Abstract
Background/objectives: Considering the diverse symptomatology of COVID-19—ranging from mild to severe cases—multi-professional interventions are crucial for enhancing physical recovery, nutritional status, and mental health outcomes in affected patients. Thus, this study aimed to investigate the effects of such an intervention on health-related physical fitness and biomarkers in overweight COVID-19 survivors with varying degrees of symptom severity after 8 weeks and 16 weeks. Methods: This non-randomized clinical trial included 59 overweight COVID-19 survivors (32 males and 27 females) divided into three groups: mild (n = 31), moderate (n = 13), and severe/critical (n = 15). The participants underwent a multi-professional program and were assessed for anthropometric and body composition (primary outcome), as well as physical fitness and biochemical markers (secondary outcome) 8 and 16 weeks before the intervention. Results: After 8 weeks, time effects were observed for the maximum isometric handgrip strength (p < 0.001), maximum isometric lumbar-traction strength (p = 0.01), flexibility (p < 0.001), abdominal strength–endurance (p < 0.001), the sit-and-stand test (p < 0.001), maximum oxygen consumption (p < 0.001), and distance covered in the 6 min walk test (p < 0.001). Additionally, time effects were also observed for fat mass (p = 0.03), body fat percentage (p = 0.02), abdominal circumference (p = 0.01), total cholesterol (p < 0.001), low-density lipoproteins (p < 0.001), and glycated hemoglobin (p < 0.001), with lower values after multi-professional interventions. After 16 weeks, the systolic and diastolic blood pressure showed significant reductions independently of the intervention group (p < 0.001). Conclusion: These findings suggest that multi-professional interventions can provide substantial benefits for post-COVID-19 patients, regardless of the severity of their initial symptoms.
1. Introduction
Coronavirus-19 (COVID-19) is an acute respiratory infection caused by the SARS-CoV-2 coronavirus, which is potentially severe, highly transmissible, and globally distributed [1]. Some COVID-19 survivors have presented sequelae of a respiratory order; physical deconditioning, with a loss of musculoskeletal mass and reduced muscle strength and endurance; a decreased cardiorespiratory capacity and reduced quality of life; and emotional problems, among others [1]. Unlike the signs and symptoms of COVID-19, the complications of acute post-COVID-19 are not fully understood [2]. For some people, complications may affect multiple organs and persist for months, regardless of the severity of the disease at onset. However, persons with a higher degree of COVID-19 involvement have a higher risk of death within 12 months of illness [3].
The severity of the disease depends on the intensity of the immune response triggered by the virus. Therefore, long-term signs and symptoms are subject to the extent and severity of the viral infection, the organs affected, and the so-called “cytokine storm” during the acute phase of COVID-19 [4]. The acute post-COVID-19 syndrome or post-COVID-19 state includes fatigue, dyspnea, chest pain, a loss of taste and/or smell, cognitive changes, and arthralgias, which are factors that affect work and daily functioning for a long time due to COVID-19 [5,6]. Thus, rehabilitation strategies for COVID-19 survivors are indispensable to combat a condition with different sequelae, minimize damage, and significantly impact the population, the health of persons, and the economy [5,6,7,8].
In addition, an intervention applied to COVID-19 survivors must consider that it is well established in the scientific literature that obesity is a risk factor for severe COVID-19, with a dose–response relationship between a higher body mass index (BMI) and worsening of the disease [6,7,9]. Obesity is a low-grade pro-inflammatory disease correlated with the increased prevalence of other chronic non-communicable diseases (NCDs), such as type 2 diabetes mellitus, hypertension, dyslipidemia, and metabolic syndrome, among other pathologies [10]. The scientific literature showed a higher prevalence of hospitalization in obese patients with COVID-19. Rottoli et al. [11] carried out a study in a hospital in Bologna, Italy; among the obese individuals, 51.9% had respiratory failure, 36.4% were admitted to the intensive care unit, 25% required mechanical ventilation, and 29.8% died within 30 days of the onset of symptoms. Gao et al. [12] investigated the association between obesity and the severity of COVID-19 in three Chinese hospitals, and found that obese individuals had more extended hospital stays and progressed to the severe form of the disease.
In addition, biochemical markers could be altered in COVID-19 survivors to different degrees, like fasting glucose, glycated hemoglobin, triglycerides, C-reactive protein, and other biomarkers [5]; however, multi-professional interventions may promote benefits in the biochemical responses for health for 8 weeks of intervention [5]. Therefore, the application of interventions aimed at patient recovery by providing physical activity, healthy eating, and psychoeducation could reduce the sequelae of the disease and the complications resulting from the post-COVID syndrome in overweight persons [5,6,7,8].
In this sense, interventions with physical exercise, dietary re-education, and psychoeducation in post-COVID-19 patients after 8 weeks showed promising responses of multi-professional interventions with a significant reduction in the C-reactive protein, a significant increase in serum albumin, and a significant improvement in the sit-to-stand test in those who were hospitalized [5]. Additionally, other studies also explored the function of physical exercise as a possible approach to mitigate the harmful impacts of COVID-19. However, they have not yet considered the various multi-professional elements related to public health promotion strategies and the severity of COVID-19 in different symptomatologies (mild, moderate, and severe/critical cases) [13,14]. Additionally, the multi-professional intervention for patients surviving COVID-19 for more than 12 weeks, considering the specific symptoms (mild, moderate, and severe/critical) [13,14], is still unknown to the present study’s authors. Consequently, considering the symptoms between the groups may be relevant to promoting assertive interventions and recovery prognoses.
Thus, this study aimed to investigate the effects of a multi-professional intervention on health-related physical fitness and biochemical parameters in overweight and obese COVID-19 survivors (in different degrees of severity) for 8 and 16 weeks. Drawing from earlier research [5,13,14], the authors of this study suggest, as a primary outcome, that an 8-week multidisciplinary intervention model can enhance anthropometric and body composition parameters. As a secondary outcome, it can improve physical fitness and metabolic parameters, irrespective of the disease’s symptomatology. Furthermore, extending the intervention to 16 weeks may yield more consistent results than the 8-week program.
2. Materials and Methods
2.1. Experimental Approach to the Problem
This study is an uncontrolled parallel-group non-randomized clinical trial, with repeated measures over 8 and 16 weeks, carried out from January to October 2022, following Consolidated Standards of Reporting Trials (CONSORT) [15]. The experimental groups (mild COVID-19 group, moderate COVID-19 group, and severe/critical COVID-19 group) underwent a multi-professional program consisting of physical exercises (muscle strength and aerobic exercises, i.e., concurrent training), nutritional intervention, and psychoeducation. Participants were assessed at the beginning of the study (pre-intervention), after 8 weeks (post-8 weeks), and after 16 weeks of intervention (post-16 weeks). Those interested contacted the multi-professional team from the Interdisciplinary Health Promotion Intervention Laboratory in the Cesumar University facilities.
The assessments included anthropometry and body composition (primary outcome), flexibility, maximal isometric strength tests, dynamic muscle strength–endurance, cardiorespiratory fitness, and biochemical parameters (secondary outcome). All assessments were conducted in the morning (between 7:00 and 11:30 h) and in the exact location (laboratory, with the control of variables, temperature, and investigators that applied the procedures). The patients did not present pain before the assessments or during the training sessions without presenting musculoskeletal and/or cardiorespiratory injuries during the intervention. The present study was approved by the Local Research Ethics Committee (protocol n° 4.546.726/2021) and followed the Declaration of Helsinki. This study was registered in the Brazilian Clinical Trials Registry Platform (REBEC) under RBR-4mxg57b. All subjects were informed about the purposes of the study and signed an informed consent form.
Baseline measurements were taken over two days. First, the subjects underwent a clinical assessment by a pulmonologist and an intensive care physician, consisting of the participant’s clinical history (history of surgery, pre-existing chronic non-communicable diseases, continuous use of medication, main signs and symptoms showing possible sequelae of COVID-19, and type and length of stay in hospital (ward/room or intensive care unit)), anthropometric and body composition assessment, and blood collection for biochemical analyses. Figure 1 shows the methodological design of the present study.
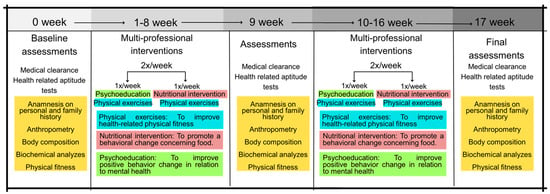
Figure 1.
Methodological design of the present study.
On the second day, the following data were collected: (i) blood pressure [BP—systolic blood pressure (SBP) and diastolic blood pressure (DBP)] after 5 min of rest, according to the VIII Guideline on Arterial Hypertension [15]; (ii) measurement of heart rate (HR) and peripherical oxygen saturation (%SpO2), both at rest; (iii) posterior chain flexibility test on the Wells bench (sit and reach test); (iv) maximal isometric strength tests with specific dynamometers; (v) sit-up test; (vi) 30 s chair–stand test; (vii) push-up (adapted); and (viii) 6 min walk test (6MWT). The physical fitness tests were performed following the recommendations published earlier by Sordi et al. [5] in a clinical trial with post-COVID-19 patients. After the 6MWT, the following variables were collected: BP, HR, and %SpO2. All tests are described in the sections below. Moreover, all evaluators had experience carrying out measurements and had already participated in other studies measuring the same indicators presented in the present study, with an intra-evaluator reproducibility greater than 0.95 with data from our research laboratory. After the clinical assessment, the self-reported signs and symptoms were considered for the non-randomized allocation of participants in the experimental COVID-19 groups according to the “Clinical Management of COVID-19: Living Guidance” [16]. The clinical manifestations of COVID-19 are divided according to their severity: mild (no evidence of viral pneumonia or hypoxia); moderate (clinical signs of pneumonia, but no signs of severe pneumonia); severe (clinical signs of pneumonia plus one of the following: respiratory rate >30 breaths/min, severe respiratory distress, or SatO2 < 90%); and critical (acute respiratory distress syndrome, sepsis, septic shock, or acute thrombosis) [16]. Over the 16 weeks, 16 participants dropped out of the study for different reasons. Figure 2 presents the flowchart of the present study’s participants based on the CONSORT Guidelines [17].
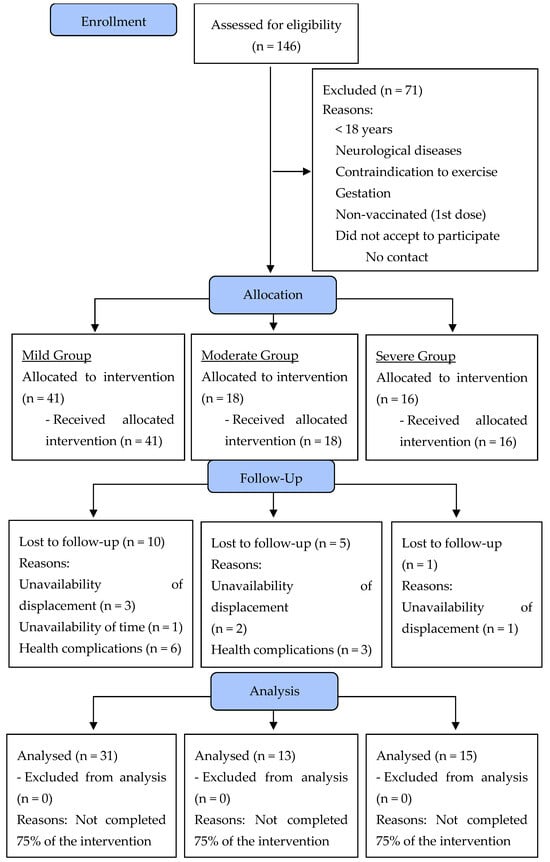
Figure 2.
Flowchart diagram of the participants of the present study.
2.2. Study Participants
Participants were recruited through the Maringá, Paraná, Brazil, Municipal Health Department, Municipal Hospital, and TV, radio, and social media advertising. One hundred and forty-six volunteers of both sexes were invited to take part in the study according to the following inclusion criteria: (i) aged between 19 and 65 years; (ii) BMI ≥ 25.0 kg/m2; (iii) positive diagnosis confirmed by RT-PCR (reverse transcriptase polymerase chain reaction) for COVID-19; (iv) medical authorization to participate in this study; (v) first dose of the COVID-19 vaccine; (vi) being available to participate in multi-professional interventions twice a week for sixteen weeks; and (vii) having contracted COVID-19 between 2 January 2021 and 22 September 2021. Exclusion criteria included the following: (i) debilitating neurological diseases (i.e., Alzheimer’s or Parkinson’s); (ii) contraindications for physical exercise; and (iii) pregnancy. Following Jensen et al. [18], a minimum of 15 participants per group would be sufficient to achieve a statistical power of 80% with an alpha error of 5%. One hundred and forty-six volunteers randomized in three experimental groups were eligible, but seventy-one were excluded because they did not meet the inclusion criteria. In total, seventy-five volunteers were accepted to participate in the program and were allocated according to their COVID-19 symptoms: fifteen volunteers were allocated to the mild COVID-19 group, eighteen volunteers to the moderate COVID-19 group, and sixteen volunteers to the COVID-19 severe/critical group. At the end (after 16 weeks), 59 volunteers were analyzed: mild COVID-19 (n = 31), moderate COVID-19 (n = 13), and severe/critical COVID-19 (n = 15) (Figure 1).
2.3. Medical Clearance
First, all patients underwent a medical consultation with an intensive care physician/thoracic surgeon. The physician reviewed the patient’s medical records, blood tests, computed tomography scans, type of hospitalization, use of ventilators, %SatO2, blood pressure, and cardiological exams (to assess for possible myocarditis or pericarditis) and performed pulmonary and cardiac auscultation. Additionally, the physician conducted an anamnesis to identify any prolonged symptoms of COVID-19, such as fatigue, dyspnea, or other lingering effects. Based on this comprehensive evaluation, the physician determined whether the patient could engage in physical activity. Patients deemed unfit for physical activity were referred for cardiopulmonary or neurological physiotherapy rehabilitation, as previously described by Lemos et al. [7] in an earlier study conducted by the same research group.
2.4. Procedures
The participants’ stature was measured using a stadiometer attached to a scale with a capacity of 2.2 m and an accuracy of 0.1 cm (Welmy R-110®, Santa Bárbara D’ Oeste, São Paulo, Brazil). Abdominal circumference was measured using a tape measure (model T87-2®, Florianopolis, Santa Catarina, Brazil), with a measuring capacity of 2 m and precision of 0.1 cm, following the specifications proposed by Heyward [19]. The participant’s body composition was measured using tetrapolar bioimpedance (InBody 570®, Bio space Co., Ltd., Seoul, Republic of Korea), with a capacity of 250 kg and an accuracy of 100 g, according to the manufacturer’s instructions, following recommendations to improve validity [20]. All participants were previously instructed on the recommendations. The following parameters were measured: BMI (kg/m2), lean mass (kg), fat mass (kg), body fat percentage (%), and skeletal muscle mass (kg) [21].
2.5. Biochemical Analyses
The blood collection procedures followed the guidelines of the Clinical and Laboratory Standards Institute [22]. Participants were previously instructed on how to prepare for the collections at the Clinical Analysis Laboratory of the Cesumar University facilities, and after collection, participants were instructed to press on the puncture site to avoid bruising. The collected blood samples were dispensed into Vacuplast® collection tubes containing stacking gel with an activator and tubes with the anticoagulant ethylene diamine tetra acetic acid (EDTA) K2. Subsequently, to obtain serum, the samples containing an activator were centrifuged in a Centrilab® analog centrifuge at 3.500 rpm (relative centrifugal force) for 15 min at room temperature. The following laboratory tests were analyzed: glycemic control [glycated hemoglobin: (HbA1c)]; lipid profile [total cholesterol, high-density lipoprotein (HDL-c), low-density lipoprotein (LDL-c), and triglycerides (TGL)], liver enzymes [alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), and albumin], C-reactive protein (CRP), markers of renal function (creatinine and urea), electrolytes (magnesium, total calcium, and phosphorus), and markers of pancreatic function (amylase and lipase). The analyses used the Gold Análise Diagnóstica kits (Belo Horizonte, Minas Gerais, Brazil) in the automatic biochemical and turbidimetric analyzer device URIT 8021® from MHLab. All analyses were performed in triplicate. The Finecare® FIA Meter Plus analyzer from WONDFO was used for HbA1c.
2.6. Physical Fitness
2.6.1. Health-Related Physical Fitness Tests
The chosen physical tests to evaluate the outcomes of the COVID-19 survivors follow the order: (i) sit and reach test; (ii) maximal isometric handgrip strength (MIHS), maximal isometric lumbar-traction strength (MILTS); (iii) sit-up test for abdominal strength resistance; (iv) 30 s chair–stand test for lower limbs; (v) push-up test for upper limbs; and (vi) 6MWT [1]. The participants were instructed about the procedures for all physical tests, and the researchers respected the remaining 5–10 min between the tests (in each test, the participants were familiarized with performing the tests), conforming to the orientation of Sordi et al. [5]. Furthermore, the choice of physical tests was based on promoting the assessment of physical fitness test parameters in places with low resources, clinics, public hospitals, gyms, and others.
Flexibility Assessment
The sit and reach test was employed to evaluate the flexibility of the posterior chain using the Wells Bench. Participants were instructed according to previously described procedures [23]. The test was repeated three times, with a 60 s interval between attempts. The highest value obtained was recorded and expressed in cm.
Maximal Isometric Strength Tests (MIHS and MILTS)
To assess MIHS, a TKK 5101 dynamometer (Takei Physical Fitness Test®, Tokyo, Japan) with a capacity of 100 kg was used. MILTS was evaluated using a Takei dynamometer (Takei Physical Fitness Test®, Back Strength Dynamometer, type 2, Japan) with a capacity of 300 kg. According to previous recommendations, three trials were performed for both tests, lasting 3–5 s with a 1 min rest between trials [24]. The highest value was recorded in kgf.
Dynamic Muscle Strength–Endurance Assessment
To assess dynamic muscle, sit-up, 30 s chair–stand, and push-up tests for upper limbs were performed according to the procedures described in previous studies [25,26]. For the sit-up and push-up tests, the maximum number of repetitions achieved in 60 s was recorded, and for the 30 s chair–stand test, the muscular endurance of the lower limbs was evaluated from the maximum number of repetitions performed.
Cardiorespiratory Fitness Test
The 6MWT was applied to verify the cardiorespiratory fitness of the present study participants. The 6MWT test was performed per American Thoracic Society guidelines [27]. Volunteers were instructed to walk as fast as possible to achieve the greatest distance at the end of 6 min. [27]. Before and after the 6MWT, distance, heart rate, and systolic and diastolic pressure were collected. The peak oxygen consumption (VO2peak) was calculated using a previous study [28].
2.7. Multi-Professional Intervention
2.7.1. Food Re-Education Protocol
Nutritionists held nutritional orientation meetings in groups, with theoretical and practical activities to promote the change in eating behavior. The classes, inspired by the Food Guide for the Brazilian Population [29], were adapted to the new scenario experienced by COVID-19. The aim was to educate participants with dynamic classes on the benefits of healthy eating for health and how to deal with the risks associated with chronic NCDs and long-term symptoms of COVID-19 [5]. The meetings took place once a week for approximately 45 min for 16 consecutive weeks. In addition to dynamic classes, printed materials on the theme were prepared to be delivered. The interventions addressed the following themes, conforming to Table 1.

Table 1.
Nutritional activities that were developed throughout the research project.
2.7.2. Psychoeducation Protocol
The psychologists held psychoeducation meetings to prevent and treat mental illness with an educational character. The protocol is applied using concepts and information from psychology, based on cognitive–behavioral theory (CBT), combined with other areas so that the individual can gain a broad understanding of their situation and other mental illnesses related to their condition, as well as focus on prevention and health promotion [8,30,31]. The meetings took place in a group once a week, for approximately 45 min, for 16 consecutive weeks. The interventions were based on content presentations, conversation circles, and dynamics. The psychoeducational interventions were adapted to the participants’ reality according to the feedback provided during the interventions. In addition to the dynamic lessons, printed materials on the subject were prepared for delivery. The psychoeducation interventions addressed the following themes, conforming to Table 2.

Table 2.
Psychological activities that were developed throughout the research project.
To reinforce the interventions applied and disseminate support material to participants, family members, and the community, we used printed and digital information papers delivered and sent after each intervention [30,31,32,33]. The interventions aimed to provide knowledge and enable changes about the psychological consequences of the COVID-19 pandemic, in addition to providing guidance on the essential themes of our century and helping participants to become more aware of the mental disorders caused by the contagion process and the COVID-19 pandemic.
2.7.3. Physical Exercise Protocol
The physical exercise intervention sessions were held at the Cesumar University facilities twice weekly, lasting 60 min each, with alternating training programs A and B on different days. The training protocol consisted of cardiorespiratory and muscle strength exercises (concurrent training) to increase muscle strength and, if necessary, motor coordination and balance. The concurrent training plan consisted of 4 weeks of anatomical adaptation with low volume and intensity, that is, 3 sets of 15 repetitions, with no aerobic exercise at the end of the session, and the remaining weeks of physical exercise (12 more in total) had a gradual progression of volume and intensity (via the classic linear periodization); in other words, the loads used were readjusted over the weeks, as well as the number of sets and repetitions. In weeks 5 to 8, 3 sets of 12 repetitions were performed; in weeks 9 to 12, the training sessions consisted of 4 sets of 12 repetitions; and finally, in weeks 13 to 16, 4 sets of 20 repetitions were performed. The rating of perceived exertion (RPE) for resistance training was used to regulate the intensity of the training sessions [34], where 0 = rest and 10 = maximal effort, with values measured in arbitrary units (a.u.). The study participants were familiarized with the scale. The RPE scale remained in the strength training laboratory, allowing patients to monitor the intensity of their sessions. During the first four weeks, the session intensity ranged from 1 a.u. to 4 a.u.; in weeks 5 to 8, the intensity gradually increased from 4 a.u. to 6 a.u.; between the 8th and 12th weeks, the intensity ranged from 6 a.u. to 7 a.u.; and from the 13th to the 16th week, the intensity fluctuated between 5 a.u. and 8 a.u. This protocol was based on/adapted from the interventions with post-COVID-19 patients conducted earlier by Sordi et al. [5]. About aerobic exercise, 1 set of 5 and 1 set of 10 min was performed on weeks 5 to 8; 1 set of 10 and 1 set of 15 min was performed on weeks 9 to 12; and finally, on weeks 13 to 16, 1 set of 10 and 1 set of 20 min was performed. The training was carried out with resistance exercises focused on large muscle groups and cardiorespiratory fitness, which were performed on a treadmill, vertical/horizontal bicycle, or rowing ergometer, according to the preference and physical condition of the patients. The rest interval between exercises varies for each participant, depending on their physical condition. The rest intervals were individualized based on each participant’s readiness to perform the exercise. As a result, rest periods were self-controlled and tailored to each participant. After each exercise and set, participants were asked if they were ready to continue, with rest times varying between 45 and 90 s. Due to restrictions imposed by the COVID-19 pandemic and/or the risk of infections, it was decided to conduct physical activities only twice a week to minimize the exposure of participants and study staff to potential risks of contracting SARS-CoV-2.
Consequently, the exercise sessions were designed at low volume and frequency and performed twice weekly. This approach has shown therapeutic benefits across various chronic diseases, as evidenced by Pedersen and Saltin’s review [35]. It has been effective in improving symptoms, functional capacity, and quality of life in patients with psychiatric, neurological, metabolic, cardiovascular, pulmonary, and musculoskeletal disorders and cancer. In addition, ten percent of severe/critical COVID-19 patients had their warm-up routines adapted to include coordination and balance activities. The activities performed were standing on one leg, swinging the legs, raising the legs backward, raising the arms, standing on tiptoes, walking in a straight line, sitting down and standing up from a chair, jumping over objects, using a Pilates ball, walking on a slackline, and standing on a Bosu ball—after that, they performed the physical exercises conforming to Table 3. Table 3 presents the training program performed by the experimental groups during the 16 weeks of multi-professional intervention.

Table 3.
Physical exercise program for mild, moderate, and severe/critical COVID-19 survivors.
2.8. Statistical Analysis
The statistical analyses were conducted using the SPSS 24 software version (IBM, Armonk, NY, USA). Data are presented as the mean ± standard deviation (SD) and 95% confidence interval (95% CI). Data normality was tested using the skewness–kurtosis test, considering values from 2 to −2 to indicate a need for parametric statistical analyses. The main effect and interaction between groups and time were performed via a two-way mixed-measure ANOVA (for repeated measures). Bonferroni’s post hoc test was used when a significant difference was found. The significance level established for all tests was p < 0.05. The partial eta square (η2) was calculated according to the classification by Richardson [36] using the following interpretation scale: 0.0099 [small], 0.0588 [moderate], and 0.1379 [large]. Cohen’s (d) was also calculated for effect size using the following rating: 0.20 [small], 0.80 [moderate], and >0.80 [large] [37].
3. Results
Table 4 shows the clinical characteristics of the participants stratified by COVID-19 symptoms. No differences were observed for age, sex, BMI, resting heart rate, SBP, BPD, and %SpO2 (p > 0.05 for all comparisons). Regarding the persistent symptoms self-reported by COVID-19 patients, a memory deficit (mild: 71.0%; moderate: 69.2%; severe/critical: 60.0%), fatigue (mild: 41.9%; moderate: 53.8%; severe/critical: 46.7%), and muscle pain (mild: 32.3%; moderate: 46.2%; severe/critical: 53.3%) were more prevalent. However, there was no significant difference between the groups for a memory deficit, fatigue, and muscle pain (p > 0.05 for all comparisons). Regarding the participants’ medical history, a difference was only observed for heart disease (mild: 19.4%; moderate: 0%; severe/critical: 40.0%; with higher values for the severe/critical group, p = 0.03). In addition, no differences were detected for the other clinical characteristics (self-reported post-COVID-19 symptoms and physical activity: p > 0.05). In addition to the results presented in the article, Supplementary Tables were created to present data by sex: anthropometry, body composition, physical tests and biochemical variables for men and women, without considering the symptoms of COVID-19 and tables making the distinction by sex, considering symptoms of COVID-19).

Table 4.
Clinical characteristics of patients of three COVID-19 survivors intervention groups.
3.1. Anthropometric and Body Composition Measurements
A time effect was observed for the abdominal circumference (F = 5.08; p = 0.01; η2p = 0.08—moderate effect), with a significant reduction after 8 weeks (p = 0.01) and no significant changes after 16 weeks (p > 0.05). No time effects were observed for the weight, BMI, fat-free mass, musculoskeletal mass, fat mass, and body fat percentage (p > 0.05). A group effect was observed for fat mass (F = 3.91; p = 0.03; η2p = 0.12—moderate effect), with significantly higher values for the severe/critical group compared to the mild group (p = 0.02), and for the body fat percentage (F = 4.54; p = 0.02; η2p = 0.14—large effect), with significantly higher values for the severe group when compared to the mild group (p = 0.04). No group effects were observed for weight, BMI, and abdominal circumference (p > 0.05). None of the variables showed an interaction effect between the group and time measurements (p > 0.05).
Table 5 presents the anthropometry and body composition pre-test after 8 and 16 weeks of intervention.

Table 5.
Anthropometry and body composition response pre-test after 8 and 16 weeks of intervention in the three COVID-19 survivors’ groups.
3.2. Health-Related Physical Fitness Tests Responses
A time effect was observed for all tests: MIHS of the right side (F = 14.00; p < 0.001; η2p = 0.22—large effect), with a significant increase after 8 weeks (p = 0.01); MIHS of the left side (F = 10.34; p < 0.001; η2p = 0.17—large effect), with a significant increase after 8 weeks (p = 0.01); flexibility (F = 25.43; p < 0.001; η2p = 0.35—large effect), with a significant increase after 8 weeks (p = 0.00) and 16 weeks (p < 0.001); MILTS (F = 4.90; p = 0.01; η2p = 0.09—moderate effect), with a significant increase after 8 weeks (p = 0.029); the push-up test (F = 18.15; p < 0.001; η2p = 0.28—large effect), with a significant increase after 8 weeks (p < 0.001); abdominal strength–endurance (F = 19.54; p < 0.001; η2p = 0.30—large effect), with a significant increase after 8 weeks (p < 0.001); and the sit-stand test (F = 17.78; p < 0.001; η2p = 0.26—large effect), with a significant increase after 8 weeks (p = 0.00). None of the variables showed differences between the groups or interaction effects between the group and time measurements (p > 0.05).
Table 6 also shows the evolution of the cardiorespiratory fitness parameters assessed in the 6MWT during the intervention period. A time effect was observed for the VO2peak (F = 10.94; p < 0.001; η2p = 0.17—large effect), with a significant increase after 8 weeks (p = 0.00); distance covered (F = 16.35; p < 0.001; η2p = 0.25—large effect), with a significant increase after 8 weeks (p < 0.001); pre-test DBP (F = 12.23; p < 0.001; η2p = 0.20—large effect), with a significant reduction after 16 weeks (p < 0.001); and final DBP (F = 16.02; p < 0.001; η2p = 0.24—large effect), with a significant reduction after 16 weeks (p < 0.001). None of the variables showed significant differences between the groups, and the interaction effect between the group and time was insignificant (p > 0.05).

Table 6.
Physical and cardiorespiratory fitness responses pre-test and after 8 and 16 weeks of intervention in the three COVID-19 survivors’ groups.
Table 6 shows the physical and cardiorespiratory tests pre-test and after 8 and 16 weeks of intervention.
3.3. Biochemical Parameters
A time effect was observed for the total cholesterol (F = 12.17; p < 0.001; η2p = 0.18—large effect), with a significant reduction after 8 weeks (p < 0.001); LDL-c (F = 18.62; p < 0.001; η2p = 0.25—large effect), with a significant reduction after 8 weeks (p < 0.001); HbA1c (F = 11.71; p < 0.001; η2p = 0.19—large effect), with a significant reduction after 8 weeks (p = 0.03); urea (F = 3.77; p = 0.03; η2p = 0.07—moderate effect), with a significant reduction after 8 weeks (p = 0.04); gamma-glutamyl transferase (F = 7.28; p = 0.001; η2p = 0.12—moderate effect), with a significant reduction after 16 weeks (p = 0.00); lipase (F = 4.47; p = 0.01; η2p = 0.08—moderate effect), with a significant reduction after 16 weeks (p = 0.00); and magnesium (F = 10.30; p < 0.001; η2p = 0.19—large effect), with a significant reduction after 16 weeks (p < 0.001).
Group effects were observed for creatinine (F = 3.18; p = 0.04; η2p = 0.11—moderate effect), which were significantly higher in the severe/critical group when compared to the moderate group (p = 0.04), and CRP (F = 3.46; p = 0.01; η2p = 0.19—large effect), which were significantly higher in the severe/critical group when compared to the mild group (p = 0.01). None of the variables showed an interaction effect between the group and time measurements (p > 0.05).
Table 7 shows the responses of biochemical parameters pre-test and after 8 and 16 weeks of intervention.

Table 7.
Biochemical parameter responses pre-test and after 8 and 16 weeks of intervention in the three COVID-19 survivors’ groups.
4. Discussion
This study aimed to analyze the effects of multi-professional interventions on health-related physical fitness and biochemical parameters in overweight and obese COVID-19 survivors after 16 weeks of discharge from COVID-19 at different degrees of impairment. The following outcomes were observed: (i) 8 weeks of intervention showed improvements in MIHS values for the right and left sides, MILTS, push-ups, abdominal strength–endurance repetitions, sit and stand test, VO2peak, and distance covered in the 6MWT; (ii) increased flexibility values after 8 and 16 weeks; (iii) the pre-test and final DBP showed a significant reduction after 16 weeks and an abdominal circumference after 8 weeks; (iv) the severe group showed an increase in fat mass values and body fat percentage compared to the mild group; (v) 8 weeks showed improvements in the total cholesterol, LDL-c, HbA1c, urea, and in GGT, lipase, and magnesium reduction values after 16 weeks; (vi) the severe group showed an increase in creatinine values compared to the moderate group; (vii) CRP had a significant increase in the severe/critical group compared to the mild group; and (viii) it was observed that the 8- and 16-week interventions showed significant improvements in the body composition parameters, physical fitness, and biomarkers.
In contrast, no significant differences were detected in body mass, BMI, final HR, pre-test SBP, final SBP, HDL-c, TGL, ALT, AST, ALP, albumin, amylase, calcium, and phosphorus. These differences were also not found when investigating intergroup differences (time effect) and the degree of COVID-19 impairment (group effect). There was no significant difference in the BMI between the different groups, although there was a significant difference in the fat mass and body fat percentage, with lower values for the mild group. In addition, no significant differences were observed between the self-reported symptoms of COVID-19 in different degrees; these responses were observed in previous studies that analyzed possible differences under mild, moderate, and severe/critical symptoms in COVID-19 survivors [5,6]. These factors reinforce that some sequels are independent of the disease severity [5,6].
To analyze the outcome of COVID-19, all anthropometric and body composition parameters must be analyzed, not just the BMI alone [6,7]. It is known that excess body fat promotes the secretion of pro-inflammatory mediators, with a consequent reduction in the immune response [10]. Therefore, it is possible to state that being overweight and obese significantly worsens the symptoms of COVID-19 [5,6,7]. A previous study reported that patients with a severe/critical form of COVID-19 showed higher values of fat mass and a body fat percentage than those with a mild form of COVID-19 with the same BMI [7]. Fat mass was significantly higher in severe/critical COVID-19 compared to the mild group; similar data were found by Perli et al. [6] when comparing the different symptoms of the disease. The authors also observed that this difference persisted 1 year after the disease. In this line, regular physical exercise can help control these parameters and promote a better immunological response against COVID-19 infection [38], regardless of the symptoms of the disease. This study did not collect food records before or after the multi-professional interventions. Therefore, it is impossible to establish a relationship between the participant’s body composition and food intake.
People who develop the severe form of COVID-19 need long periods of hospitalization, associated with the frequent use of corticosteroids, the use of prolonged mechanical ventilation, and the use of neuromuscular blockers, causing direct impacts on the musculoskeletal system after hospitalization [39]. The practice of physical activity is considered one of the main components of a healthy life, promoting the prevention of overweight, systemic inflammation, and transmissible viral diseases, proving to be an effective therapeutic strategy to reduce a series of metabolic disorders, thus reducing the effects against the “cytokine storm” reported in patients with COVID-19 [38,39,40]. Regarding health-related physical fitness tests, significant improvements in hand pressure strength were observed for the mild, moderate, and severe/critical COVID-19 groups after 8 weeks of intervention, corroborating the findings of Everaerts et al. [41], who found an improvement in MISH after 16 weeks of post-hospital discharge intervention. The 30 s sit-up and chair–stand test for the lower limbs also showed improvement after 8 weeks of intervention for all COVID-19 groups, reinforcing the findings of Li et al. [42] after 8 and 9 weeks of tele-exercises. MILTS showed an improvement in the time effect after 8 weeks (pre-test vs. post-8w) in the mild, moderate, and severe/critical COVID-19 groups; the present study partially corroborates the results of Sordi et al. [5], showing improvement only in the moderate COVID-19 group.
Cardiorespiratory health was assessed using VO2peak, HR, SpO2, and blood pressure to verify the physical capacity, effort tolerance, and possible cardiopulmonary changes [5,27]. The VO2peak of the mild, moderate, and severe/critical COVID-19 groups after 8 weeks was higher when compared to after 16 weeks of intervention, in line with Rinaldo et al. [43], showing improvement in the VO2peak in response to concurrent training after hospital discharge for COVID-19. No significant improvement was detected in SpO2. This finding does not corroborate those of Lemos et al. [7], who found an improvement in SpO2 values due to the impact of this disease.
Some biochemical analyses did not show significant changes after the intervention: HDL-c, TGL, ALT, AST, ALP, albumin, amylase, calcium, and phosphorus. However, the patient’s biochemical analyses were among normative values at pre-intervention [42,43,44]. Significant changes were verified after intervention for total cholesterol, LDL-c, HbA1c, urea, creatinine, GGT, lipase, magnesium, and CRP. It was observed that dyslipidemia is a risk factor for severe manifestations of COVID-19 [45]. It is known that the lipid profile can change viral infections due to the neutralizing role of lipoproteins, protecting the host [42]; however, in patients with COVID-19, this protection does not appear to occur. After the interventions, there was a significant reduction in the total cholesterol and LDL-c levels after 8 weeks in all groups (mild, moderate, and severe/critical) and an increase in HDL-c levels in the mild and moderate groups compared to the 16 weeks of intervention. This study corroborates other studies that show that the decrease in HDL-c levels correlates with the severity of COVID-19 cases [45,46,47].
Another risk factor for COVID-19 is the increase in serum HbA1c levels. Diabetic individuals are at an increased risk for several infections, including more severe cases of COVID-19 [47,48,49]. A high level of glucose in the bloodstream facilitates the hyper inflammation observed in the cytokine storm [50,51], and the SARS-CoV-2 virus can also cause damage to the pancreatic islets, which are responsible for glucose regulation [50,51]. In the present study, HbA1c levels in the bloodstream were higher in persons with severe COVID-19 even after 16 weeks of intervention; therefore, people with increased blood glucose levels are more likely to progress to severe cases.
Due to the presence of the angiotensin-converting enzyme 2 (ACE2) receptor in several organs, liver dysfunction resulting from COVID-19 may be related to severe infection [49,50,51,52]. Chen et al. [53] observed that ALT, AST, total bilirubin, ALP, and GGT concentrations were higher in deceased persons than in those recovered from COVID-19. Hepatocyte steatosis is derived from the accumulation of lipids in hepatocytes, altering serum levels of triglycerides and HDL-c, and is considered one of the most common causes of chronic liver disease in adults [54]; therefore, interventions with physical exercise are necessary for a better prognosis of HS. GGT levels in this study were reduced only after 16 weeks of intervention in the mild, moderate, and severe/critical COVID-19 groups, showing that just eight weeks is insufficient to improve liver parameters.
Studies on COVID-19 indicate that electrolyte abnormalities, including sodium, potassium, chloride, and calcium abnormalities, are also associated with disease severity in persons with COVID-19 [54,55,56], presenting an association where patients with more severe COVID-19 tend to present hypocalcemia compared to those with less severe forms of the disease [54]. Due to the severity of the disease, many people tend to remain hospitalized for longer, resulting in a loss of muscle mass and bone mineral mass; in females, this process accelerates after menopause due to low estrogen production [56]. In this study, magnesium levels after interventions significantly improved in patients with mild, moderate, and severe/critical COVID-19 after 16 weeks.
There was no statistical difference in serum CRP levels, although there was a significant level reduction when comparing the three intervention moments. At the pre-intervention moment, a high concentration of CRP was found in the experimental groups about the reference values (>5.1 mg/dL), corroborating a previous study that revealed a high concentration of CRP in persons with severe COVID-19 due to the innate system deregulated by the presence of inflammatory cytokines [57,58]. The CRP concentration was reduced in the severe/critical group (pre-test vs. severe/critical) in response to physical exercise, approaching the reference values (<5.0 mg/dL). High levels in the bloodstream can be found in response to active infections or acute inflammatory processes [58,59], but high levels of this marker have been associated with obesity, as, in these persons, the inflammatory response can be precise [58]. Considering the aspects listed, multi-professional interventions that aim to recover the health conditions of overweight and obese people are relevant for promoting health in this significant portion of the Brazilian population, which already has a prevalence of overweight of 61.4% and obesity of 24.3% in people aged 18 or over. Public policies must guide change by integrating multi-professional teams to promote a healthy lifestyle for the better rehabilitation of COVID-19 survivors [5,60].
The absence of significant differences after 16 weeks in the general variables investigated in this research could be related to a lower volume and frequency of physical exercise, i.e., twice a week. The primary physical training adaptations during the first weeks (8 and 12) are related to neural adaptations with subsequent plateaus [61]. Thus, the improvement in the MIHS, MILTS, push-ups, abdominal strength–endurance, and sit-and-reach tests could be explained by neural adaptations after 8 weeks of physical exercise [61]. Considering muscle hypertrophy, the lack of significant differences in skeletal muscle mass and fat-free mass is probably related to a lower volume and lower frequency of strength training [62], in which the main muscle groups must be trained at least twice a week to maximize muscle growth, within a volume and intensity suitable for each person. Detrained people expend more energy doing physical exercises than trained people [63]. Therefore, when detrained people start physical exercises, energy expenditure could be higher in the first weeks, but with time, the expenditure tends to reduce [63]. Given this, the stabilization of the fat mass and body fat percentage after 16 weeks may be justified by a body adaptation [62] or a lower level of manipulation in the volume, frequency, and intensity of the concurrent training [61]. However, considering the health status of the study participants, we were cautious about manipulating some aspects linked to physical exercise.
After 16 weeks, a relevant aspect occurred with the three experimental groups. SBP and DBP were significantly reduced at rest. Pescatello et al. [64] pointed out that chronic exercise may reduce the BP around ~5–7 mmHg with the following mechanisms: a decrease in the cardiac output and/or total peripheral resistance, less sympathetic neural influence, greater local vasodilator influence, higher lumen diameter, and bigger distensibility of the vasculature are structural adaptations to physical training promoting lower peripheral resistance, as well as genetic factors.
Eight limitations can be highlighted in this study: (i) a loss of follow-up between groups because participants did not return for the final assessments; (ii) the application of the food record before the intervention, after 8 and 16 weeks; (iii) the absence of other biochemical measures, such as pro-inflammatory cytokines and anti-inflammatory cytokines, coagulation factors, and cardiac markers; (iv) the monitoring of the body composition and biomarkers after 16 weeks of this study to verify whether changes in eating habits and physical activity were persistent; (v) to perform fine-tuning in the volume, intensity, and frequency of physical exercise for study participants; (vi) including normal-weight participants to investigate these same relationships in a “healthy” comparison group; (vii) difficulty in composing a control group, as many persons were asymptomatic (another problem in recruiting a control group at this stage of COVID-19 in Brazil refers to providing care to the control group since these people could contract SARS-CoV-2 at any time, and another point would be not to assist survivors of COVID -19, as this would be unethical and could promote worse effects on the biopsychosocial health of these survivors); and (viii) include normal-weight participants to investigate these same relationships in a “healthy” comparison group.
Another point that needs to be considered is dropouts (health problems; n = 9) from the present study related to low %SpO2 during physical exercise and at rest and medical advice for suspending practical activities. This study highlights the importance of a 16-week multi-professional intervention for addressing COVID-19 sequelae in overweight or obese individuals, following WHO guidelines [16]. It emphasizes the role of concurrent training combined with a team of physicians, nutritionists, exercise physiologists, psychologists, physiotherapists, and biomedical professionals in aiding the recovery and resumption of daily activities. The study suggests that future research should focus on long-term monitoring, differences in response by sex and age, and exploring various training combinations (aerobic vs. resistance) to understand pathophysiological responses better. Additionally, including a control group without the disease could enhance the effectiveness of rehabilitation strategies.
The severity of COVID-19 symptoms directly influences the progression of physical exercise programs, necessitating individualized training based on initial evaluations. Professionals must conduct comprehensive assessments, including physical fitness and recent blood results, to devise effective strategies for improving quality of life. Addressing individual needs and sequelae is crucial for recovering health-related physical fitness in COVID-19 survivors. Continuous monitoring and follow-up are essential and urgent. This study underscores the importance of developing strategies to improve health conditions through physical activity, nutrition, and psychoeducation, aiming for comprehensive care and support for COVID-19 survivors.
5. Conclusions
This study concludes that an 8-week intervention significantly improved the anthropometrics, body composition, and physical fitness and influenced reductions in the lipid profile and glycated hemoglobin. After 16 weeks, these variables stabilized, indicating lasting benefits. The multi-professional intervention model proved beneficial for post-COVID-19 patients, regardless of the symptom severity. The study’s clinical trial design, incorporating patients with varying COVID-19 symptoms, enhances the validity and broadens the understanding of a multi-professional program’s effects. This approach combines nutritional education to promote healthy eating, psychoeducation for knowledge and behavior changes, and biochemical tests to assess the impacts on overweight and obese individuals.
6. Practical Applications
To thoroughly assess various health indicators—such as health-related physical fitness, vital signs, nutritional aspects, biochemical markers, and mental health—and to conduct a detailed medical history is essential for understanding the potential persistent sequelae of COVID-19. This comprehensive evaluation is crucial for guiding health recovery efforts for these patients. Given the heterogeneous nature of complaints and symptoms, a skilled team’s individualized direction of multi-professional interventions is indispensable for recovering post-COVID-19 patients. Prolonged interventions exceeding 16 weeks are recommended to improve various biopsychosocial aspects of these patients. Furthermore, periodic evaluations can more accurately guide rehabilitation programs for post-COVID patients. A significant portion of patients with post-COVID syndrome continue to experience physical and psychological limitations that hinder their ability to perform daily and occupational activities. Therefore, promoting multidisciplinary interventions is imperative and urgent for a substantial population affected by COVID-19.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare12202034/s1, In addition to the results presented in the article, supplementary tables were created to present data by sex: anthropometry, body composition, physical tests and biochemical variables for men and women, without considering the symptoms of COVID-19 and tables making the distinction by sex, considering symptoms of COVID-19). Supplementary Table S1 refers to anthropometry and body composition responses pre-test, after 8, and after 16 weeks of intervention in the three COVID-19 survivors’ groups per sex (males and females), supplementary Table S2 refers to physical and cardiorespiratory fitness responses pre-test, after 8 and 16 weeks of intervention in the three COVID-19 survivors’ groups per sex (males and females), supplementary Table S3 refers to biochemical parameters responses pre-test, after 8, and after 16 weeks of intervention in the three COVID-19 survivors’ groups per sex (males and females), supplementary Table S4 refers to anthropometry and body composition responses pre-test, after 8, and after 16 weeks of intervention in the three COVID-19 male survivors’ groups considering the symptoms (mild, moderate, and severe/critical), supplementary Table S5 refers to anthropometry and body composition responses pre-test, after 8, and after 16 weeks of intervention in the three COVID-19 female survivors’ groups considering the symptoms (mild, moderate, and severe/critical), supplementary Table S6 refers to physical and cardiorespiratory fitness responses pre-test, after 8 and 16 weeks of intervention in the three COVID-19 male survivors’ groups considering the symptoms (mild, moderate, and severe/critical), supplementary Table S7 refers to Physical and cardiorespiratory fitness responses pre-test, after 8 and 16 weeks of intervention in the three COVID-19 female survivors’ groups considering the symptoms (mild, moderate, and severe/critical), supplementary Table S8 refers to biochemical parameters responses pre-test, after 8, and after 16 weeks of intervention in the three male COVID-19 survivors’ groups and supplementary Table S9 refers to biochemical parameters responses pre-test, after 8, and after 16 weeks of intervention in the three female COVID-19 survivors’ groups.
Author Contributions
Conceptualization, M.P.d.P.S.-L., D.C.d.S.M., J.J.R., M.G.d.S.M., V.A.S.P. and B.H.M.B.; methodology, M.P.d.P.S.-L., D.C.d.S.M., J.J.R., M.G.d.S.M., A.F.S. and B.H.M.B.; software, M.P.d.P.S.-L., V.A.S.P. and B.H.M.B.; validation, M.P.d.P.S.-L., S.M.F.d.M., P.V.-B. and B.H.M.B.; formal analysis, M.P.d.P.S.-L., V.A.S.P. and BHM, investigation, M.P.d.P.S.-L., D.C.d.S.M., J.J.R., M.G.d.S.M., V.A.S.P., A.F.S. S.M.F.d.M., P.V.-B. and B.H.M.B.; resources, M.P.d.P.S.-L. and B.H.M.B.; data curation, M.P.d.P.S.-L., writing—original draft preparation, M.P.d.P.S.-L., D.C.d.S.M., J.J.R., M.G.d.S.M., V.A.S.P. and B.H.M.B.; writing—review and editing, M.P.d.P.S.-L., S.M.F.d.M., P.V.-B., L.V.A. and B.H.M.B.; visualization, M.P.d.P.S.-L.; supervision, M.P.d.P.S.-L., S.M.F.d.M., P.V.-B., L.V.A. and B.H.M.B.; project administration, B.H.M.B.; funding acquisition, L.V.A. and B.H.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study received financial support from “Fundação Araucária”—means by “Programa de Pesquisa para o Sistema Único de Saúde (PPSUS)” under number 2020/2021 and Cesumar Institute of Science, Technology, and Innovation (ICETI) of Cesumar University (UniCesumar), under number 2024.
Institutional Review Board Statement
This study was conducted following the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the Committee of Ethics in Research of the UniCesumar, who approved this study through opinion number 4.546.726/20211, 18 February 2021. After approval by the committee, the project was registered with the Brazilian Clinical Trials Registry Platform (ReBEC) under the number RBR-4mxg57b.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data generated during the study will be informed when requested.
Acknowledgments
The authors thank the project participants, the members of the laboratory, and especially the authors who worked to make the completion of this project possible. L.V.A. thanks the National Council for Scientific and Technological Development (CNPq) for the research scholarship. All individuals included in this section consented to the acknowledgement.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Centers for Disease Control and Prevention (CDC). Post-COVID Conditions: An Overview for Healthcare Providers. Available online: https://archive.cdc.gov/#/details?archive_url=https://archive.cdc.gov/www_cdc_gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-science.html (accessed on 18 May 2024).
- De Miranda, D.A.P.; Gomes, S.V.C.; Filgueiras, P.S.; Corsini, C.A.; Almeida, N.B.F.; Silva, R.A.; Medeiros, M.I.V.A.R.C.; Vilela, R.V.R.; Fernandes, G.R.; Grenfell, R.F.Q. Long COVID-19 syndrome: A 14-months longitudinal study during the two first epidemic peaks in Southeast Brazil. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 1007–1014. [Google Scholar] [CrossRef]
- Mainous, A.G., 3rd; Rooks, B.J.; Wu, V.; Orlando, F.A. COVID-19 Post-acute Sequelae Among Adults: 12 Month Mortality Risk. Front. Med. 2021, 8, 778434. [Google Scholar] [CrossRef]
- Landi, F.; Barillaro, C.; Bellieni, A.; Brandi, V.; Carfì, A.; D’Angelo, M.; Fusco, D.; Landi, G.; Lo Monaco, R.; Martone, A.M.; et al. The New Challenge of Geriatrics: Saving Frail Older People from the SARS-COV-2 Pandemic Infection. J. Nutr. Health Aging 2020, 24, 466–470. [Google Scholar] [CrossRef]
- Sordi, A.F.; Lemos, M.M.; De Souza Marques, D.C.; Ryal, J.J.; Priscila de Paula Silva Lalucci, M.; Marques, M.G.; Amaro Camilo, M.L.; De Paula Ramos, S.; Franzói De Moraes, S.M.; Valdés-Badilla, P.; et al. Effects of a multi-professional intervention on body composition, physical fitness and biochemical markers in overweight COVID-19 survivors: A clinical trial. Front. Physiol. 2023, 14, 1219252. [Google Scholar] [CrossRef]
- Perli, V.A.S.; Sordi, A.F.; Lemos, M.M.; Fernandes, J.S.A.; Capucho, V.B.N.; Silva, B.F.; De Paula Ramos, S.; Valdés-Badilla, P.; Mota, J.; Branco, B.H.M. Body composition and cardiorespiratory fitness of overweight COVID-19 survivors in different severity degrees: A cohort study. Sci. Rep. 2023, 13, 17615. [Google Scholar] [CrossRef]
- Lemos, M.M.; Cavalini, G.R.; Pugliese Henrique, C.R.; Perli, V.A.S.; De Moraes Marchiori, G.; Marchiori, L.L.M.; Sordi, A.F.; Franzói de Moraes, S.M.; De Paula Ramos, S.; Valdés-Badilla, P.; et al. Body composition and cardiorespiratory fitness in overweight or obese people post COVID-19: A comparative study. Front. Physiol. 2022, 13, 949351. [Google Scholar] [CrossRef]
- Ryal, J.J.; Perli, V.A.S.; Marques, D.C.S.; Sordi, A.F.; Marques, M.G.S.; Camilo, M.L.; Milani, R.G.; Mota, J.; Valdés-Badilla, P.; Magnani Branco, B.H. Effects of a Multi-Professional Intervention on Mental Health of Middle-Aged Overweight Survivors of COVID-19: A Clinical Trial. Int. J. Environ. Res. Public Health 2023, 20, 4132. [Google Scholar] [CrossRef]
- Pranata, R.; Lim, M.A.; Yonas, E.; Vania, R.; Lukito, A.A.; Siswanto, B.B.; Meyer, M. Body mass index and outcome in patients with COVID-19: A dose-response meta-analysis. Diabetes Metab. 2021, 47, 101178. [Google Scholar] [CrossRef]
- Maffetone, P.B.; Laursen, P.B. The Perfect Storm: Coronavirus (COVID-19) Pandemic Meets Overfat Pandemic. Front. Public Health 2020, 8, 135. [Google Scholar] [CrossRef]
- Rottoli, M.; Bernante, P.; Belvedere, A.; Balsamo, F.; Garelli, S.; Giannella, M.; Cascavilla, A.; Tedeschi, S.; Ianniruberto, S.; Rosselli Del Turco, E.; et al. How important is obesity as a risk factor for respiratory failure, intensive care admission and death in hospitalised COVID-19 patients? Results from a single Italian centre. Eur. J. Endocrinol. 2020, 183, 389–397. [Google Scholar] [CrossRef]
- Gao, F.; Zheng, K.I.; Wang, X.B.; Sun, Q.F.; Pan, K.H.; Wang, T.Y.; Chen, Y.P.; Targher, G.; Byrne, C.D.; George, J.; et al. Obesity Is a Risk Factor for Greater COVID-19 Severity. Diabetes Care. 2020, 43, e72–e74. [Google Scholar] [CrossRef] [PubMed]
- Dalbosco-Salas, M.; Torres-Castro, R.; Rojas Leyton, A.; Morales Zapata, F.; Henríquez Salazar, E.; Espinoza Bastías, G.; Beltran Diaz, M.E.; Tapia Allers, K.; Mornhinweg Fonseca, D.; Vilaro, J. Effectiveness of a primary care telerehabilitation program for post-COVID-19 patients: A feasibility study. J. Clin. Med. 2021, 10, 4428. [Google Scholar] [CrossRef] [PubMed]
- Jimeno-Almazán, A.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Sánchez-Alcaraz, B.J.; Courel-Ibáñez, J.; Pallarés, J.G. Effects of a concurrent training, respiratory muscle exercise, and self-management recommendations on recovery from post-COVID-19 conditions: The RECOVE trial. J. Appl. Physiol. 2023, 134, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Barroso, W.K.S.; Rodrigues, C.I.S.; Bortolotto, L.A.; Mota-Gomes, M.A.; Brandão, A.A.; Feitosa, A.D.d.M.; Machado, C.A.; Poli-de-Figueiredo, C.E.; Amodeo, C.; Júnior, D.M.; et al. VIII Guideline on Arterial Hypertension—2020. Arq. Bras. Cardiol. 2021, 116, 516–658. [Google Scholar] [CrossRef]
- World Health Organization (WHO). COVID-19 Clinical Management: Living Guidance; WHO: Geneva, Switzerland, 2023; pp. 1–116. [Google Scholar]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. BMJ 2012, 340, c332. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation 2014, 129 (Suppl. S2), S102–S138. [Google Scholar] [CrossRef]
- Heyward, V. ASEP Methods recommendation: Body composition assessment. J. Exerc. Physiol. Online. 2001, 4, 1–12. [Google Scholar]
- Miller, R.M.; Chambers, T.L.; Burns, S.P. Validating InBody® 570 Multi-frequency Bioelectrical Impedance Analyzer versus DXA for Body Fat Percentage Analysis. J. Exerc. Physiol. Online 2016, 19, 71–78. [Google Scholar] [CrossRef]
- Branco, B.H.M.; Bernuci, M.P.; Marques, D.C.; Carvalho, I.Z.; Barrero, C.A.L.; De Oliveira, F.M.; Ladeia, G.F.; Júnior, N.N. Proposal of a normative table for body fat percentages of Brazilian young adults through bioimpedanciometry. J. Exerc. Rehabil. 2018, 14, 974–979. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Interference Testing in Clinical Chemistry. In Approved Guideline, 2nd ed.; CLSI document EP7-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2005; pp. 1–12. [Google Scholar]
- Wells, K.F.; Dillon, E.K. The sit and reach—A test of back and leg flexibility. Res. Q. Am. Assoc. Health Phys. Educ. Recreat. 1952, 23, 115–118. [Google Scholar] [CrossRef]
- Branco, B.H.M.; Andreato, L.V.; Ribeiro, E.D.; De Oliveira, H.G.; Almeida, F.N.; Nardo Junior, N. Correction to: Development of tables for classifying judo athletes according to maximal isometric strength and muscular power, and comparisons between athletes at different competitive levels. Sport Sci. Health. 2021, 17, 265–266. [Google Scholar] [CrossRef]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport. 1999, 70, 113–119. [Google Scholar] [CrossRef]
- Cuenca-Garcia, M.; Marin-Jimenez, N.; Perez-Bey, A.; Sánchez-Oliva, D.; Camiletti-Moiron, D.; Alvarez-Gallardo, I.C.; Ortega, F.B.; Castro-Piñero, J. Reliability of field-based fitness tests in adults: A systematic review. Sports Med. 2022, 52, 1961–1979. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Cahalin, L.P.; Mathier, M.A.; Semigran, M.J.; Dec, G.W.; DiSalvo, T.G. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest 1996, 110, 325–332. [Google Scholar] [CrossRef]
- Brazil. Food Guide for the Brazilian Population (Portuguese); Ministry of Health, Department of Primary Health Care: Brasília, Brazil, 2014; pp. 1–158. [Google Scholar]
- Authier, J. The psychoeducation model: Definition, contemporary roots and content. Can. Couns. 1997, 12, 15–22. [Google Scholar]
- Beck, J.S. Cognitive-Behavioral Therapy: Theory and Practice (Portuguese), 2nd ed.; Artmed: Porto Alegre, Portugal, 2013; pp. 1–384. [Google Scholar]
- Mccrorie, A.; Donnelly, C.; Mcglade, K. Infographics: Healthcare Communication for the Digital Age. Ulst. Med. J. 2016, 85, 71–75. [Google Scholar]
- De Oliveira, D.A.; Lessa, R.S.; Ribeiro, S.C.S.; De Vasconcelos, P.F. The Visual Practice: The Infographic as a Facilitating Tool for Learning in Medical School. Rev. Bras. Educ. Med. 2020, 44, e109. [Google Scholar] [CrossRef]
- McGuigan, M.R.; Foster, C. A New Approach to Monitoring Resistance Training. Strength Cond. J. 2004, 26, 42–47. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scan J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef]
- Richardson, J.T. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power for the Social Sciences, 2nd ed.; Laurence Erlbaum and Associates: Hillsdale, NJ, USA, 1988; pp. 1–400. [Google Scholar]
- Queiroz, C.O.; Conceição, A.F.; Aristides, P.R.S.; Alves, L.S.; Almeida, R.T. Physical Activity, Obesity, and COVID-19: What can we Expect from his Relationship? Int. J. Cardiovasc. Sci. 2022, 35, 123–126. [Google Scholar] [CrossRef]
- Núñez-Seisdedos, M.N.; Lázaro-Navas, I.; López-González, L.; López-Aguilera, L. Intensive Care Unit- Acquired Weakness and Hospital Functional Mobility Outcomes Following Invasive Mechanical Ventilation in Patients with COVID-19: A Single-Centre Prospective Cohort Study. J. Intensive Care Med. 2022, 37, 1005–1014. [Google Scholar] [CrossRef]
- Da Silveira, M.P.; Da Silva Fagundes, K.K.; Bizuti, M.R.; Starck, É.; Rossi, R.C.; De Resende, E.; Silva, D.T. Physical exercise as a tool to help the immune system against COVID-19: An integrative review of the current literature. Clin. Exp. Med. 2021, 21, 15–28. [Google Scholar] [CrossRef]
- Everaerts, S.; Heyns, A.; Langer, D.; Beyens, H.; Hermans, G.; Troosters, T.; Gosselink, R.; Lorent, N.; Janssens, W. COVID-19 recovery: Benefits of multi-professional respiratory rehabilitation. BMJ Open Respir. Res. 2021, 8, e000837. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, W.; Zhan, C.; Liu, S.; Yin, Z.; Wang, J.; Chong, Y.; Zheng, C.; Fang, X.; Cheng, W.; et al. A telerehabilitation programme in post-discharge COVID-19 patients (tereco): A randomised controlled trial. Thorax 2021, 77, 697–706. [Google Scholar] [CrossRef]
- Rinaldo, R.F.; Mondoni, M.; Parazzini, E.M.; Baccelli, A.; Pitari, F.; Brambilla, E.; Luraschi, S.; Balbi, M.; Guazzi, M.; Di Marco, F.; et al. Severity does not impact on exercise capacity in COVID-19 survivors. Respir. Med. 2021, 187, 106577. [Google Scholar] [CrossRef] [PubMed]
- Williamson, M.A.; Synder, L.M. Wallach’s Interpretation of Diagnostic Tests (Portuguese), 10th ed.; Guanabara Koogan: Rio de Janeiro, Brasil, 2015; pp. 1–1288. [Google Scholar]
- Wang, H.; Yuan, Z.; Pavel, M.A.; Jablonski, S.M.; Jablonski, J.; Hobson, R.; Valente, S.; Reddy, C.B.; Hansen, S.B. The role of high cholesterol in SARS-CoV-2 infectivity. J. Biol. Chem. 2023, 299, 104763. [Google Scholar] [CrossRef]
- Kimura, L.F.; Sant’Anna, M.B.; Andrade, S.A.; Ebram, M.C.; Lima, C.F.G.; Celano, R.M.G.; Viégas, R.F.M.; Picolo, G. COVID-19 induces proatherogenic alterations in moderate to severe non-comorbid patients: A single-center observational study. Blood Cells Mol. Dis. 2021, 92, 102604. [Google Scholar] [CrossRef]
- Masana, L.; Correig, E.; Ibarretxe, D.; Anoro, E.; Arroyo, J.A.; Jericó, C.; Guerrero, C.; Miret, M.; Näf, S.; Pardo, A.; et al. Low HDL and high triglycerides predict COVID-19 severity. Sci. Rep. 2021, 11, 7217. [Google Scholar] [CrossRef]
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Correia de Sá, T.; Soares, C.; Rocha, M. Acute pancreatitis and COVID-19: A literature review. World J. Gastrointest. Surg. 2021, 13, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Silva-Lalucci, M.P.P.; Souza-Marques, D.C.; Valdés-Badilla, P.; Andreato, L.V.; Branco, B.H.M. Obesity as a risk factor for complications and mortality in individuals with SARS-CoV-2: A systematic review. Nutrients 2024, 16, 543. [Google Scholar] [CrossRef] [PubMed]
- Logette, E.; Lorin, C.; Favreau, C.; Oshurko, E.; Coggan, J.S.; Casalegno, F.; Sy, M.F.; Monney, C.; Bertschy, M.; Delattre, E.; et al. A Machine-Generated View of the Role of Blood Glucose Levels in the Severity of COVID-19. Front. Public Health 2021, 9, 695139. [Google Scholar] [CrossRef] [PubMed]
- Leowattana, W. Angiotensin-converting enzyme 2 receptors, chronic liver diseases, common medications, and clinical outcomes in coronavirus disease 2019 patients. World J. Virol. 2021, 25, 86–96. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1295. [Google Scholar] [CrossRef]
- Pinto, C.G.S.; Marega, M.; De Carvalho, J.A.M.; Carmona, F.G.; Lopes, C.E.F.; Ceschini, F.L.; Bocalini, D.S.; Figueira Junior, A.J. Physical activity as a protective factor for development of non-alcoholic fatty liver in men. Einstein 2015, 13, 34–40. [Google Scholar] [CrossRef]
- Malieckal, D.A.; Uppal, N.N.; Ng, J.H.; Jhaveri, K.D.; Hirsch, J.S. Electrolyte abnormalities in patients hospitalized with COVID-19. Clin. Kidney J. 2021, 14, 1704–1707. [Google Scholar] [CrossRef]
- Qian, G.Q.; Yang, N.B.; Ding, F.; Ma, A.H.Y.; Wang, Z.Y.; Shen, Y.F.; Shi, C.W.; Lian, X.; Chu, J.G.; Chen, L.; et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: A retrospective, multi-centre case series. QJM Int. J. Med. 2020, 113, 474–481. [Google Scholar] [CrossRef]
- Wang, G.; Wu, C.; Zhang, Q.; Wu, F.; Yu, B.; Lv, J.; Li, Y.; Li, T.; Zhang, S.; Wu, C.; et al. C-Reactive Protein Level May Predict the Risk of COVID-19 Aggravation. Open Forum Infect. Dis. 2020, 7, ofaa153. [Google Scholar] [CrossRef]
- Aguiar, F.J.; Ferreira-Júnior, M.; Sales, M.M.; Cruz-Neto, L.M.; Fonseca, L.A.M.; Sumita, N.M.; Duarte, N.J.C.; Lichtenstein, A.; Duarte, A.J.S. C-reactive protein: Clinical applications and proposals for a rational use. Rev. Assoc. Med. Bras. 2013, 59, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Wagmacker, D.S.; Petto, J.; Silva, F.L.; dos Santos, A.C.N.; Ladeira, A.M.T. C-Reactive protein in the initial phase of postprandial lipemia in subjects with central obesity. Int. J. Cardiovasc. Sci. 2015, 28, 9–15. [Google Scholar] [CrossRef]
- Brazil. Vigitel Brasil 2023: Surveillance of Risk and Protective Factors for Chronic Diseases by Telephone Survey: Estimates of the Frequency and Sociodemographic Distribution of Risk and Protective Factors for Chronic Diseases in the Capitals of the 26 Brazilian States and the Federal District in 2023 (Portuguese); Ministry of Health, Secretariat of Health and Environmental Surveillance, Department of Epidemiological Analysis and Surveillance of Non-Communicable Diseases; Ministry of Health: Brasília, Brazil, 2023; pp. 1–133. [Google Scholar]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to Endurance and Strength training. Cold Spring Harb. Perspect. Med. 2018, 8, a029769. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Ogborn, D.; Krieger, J.W. Effects of Resistance Training Frequency on Measures of Muscle Hypertrophy: A Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 1689–1697. [Google Scholar] [CrossRef]
- Westerterp, K.R. Control of energy expenditure in humans. Eur. J. Clin. Nutr. 2017, 71, 340–344. [Google Scholar] [CrossRef]
- Pescatello, L.S.; Franklin, B.A.; Fagard, R.; Farquhar, W.B.; Kelley, G.A.; Ray, C.A.; American College of Sports Medicine. American College of Sports Medicine position stand. Exercise and Hypertension. Med. Sci. Sports Exerc. 2004, 36, 533–553. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).