Exploring the Knowledge and Perception of Generic Medicines among Final Year Undergraduate Medical, Pharmacy, and Nursing Students in Sierra Leone: A Comparative Cross-Sectional Approach
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Respondent Demographics
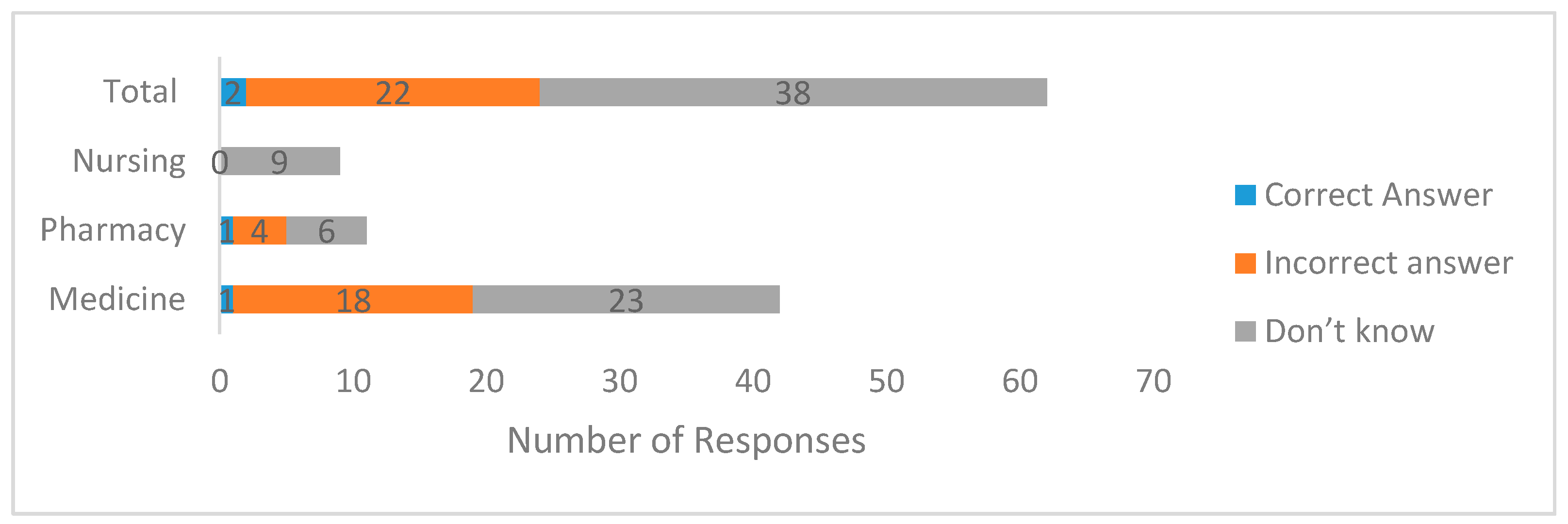
3.2. Knowledge of Regulatory Bioequivalence Limit among Final Year Undergraduate Medicine, Pharmacy, and Nursing Student
3.3. Knowledge and Perceptions about Generic Equivalent According to Course of Study
3.4. Knowledge and Perceptions of the Quality, Safety, and Efficacy of Generic Medicines versus Brand Name Drug According to Course of Study
3.5. The Perceptions of Students about Generic Medicines According to Course of Study
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PBSL | Pharmacy Board of Sierra Leone |
| COMAHS-USL | College of Medicine and Allied Health Sciences, University of Sierra Leone |
References
- Lu, Y.; Hernandez, P.; Abegunde, D.; Edejer, T. The World Medicines Situation 2011. Medicine Expenditures; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Hogerzeil, H.V.; Mirza, Z. The World Medicines Situation 2011: Access to Essential Medicines as Part of the Right to Health; World Health Organization: Geneva, Switzerland, 2011; pp. 680–689. [Google Scholar]
- Cameron, A.; Mantel-Teeuwisse, A.K.; Leufkens, H.G.M.; Laing, R.O. Switching from originator brand medicines to generic equivalents in selected developing countries: How much could be saved? Value Health 2012, 15, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Sanitation Sierra Leone. National Medicine Policy Ministry of Health and Sanitation Freetown; Ministry of Health and Sanitation Sierra Leone: Freetown, Sierra Leone, 2012.
- WHO. Global Health Expenditure Database; World Health Organisation: Geneva, Switzerland, 2014. [Google Scholar]
- Witter, S.; Brikci, N.; Harris, T.; Williams, R.; Keen, S.; Mujica, A.; Jones, A.; Murray-Zmijewski, A.; Bale, B.; Leigh, B. The Sierra Leone Free Health Care Initiative (FHCI): Process and Effectiveness Review. 2016. Available online: http://eresearch.qmu.ac.uk/4358/1/eResearch%204358.pdf (accessed on 24 August 2017).
- Government of Sierra Leone. National Essential Medicine List; Ministry of Health and Sanitation Freetown: Freetown, Sierra Leone, 2012.
- Cole, C.P.; James, P.B.; Kargbo, A.T. An evaluation of the prescribing patterns for under-five patients at a tertiary paediatric hospital in Sierra Leone. J Basic Clin. Pharm. 2015, 6, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Chua, G.N.; Hassali, M.A.; Shafie, A.A.; Awaisu, A. A survey exploring knowledge and perceptions of general practitioners towards the use of generic medicines in the northern state of Malaysia. Health Policy 2010, 95, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Hassali, M.A.; Kong, D.; Stewart, K. A comparison between senior medical students’ and pharmacy pre-registrants’ knowledge and perceptions of generic medicines. Med. Educ. 2007, 41, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Auta, A.; Bala, E.T.; Shalkur, D. Generic medicine substitution: A cross-sectional survey of the perception of pharmacists in North-Central, Nigeria. Med. Princ. Pract. 2014, 23, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Hassali, M.A.; Shafie, A.A.; Awaisu, A.; Ibrahim, M.I.; Ping, C.C.; Jamshed, S. Physicians’ views on generic medicines: A narrative review. J. Generic Med. 2010, 7, 30–39. [Google Scholar] [CrossRef]
- Siam, M.K.S.; Khan, A.; Khan, T.M. Medical and pharmacy students’ knowledge and perceptions about generic medicines in Bangladesh. J. Pharm. Health Serv. Res. 2013, 4, 57–61. [Google Scholar] [CrossRef]
- Patel, A.; Gauld, R.; Norris, P.; Rades, T. Quality of generic medicines in South Africa: Perceptions versus reality—A qualitative study. BMC Health Serv. Res. 2012, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- University of Sierra Leone. College of Medicine and Allied Health Sciences. Available online: http://www.usl.edu.sl/?page_id=786 (accessed on 17 May 2017).
- College of Medicine and Allied Health Sciences University of Sierra Leone. Revised Curriculum for the Bachelor of Medicine and Pharmacy Programs; University of Sierra Leone: Freetown, Sierra Leone, 2014. [Google Scholar]
- Jamshed, S.Q.; Ibrahim, M.I.M.; Hassali, M.A.; Sharrad, A.K.; Shafie, A.A.; Babar, Z.-U.-D. Understanding and perceptions of final-year doctor of pharmacy students about generic medicines in Karachi, Pakistan: A quantitative insight. Adv. Med. Educ. Pract. 2015, 6, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Hassali, M.A.; Alrasheedy, A.A.; McLachlan, A.; Nguyen, T.A.; AL-Tamimi, S.K.; Ibrahim, M.I.M.; Aljadhey, H. The experiences of implementing generic medicine policy in eight countries: A review and recommendations for a successful promotion of generic medicine use. Saudi Pharm. J. 2014, 22, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, S.; Hassali, M.A.; Saha, A. A survey exploring the knowledge and perceptions of senior medical students in Nepal toward generic medicines. SAGE Open Med. 2016, 4, 2050312116662570. [Google Scholar] [CrossRef] [PubMed]
- Shankar, P.R.; Herz, B.L.; Dubey, A.K.; Hassali, M.A. Assessment of knowledge and perceptions toward generic medicines among basic science undergraduate medical students at Aruba. Indian J. Pharmacol. 2016, 48, S29–S32. [Google Scholar] [CrossRef] [PubMed]
- Othman, G.Q.; Abdulghani, M.A. Assessment of knowledge and perceptions of generic medicines among pharmacy students in Yemeni universities. Pharm. Educ. 2015, 15, 93–98. [Google Scholar]
- Hassali, M.A.; Kong, D.C.; Stewart, K. Knowledge and perceptions of recent pharmacy graduates about generic medicines. Pharm. Educ. 2007, 7, 89–95. [Google Scholar] [CrossRef]
- Pharmacy Board of Sierra Leone. Guidlines for Registration Medicinal Product Freetown; Pharmacy Board of Sierra Leone: Freetown, Sierra Leone, 2014.
- Lee, S.W.; Hassali, M.A.; Shafie, A.A. Knowledge and perception of senior year pharmacy students about generic medicines in public universities of Malaysia. Arch. Pharm. Pract. 2014, 5, 108–112. [Google Scholar] [CrossRef]
- Hassali, M.A.; Wong, Z.Y.; Alrasheedy, A.A.; Saleem, F.; Mohamad Yahaya, A.H.; Aljadhey, H. Does educational intervention improve doctors’ knowledge and perceptions of generic medicines and their generic prescribing rate? A study from Malaysia. SAGE Open Med. 2014, 2, 2050312114555722. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.E. The Founding Of a Medical College: Overcoming Obstacles to Academic Development in Sierra Leone. Res. Sierra Leone Stud. (RISLS) Weave 2013, 1, 2. [Google Scholar]
| Variables | Frequency | Percentages | |
|---|---|---|---|
| Age Group | 20–29 years | 46 | 74.2 |
| 30–39 years | 16 | 25.8 | |
| Sex | Male | 39 | 62.9 |
| Female | 23 | 37.1 | |
| Program of study | Medicine | 42 | 67.7 |
| Pharmacy | 11 | 17.7 | |
| Nursing | 9 | 14.5 |
| Statements | Program of Study | SA n (%) | A n (%) | N n (%) | D n (%) | SD n (%) | p-Value 1 |
|---|---|---|---|---|---|---|---|
| All generic products of a particular medicine that are rated as generic equivalents are therapeutically equivalent to the innovator brand product | Medicine n = 42 | 2 (4.8) | 21 (50.0) | 10 (23.8) | 8 (19.0) | 1 (2.4) | 0.056 |
| Pharmacy n = 11 | 2 (18.2) | 6 (54.5) | 2 (18.2) | 1 (9. 1) | 0 (0.0) | ||
| Nursing n = 9 | 3 (33.3) | 4 (44.4) | 2 (22.2) | 0 (0.0) | 0 (0.0) | ||
| Total n (%) | 7 (11.3) | 31 (50.0) | 14 (22.6) | 9 (14.5) | 1 (1.6) | ||
| All generic products of a particular medicine that are rated as generic equivalents are therapeutically equivalent to each other | Medicine n = 42 | 1 (2.4) | 19 (45.2) | 9 (21.4) | 12 (28.6) | 1 (2.4) | 0.002 |
| Pharmacy n = 11 | 3 (27.3) | 5 (45.5) | 1 (9.1) | 2 (18.2) | 0 (0.0) | ||
| Nursing n = 9 | 3 (33.3) | 6 (66.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Total n (%) | 7 (11.3) | 30 (48.4) | 10 (16.1) | 14 (22.6) | 1 (1.6) | ||
| I have not been introduced to the issues of bioequivalence for generic drugs during my undergraduate education | Medicine n = 42 | 5 (11.9) | 16 (38.1) | 3 (7.1) | 15 (35.7) | 3 (7.1) | 0.692 |
| Pharmacy n = 11 | 0 (0.0) | 4 (36.4) | 2 (18.2) | 4 (36.4) | 1 (9.1) | ||
| Nursing n = 9 | 0 (0.0) | 3 (33.3) | 2 (22.2) | 4 (44.4) | 0 (0.0) | ||
| Total n (%) | 5 (8.1) | 23 (37.1) | 7 (11.7) | 23 (37.1) | 4 (6.5) | ||
| I need more information on how bioequivalence tests are conducted for generic medicines | Medicine n = 42 | 18 (42.9) | 22 (52.4) | 1 (2.4) | 1 (2.4) | 0 (0.0) | 0.237 |
| Pharmacy n = 11 | 3 (27.3) | 7 (63.6) | 1 (9.1) | 0 (0.0) | 0 (0.0) | ||
| Nursing n = 9 | 2 (22.2) | 5 (55.6) | 2 (22.2) | 0 (0.0) | 0 (0.0) | ||
| Total n (%) | 23 (37.1) | 34 (54.8) | 4 (6.5) | 1 (1.6) | 0 (0.0) |
| Statements | Program of Study | SA n (%) | A n (%) | N n (%) | D n (%) | SD n (%) | p-Value 1 |
|---|---|---|---|---|---|---|---|
| A generic medicine is bioequivalent to a brand name medicine | Medicine n = 42 | 1 (2.4) | 12 (28.6) | 15 (35.7) | 14 (33.3) | 0 (0.0) | 0.015 |
| Pharmacy n = 11 | 1 (9.1) | 5 (45.5) | 3 (27.3) | 2 (18.2) | 0 (0.0) | ||
| Nursing n = 9 | 3 (33.3) | 3 (33.3) | 3 (33.3) | 0 (0.0) | 0 (0.0) | ||
| Total n (%) | 5 (8.1) | 20 (32.3) | 21 (33.9) | 16 (25.8) | 0 (0.0) | ||
| A generic medicine must be in the same dosage form (e.g., tablet, capsule) as the brand name medicine | Medicine n = 42 | 4 (9.5) | 20 (47.6) | 5 (11.9) | 13 (31.0) | 0 (0.0) | 0.211 |
| Pharmacy n = 11 | 2 (18.2) | 3 (27.3) | 2 (18.2) | 3 (27.3) | 1 (9.1) | ||
| Nursing n = 9 | 1 (11.1) | 7 (77.8) | 1 (11.1) | 0 (0.0) | 0 (0.0) | ||
| Total n (%) | 7 (11.3) | 30 (48.4) | 8 (12.9) | 16 (25.8) | 1 (1.6) | ||
| A generic medicine must contain the same dose as the brand name medicine | Medicine n = 42 | 7 (16.7) | 21 (50.0) | 5 (11.9) | 9 (21.4) | 0 (0.0) | 0.188 |
| Pharmacy n = 11 | 3 (27.3) | 6 (54.5) | 1 (9.1) | 1 (9.1) | 0 (0.0) | ||
| Nursing n = 9 | 2 (22.2) | 7 (77.8) | 0 | 0 (0.0) | 0 (0.0) | ||
| Total n (%) | 12 (19.4) | 34 (54.8) | 6 (9.7) | 10 (16.1) | 0 (0.0) | ||
| Generic medicines are of inferior quality to branded drugs | Medicine n = 42 | 0 (0.0) | 3 (7.1) | 5 (11.9) | 28 (66.7) | 6 (14.3) | 0.004 |
| Pharmacy n = 11 | 0 (0.0) | 0 (0.0) | 2 (18.2) | 6 (54.5) | 3 (27.3) | ||
| Nursing n = 9 | 0 (0.0) | 1 (11.1) | 6 (66.7) | 2 (22.2) | 0 (0.0) | ||
| Total n (%) | 0 (0.0) | 4 (6.5) | 13 (21.0) | 36 (58.1) | 9 (14.5) | ||
| Generic medicines are less effective than brand name medicines | Medicine n = 42 | 2 (4.8) | 7 (16.7) | 4 (9..5) | 23 (54.8) | 6 (14.3) | 0.049 |
| Pharmacy n = 11 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (72.7) | 3 (27.3) | ||
| Nursing n = 9 | 0 (0.0) | 1 (11.1) | 3 (33.3) | 5 (55.6) | 0 (0.0) | ||
| Total n (%) | 2 (3.2) | 8 (12.9) | 7 (11.3) | 36 (58.1) | 9 (14.5) | ||
| Generic medicines are less safe than brand name medicines | Medicine n = 42 | 2 (4.8) | 6 (14.3) | 11 (26.2) | 17 (40.5) | 6 (14.3) | 0.562 |
| Pharmacy n = 11 | 0 (0.0) | 0 (0.0) | 3 (27.3) | 8 (72.7) | 0 (0.0) | ||
| Nursing n = 9 | 0 (0.0) | 1 (11.2) | 4 (44.4) | 4 (44.4) | 0 (0.0) | ||
| Total n (%) | 2 (3.2) | 7 (11.3) | 18 (29.0) | 29 (46.8) | 6 (9.7) | ||
| Generic medicines are less expensive than brand name medicines | Medicine n = 42 | 7 (16.7) | 11 (26.2) | 15 (35.7) | 6 (14.3) | 3 (7.1) | 0.330 |
| Pharmacy n = 11 | 2 (18.2) | 0 (0.0) | 5 (45.5) | 3 (27.2) | 1 (9.1) | ||
| Nursing n = 9 | 0 (0.0) | 3 (33.3) | 2 (22.2) | 4 (44.4) | 0 (0.0) | ||
| Total n (%) | 9 (14.5) | 14 (22.6) | 22 (35.5) | 13 (21.0) | 4 (6.5) | ||
| Brand name medicines are required to meet higher safety standards than generic medicines | Medicine n = 42 | 6 (14.3) | 15 (35.7) | 13 (31.0) | 7 (16.7) | 1 (2.4) | 0.193 |
| Pharmacy n = 11 | 0 | 5 (45.5) | 3 (27.2) | 3 (27.2) | 0 (0.0) | ||
| Nursing n = 9 | 3 (33.3) | 3 (33.3) | 3 (33.3) | 0 (0.0) | 0 (0.0) | ||
| Total n (%) | 9 (14.5) | 23 (37.1) | 19 (30.6) | 10 (16.1) | 1 (1.6) |
| Statements | Program of Study | SA n (%) | A n (%) | N n (%) | D n (%) | SD n (%) | p-Value 1 |
|---|---|---|---|---|---|---|---|
| From the knowledge I have, I’m confident in giving advice in the future by generic drug name rather than brand name | Medicine n = 42 | 9 (21.4) | 27 (64.3) | 4 (9.5) | 2 (4.8) | 0 (0.0) | <0.001 |
| Pharmacy n = 11 | 0 (0.0) | 6 (54.5) | 3 (27.3) | 2 (18.2) | 0 (0.0) | ||
| Nursing n = 9 | 0 (0.0) | 0 (0.0) | 6 (66.7) | 3 (33.3) | 0 (0.0) | ||
| Total n (%) | 9 (14.5) | 33 (53.2) | 13 (21.0) | 7 (11.3) | 0 (0.0) | ||
| I find it easier to recall a medicine’s therapeutic class using generic names rather than brand names | Medicine n = 42 | 20 (47.6) | 17 (40.5) | 2 (4.8) | 3 (7.1) | 0 (0.0) | 0.011 |
| Pharmacy n = 11 | 3 (27.3) | 5 (45.5) | 2 (18.2) | 1 (9.1) | 0 (0.0) | ||
| Nursing n = 9 | 0 (0.0) | 5 (55.6) | 3 (33.3) | 1 (11.1) | 0 (0.0) | ||
| Total n (%) | 23 (37.1) | 27 (43.5) | 7 (11.3) | 5 (8.1) | 0 (0.0) | ||
| I believe that pharmacists are one of the most important health care professionals to give advice on generic medicines | Medicine n = 42 | 25 (59.5) | 16 (38.1) | 0 (0.0) | 1 (2.4) | 0 (0.0) | <0.001 |
| Pharmacy n = 11 | 7 (63.6) | 4 (36.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Nursing n = 9 | 1 (11.1) | 2 (22.2) | 0 (0.0) | 4 (44.4) | 2 (22.2) | ||
| Total n (%) | 33 (53.2) | 22 (35.5) | 0 (0.0) | 5 (8.1) | 2 (3.2) | ||
| I believe that multinational products are of good quality than local company products | Medicine n = 42 | 8 (19.0) | 18 (42.9) | 12 (28.6) | 2 (4.8) | 2 (4.8) | 0.282 |
| Pharmacy n = 11 | 2 (18.2) | 4 (36.4) | 4 (36.4) | 1 (9.1) | 0 (0.0) | ||
| Nursing n = 9 | 0 (0.0) | 3 (33.3) | 5 (55.6) | 1 (11.1) | 0 (0.0) | ||
| Total n (%) | 10 (16.1) | 25 (40.3) | 21 (33.9) | 4 (6.5) | 2 (3.2) | ||
| I need more information on the issues pertaining to the safety and efficacy of generic medicines | Medicine n = 42 | 17 (40.5) | 22 (52.4) | 1 (2.4) | 1 (2.4) | 1 (2.4) | 0.764 |
| Pharmacy n = 11 | 3 (27.3) | 8 (72.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Nursing n = 9 | 2 (22.2) | 7 (77.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Total n (%) | 22 (35.5) | 37 (59.7) | 1 (1.6) | 1 (1.6) | 1 (1.6) | ||
| I believe my health training should include course on rational medicine use | Medicine n = 42 | 23 (54.7) | 17 (40.5) | 1 (2.4) | 0 (0.0) | 1 (2.4) | 0.110 |
| Pharmacy n = 11 | 6 (54,5) | 5 (45.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Nursing n = 9 | 2 (22.2) | 5 (55.6) | 2 (22.2) | 0 (0.0) | 0 (0.0) | ||
| Total n (%) | 31 (50.0) | 27 (43.5) | 3 (4.8) | 0 (0.0) | 1 (1.6) | ||
| I believe my health training should include course on national drug policy and essential drug list | Medicine n = 42 | 28 (66.7) | 13 (30.9) | 0 (0.0) | 0 (0.0) | 1 (2.4) | 0.208 |
| Pharmacy n = 11 | 6 (54,5) | 5 (45.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Nursing n = 9 | 3 (33.3) | 6 (66.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Total n (%) | 37 (59.7) | 24 (38.7) | 0 (0.0) | 0 (0.0) | 1 (1.6) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
James, P.B.; Bah, A.J.; Margao, E.K.; Hanson, C.; Kabba, J.A.; Jamshed, S.Q. Exploring the Knowledge and Perception of Generic Medicines among Final Year Undergraduate Medical, Pharmacy, and Nursing Students in Sierra Leone: A Comparative Cross-Sectional Approach. Pharmacy 2018, 6, 3. https://doi.org/10.3390/pharmacy6010003
James PB, Bah AJ, Margao EK, Hanson C, Kabba JA, Jamshed SQ. Exploring the Knowledge and Perception of Generic Medicines among Final Year Undergraduate Medical, Pharmacy, and Nursing Students in Sierra Leone: A Comparative Cross-Sectional Approach. Pharmacy. 2018; 6(1):3. https://doi.org/10.3390/pharmacy6010003
Chicago/Turabian StyleJames, Peter Bai, Abdulai Jawo Bah, Emmanuel Kamanda Margao, Christian Hanson, John Alimamy Kabba, and Shazia Qasim Jamshed. 2018. "Exploring the Knowledge and Perception of Generic Medicines among Final Year Undergraduate Medical, Pharmacy, and Nursing Students in Sierra Leone: A Comparative Cross-Sectional Approach" Pharmacy 6, no. 1: 3. https://doi.org/10.3390/pharmacy6010003





