we could just get the partner on the telephone and just take a general history”—Prescriber 6 |
| Process Issues | ||
| Barriers | Too accessible | “If they can access treatment a lot easier, they’re going to be a lot more reckless with their behaviour”—Prescriber 5 “… And they think, it’s easy to treat so it’s not as important to prevent”—Pharmacist 4 |
| Inability for further testing and follow up care | “You don’t know that [partners] have had an opportunity to…get tested for other STIs, because if you’ve got one, you’re more likely going to have another”—Prescriber 7 | |
| Suggested Facilitators | Provision of chlamydia self-test kits | “You could have testing kits in the pharmacy that [partners] could potentially pick up … when they’ve picked up their azithromycin”—Prescriber 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wood, H.; Hall, C.; Ioppolo, E.; Ioppolo, R.; Scacchia, E.; Clifford, R.; Gudka, S. Barriers and Facilitators of Partner Treatment of Chlamydia: A Qualitative Investigation with Prescribers and Community Pharmacists. Pharmacy 2018, 6, 17. https://doi.org/10.3390/pharmacy6010017
Wood H, Hall C, Ioppolo E, Ioppolo R, Scacchia E, Clifford R, Gudka S. Barriers and Facilitators of Partner Treatment of Chlamydia: A Qualitative Investigation with Prescribers and Community Pharmacists. Pharmacy. 2018; 6(1):17. https://doi.org/10.3390/pharmacy6010017
Chicago/Turabian StyleWood, Helen, Caroline Hall, Emma Ioppolo, Renée Ioppolo, Ella Scacchia, Rhonda Clifford, and Sajni Gudka. 2018. "Barriers and Facilitators of Partner Treatment of Chlamydia: A Qualitative Investigation with Prescribers and Community Pharmacists" Pharmacy 6, no. 1: 17. https://doi.org/10.3390/pharmacy6010017




