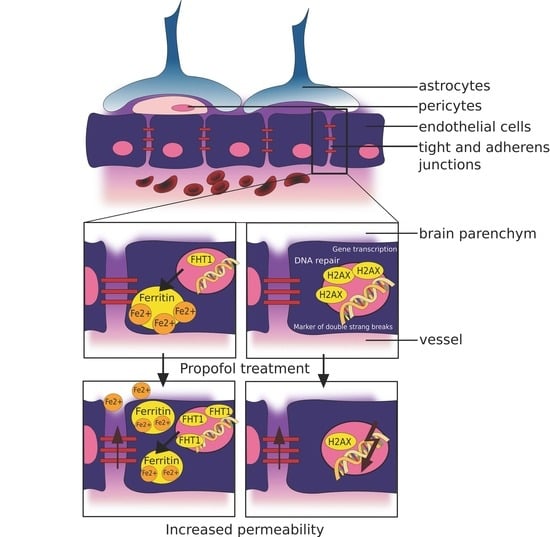
Disturbance of Key Cellular Subproteomes upon Propofol Treatment Is Associated with Increased Permeability of the Blood-Brain Barrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Cell Culture
2.2. Measurement of Fluorescein Passage through the BBB
2.3. Real Time Cell Adhesion Assay
2.4. Preparation of Cell Extracts
2.5. Mass Spectrometry
2.5.1. Sample Preparation
2.5.2. LC-MSMS
2.5.3. Data Analysis
2.6. Immunoblotting
3. Results
3.1. Propofol Increases the Permeability of the BBB
3.2. Propofol Interferes with Protein Expression of the BBB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montagne, A.; Zhao, Z.; Zlokovic, B.V. Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J. Exp. Med. 2017, 214, 3151–3169. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brightman, M.W.; Reese, T.S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969, 40, 648–677. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.W.; Sharp, M.M.; Albargothy, N.J.; Fernandes, R.; Hawkes, C.A.; Verma, A.; Weller, R.O.; Carare, R.O. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol. 2016, 131, 725–736. [Google Scholar] [CrossRef]
- Coomber, B.L.; Stewart, P.A. Morphometric analysis of CNS microvascular endothelium. Microvasc. Res. 1985, 30, 99–115. [Google Scholar] [CrossRef]
- Betz, A.L.; Goldstein, G.W. Polarity of the blood-brain barrier: Neutral amino acid transport into isolated brain capillaries. Science 1978, 202, 225–227. [Google Scholar] [CrossRef]
- Betz, A.L.; Firth, J.A.; Goldstein, G.W. Polarity of the blood-brain barrier: Distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res. 1980, 192, 17–28. [Google Scholar] [CrossRef]
- Mittapalli, R.K.; Manda, V.K.; Adkins, C.E.; Geldenhuys, W.J.; Lockman, P.R. Exploiting nutrient transporters at the blood-brain barrier to improve brain distribution of small molecules. Ther. Deliv. 2010, 1, 775–784. [Google Scholar] [CrossRef]
- Oldendorf, W.H.; Cornford, M.E.; Brown, W.J. The large apparent work capability of the blood-brain barrier: A study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann. Neurol. 1977, 1, 409–417. [Google Scholar] [CrossRef]
- Roos, D.H.; Puntel, R.L.; Santos, M.M.; Souza, D.O.; Farina, M.; Nogueira, C.W.; Aschner, M.; Burger, M.E.; Barbosa, N.B.; Rocha, J.B. Guanosine and synthetic organoselenium compounds modulate methylmercury-induced oxidative stress in rat brain cortical slices: Involvement of oxidative stress and glutamatergic system. Toxicol Vitr. 2009, 23, 302–307. [Google Scholar] [CrossRef]
- Palmer, A.M. The role of the blood-CNS barrier in CNS disorders and their treatment. Neurobiol. Dis. 2010, 37, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.C.; Couch, Y.; Sibson, N.; Turner, M.R. Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol. Cell Neurosci. 2013, 53, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Jeppsson, B.; Freund, H.R.; Gimmon, Z.; James, J.H.; von Meyenfeldt, M.F.; Fischer, J.E. Blood-brain barrier derangement in sepsis: Cause of septic encephalopathy? Am. J. Surg. 1981, 141, 136–142. [Google Scholar] [CrossRef]
- Tighe, D.; Moss, R.; Bennett, D. Cell surface adrenergic receptor stimulation modifies the endothelial response to SIRS. Systemic Inflammatory Response Syndrome. New Horiz. 1996, 4, 426–442. [Google Scholar] [PubMed]
- Pluta, R. Pathological opening of the blood-brain barrier to horseradish peroxidase and amyloid precursor protein following ischemia-reperfusion brain injury. Chemotherapy 2005, 51, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Lossinsky, A.S.; Wisniewski, H.M.; Mossakowski, M.J. Early blood-brain barrier changes in the rat following transient complete cerebral ischemia induced by cardiac arrest. Brain Res. 1994, 633, 41–52. [Google Scholar] [CrossRef]
- Zador, Z.; Stiver, S.; Wang, V.; Manley, G.T. Role of aquaporin-4 in cerebral edema and stroke. Handb. Exp. Pharmacol. 2009, 190, 159–170. [Google Scholar] [CrossRef]
- Kim, K.S.; Wass, C.A.; Cross, A.S. Blood-brain barrier permeability during the development of experimental bacterial meningitis in the rat. Exp. Neurol. 1997, 145, 253–257. [Google Scholar] [CrossRef]
- Arshi, A.; Lai, W.C.; Chen, J.B.; Bukata, S.V.; Stavrakis, A.I.; Zeegen, E.N. Predictors and Sequelae of Postoperative Delirium in Geriatric Hip Fracture Patients. Geriatr. Orthop. Surg. Rehabil. 2018, 9, 2151459318814823. [Google Scholar] [CrossRef]
- Young, G.B.; Bolton, C.F.; Archibald, Y.M.; Austin, T.W.; Wells, G.A. The electroencephalogram in sepsis-associated encephalopathy. J. Clin. Neurophysiol. 1992, 9, 145–152. [Google Scholar] [CrossRef]
- Clawson, C.C.; Hartmann, J.F.; Vernier, R.L. Electron microscopy of the effect of gram-negative endotoxin on the blood-brain barrier. J. Comp. Neurol. 1966, 127, 183–198. [Google Scholar] [CrossRef]
- Weber, V.; Olzscha, H.; Langrich, T.; Hartmann, C.; Jung, M.; Hofmann, B.; Horstkorte, R.; Bork, K. Glycation Increases the Risk of Microbial Traversal through an Endothelial Model of the Human Blood-Brain Barrier after Use of Anesthetics. J. Clin. Med. 2020, 9, 3672. [Google Scholar] [CrossRef]
- James, R.; Glen, J.B. Synthesis, biological evaluation, and preliminary structure-activity considerations of a series of alkylphenols as intravenous anesthetic agents. J. Med. Chem. 1980, 23, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Chidambaran, V.; Costandi, A.; D’Mello, A. Propofol: A review of its role in pediatric anesthesia and sedation. CNS Drugs 2015, 29, 543–563. [Google Scholar] [CrossRef] [PubMed]
- Wessen, A.; Persson, P.M.; Nilsson, A.; Hartvig, P. Concentration-effect relationships of propofol after total intravenous anesthesia. Anesth. Analg. 1993, 77, 1000–1007. [Google Scholar] [PubMed]
- Shafer, S.L. Advances in propofol pharmacokinetics and pharmacodynamics. J. Clin. Anesth. 1993, 5, 14–21. [Google Scholar] [CrossRef]
- Takizawa, E.; Hiraoka, H.; Takizawa, D.; Goto, F. Changes in the effect of propofol in response to altered plasma protein binding during normothermic cardiopulmonary bypass. Br. J. Anaesth. 2006, 96, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Angelini, G.; Ketzler, J.T.; Coursin, D.B. Use of propofol and other nonbenzodiazepine sedatives in the intensive care unit. Crit. Care Clin. 2001, 17, 863–880. [Google Scholar] [CrossRef]
- Langley, M.S.; Heel, R.C. Propofol. A review of its pharmacodynamic and pharmacokinetic properties and use as an intravenous anaesthetic. Drugs 1988, 35, 334–372. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.; Bullock, R.; Rafferty, C.; Fitch, W.; Teasdale, G.M. Propofol sedation in severe head injury fails to control high ICP, but reduces brain metabolism. Acta Neurochir. Suppl. (Wien) 1994, 60, 544–546. [Google Scholar] [CrossRef]
- Plunkett, J.J.; Reeves, J.D.; Ngo, L.; Bellows, W.; Shafer, S.L.; Roach, G.; Howse, J.; Herskowitz, A.; Mangano, D.T. Urine and plasma catecholamine and cortisol concentrations after myocardial revascularization. Modulation by continuous sedation. Multicenter Study of Perioperative Ischemia (McSPI) Research Group, and the Ischemia Research and Education Foundation (IREF). Anesthesiology 1997, 86, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Kelbel, I.; Weiss, M. Anaesthetics and immune function. Curr. Opin. Anaesthesiol. 2001, 14, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.T.; Naguib, M. Propofol: The challenges of formulation. Anesthesiology 2005, 103, 860–876. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, D.; Lei, L.; Jia, Y.; Zhou, H.; Yang, B. Propofol Prevents Renal Ischemia-Reperfusion Injury via Inhibiting the Oxidative Stress Pathways. Cell Physiol. Biochem. 2015, 37, 14–26. [Google Scholar] [CrossRef]
- Jovic, M.; Stancic, A.; Nenadic, D.; Cekic, O.; Nezic, D.; Milojevic, P.; Micovic, S.; Buzadzic, B.; Korac, A.; Otasevic, V.; et al. Mitochondrial molecular basis of sevoflurane and propofol cardioprotection in patients undergoing aortic valve replacement with cardiopulmonary bypass. Cell Physiol. Biochem. 2012, 29, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, T.B.; Engel, A.; Sakamoto, H.; O’Shea, A.; O’Callaghan-Enright, S.; Shorten, G.D. The effects of propofol on neutrophil function, lipid peroxidation and inflammatory response during elective coronary artery bypass grafting in patients with impaired ventricular function. Br. J. Anaesth. 2006, 97, 825–831. [Google Scholar] [CrossRef]
- Fischer, S.; Renz, D.; Kleinstuck, J.; Schaper, W.; Karliczek, G.F. In vitro effects of anaesthetic agents on the blood-brain barrier. Anaesthesist 2004, 53, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhuang, J.; Wang, Y.; Zhao, D.; Zhao, L.; Zhu, S.; Pu, J.; Yin, M.; Zhang, H.; Wang, Z.; et al. Propofol Alleviates DNA Damage Induced by Oxygen Glucose Deprivation and Reperfusion via FoxO1 Nuclear Translocation in H9c2 Cells. Front. Physiol. 2019, 10, 223. [Google Scholar] [CrossRef]
- Weber, V.; Bork, K.; Horstkorte, R.; Olzscha, H. Analyzing the Permeability of the Blood-Brain Barrier by Microbial Traversal through Microvascular Endothelial Cells. J. Vis. Exp. 2020, 14, e60692. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- The Gene Ontology Consortium. The Gene Ontology resource: Enriching a GOld mine. Nucleic Acids Res. 2021, 49, D325–D334. [Google Scholar] [CrossRef] [PubMed]
- Stins, M.F.; Badger, J.; Sik Kim, K. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb. Pathog. 2001, 30, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Dagley, L.F.; Infusini, G.; Larsen, R.H.; Sandow, J.J.; Webb, A.I. Universal Solid-Phase Protein Preparation (USP(3)) for Bottom-up and Top-down Proteomics. J. Proteome Res. 2019, 18, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; Garcia-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]
- Tarrade, S.; Bhardwaj, T.; Flegal, M.; Bertrand, L.; Velegzhaninov, I.; Moskalev, A.; Klokov, D. Histone H2AX Is Involved in FoxO3a-Mediated Transcriptional Responses to Ionizing Radiation to Maintain Genome Stability. Int. J. Mol. Sci. 2015, 16, 29996–30014. [Google Scholar] [CrossRef]
- Fishman, J.B.; Rubin, J.B.; Handrahan, J.V.; Connor, J.R.; Fine, R.E. Receptor-mediated transcytosis of transferrin across the blood-brain barrier. J. Neurosci. Res. 1987, 18, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Killilea, D.W.; Wong, S.L.; Cahaya, H.S.; Atamna, H.; Ames, B.N. Iron accumulation during cellular senescence. Ann. N. Y. Acad Sci. 2004, 1019, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Reiche, J.; Huber, O. Post-translational modifications of tight junction transmembrane proteins and their direct effect on barrier function. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183330. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, R.; Shigetomi, K.; Inai, T.; Sakai, M.; Ikenouchi, J. CaMKII regulates the strength of the epithelial barrier. Sci. Rep. 2015, 5, 13262. [Google Scholar] [CrossRef] [PubMed]
- Marunaka, K.; Furukawa, C.; Fujii, N.; Kimura, T.; Furuta, T.; Matsunaga, T.; Endo, S.; Hasegawa, H.; Anzai, N.; Yamazaki, Y.; et al. The RING finger- and PDZ domain-containing protein PDZRN3 controls localization of the Mg(2+) regulator claudin-16 in renal tube epithelial cells. J. Biol. Chem. 2017, 292, 13034–13044. [Google Scholar] [CrossRef]
- Heiler, S.; Mu, W.; Zoller, M.; Thuma, F. The importance of claudin-7 palmitoylation on membrane subdomain localization and metastasis-promoting activities. Cell Commun. Signal 2015, 13, 29. [Google Scholar] [CrossRef]
- Molina-Jijon, E.; Rodriguez-Munoz, R.; Namorado Mdel, C.; Pedraza-Chaverri, J.; Reyes, J.L. Oxidative stress induces claudin-2 nitration in experimental type 1 diabetic nephropathy. Free Radic. Biol. Med. 2014, 72, 162–175. [Google Scholar] [CrossRef]
- Han, G.; Ye, M.; Zhou, H.; Jiang, X.; Feng, S.; Jiang, X.; Tian, R.; Wan, D.; Zou, H.; Gu, J. Large-scale phosphoproteome analysis of human liver tissue by enrichment and fractionation of phosphopeptides with strong anion exchange chromatography. Proteomics 2008, 8, 1346–1361. [Google Scholar] [CrossRef]
- Olsen, J.V.; Vermeulen, M.; Santamaria, A.; Kumar, C.; Miller, M.L.; Jensen, L.J.; Gnad, F.; Cox, J.; Jensen, T.S.; Nigg, E.A.; et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci. Signal 2010, 3, ra3. [Google Scholar] [CrossRef]
- Shigetomi, K.; Ikenouchi, J. Regulation of the epithelial barrier by post-translational modifications of tight junction membrane proteins. J. Biochem. 2018, 163, 265–272. [Google Scholar] [CrossRef]
- Han, D.; Jin, J.; Fang, H.; Xu, G. Long-term action of propofol on cognitive function and hippocampal neuroapoptosis in neonatal rats. Int. J. Clin. Exp. Med. 2015, 8, 10696–10704. [Google Scholar]
- Kim, J.L.; Bulthuis, N.E.; Cameron, H.A. The Effects of Anesthesia on Adult Hippocampal Neurogenesis. Front. Neurosci. 2020, 14, 588356. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Längrich, T.; Bork, K.; Horstkorte, R.; Weber, V.; Hofmann, B.; Fuszard, M.; Olzscha, H. Disturbance of Key Cellular Subproteomes upon Propofol Treatment Is Associated with Increased Permeability of the Blood-Brain Barrier. Proteomes 2022, 10, 28. https://doi.org/10.3390/proteomes10030028
Längrich T, Bork K, Horstkorte R, Weber V, Hofmann B, Fuszard M, Olzscha H. Disturbance of Key Cellular Subproteomes upon Propofol Treatment Is Associated with Increased Permeability of the Blood-Brain Barrier. Proteomes. 2022; 10(3):28. https://doi.org/10.3390/proteomes10030028
Chicago/Turabian StyleLängrich, Timo, Kaya Bork, Rüdiger Horstkorte, Veronika Weber, Britt Hofmann, Matt Fuszard, and Heidi Olzscha. 2022. "Disturbance of Key Cellular Subproteomes upon Propofol Treatment Is Associated with Increased Permeability of the Blood-Brain Barrier" Proteomes 10, no. 3: 28. https://doi.org/10.3390/proteomes10030028