Effectiveness of a Lifestyle Intervention in Patients with Type 2 Diabetes: The Physical Activity and Nutrition for Diabetes in Alberta (PANDA) Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
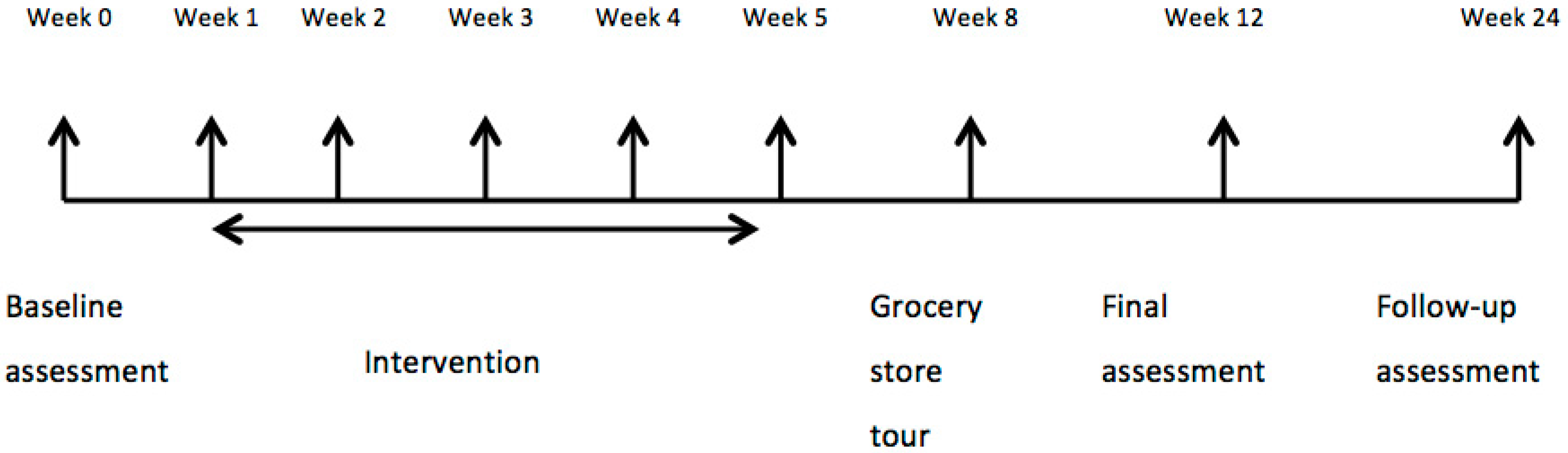
2.2. Study Design, Assessments, and Endpoints
2.3. Intervention
2.4. Statistical Analysis
3. Results
3.1. Study Participants at Baseline
3.2. Effect of PANDA–Nutrition Arm on A1c and Secondary Biological Outcomes
3.3. Effect of PANDA—Nutrition Arm on Dietary Adherence and Diet Quality at Three Months
3.4. Predictors of Changes in A1c and HEI Score
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- International Diabetes Federation. Diabetes Atlas, 6th ed. Available online: http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf (accessed on 21 May 2016).
- Public Health Agency of Canada. Diabetes in Canada: Facts and Figures from a Public Health Perspective. Available online: http://www.phac-aspc.gc.ca/cd-mc/publications/diabetes-diabete/facts-figures-faits-chiffres-2011/index-eng.php (accessed on 21 May 2016).
- Canadian Diabetes Association. An Economic Tsunami: The Cost of Diabetes in Canada 2009. Available online: https://www.diabetes.ca/CDA/media/documents/publications-and-newsletters/advocacy-reports/economic-tsunami-cost-of-diabetes-in-canada-english.pdf (accessed on 21 May 2016).
- Dworatzek, P.D.; Arcudi, K.; Gougeon, R.; Husein, H.; Sievenpiper, J.L.; Williams, S.L. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Nutrition therapy. Can. J. Diabetes 2013, 37, S45–S55. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerk, J.; Reick, K.; Meengs, L. Perceived barriers and effective strategies to diabetes self-management. J. Adv. Nurs. 2006, 54, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Assad, G.; Chan, C.B. Relationship of diet quality to food security and nutrition knowledge in low-income, community-dwelling elders with type 2 diabetes mellitus: A pilot study. Can. J. Diabetes 2012, 36, 310–313. [Google Scholar] [CrossRef]
- Jarvandi, S.; Gougeon, R.; Bader, A.; Dasgupta, K. Differences in food intake among obese and nonobese women and men with type 2 diabetes. J. Am. Coll. Nutr. 2011, 30, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Pareja, M.; Leon-Munoz, L.M.; Guallar-Castillon, M.; Graciani, A.; López-García, E.; Banegas, J.R.; Rodríguez-Artalejo, F. The diet of diabetic patients in Spain in 2008–2010: Accordance with the main dietary recommendations: A cross-sectional study. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Berry, T.R.; Anders, S.; Chan, C.B.; Bell, R.C. Communicating diabetes best practices to clients: A preliminary investigation of educator perspectives. Health Promot. Pract. 2012, 3, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Aitaoto, N.; Campo, S.; Snetselaar, L.G.; Janz, K.F.; Farris, K.B.; Parker, E.; Belyeu-Camacho, T.; Jimenez, R.P. Formative research to inform nutrition interventions in Chuuk and the US Pacific. J. Acad. Nutr. Diet. 2015, 6, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Zhang, A.; Chan, C. Acculturation, dietary acceptability, and diabetes management among Chinese in North America. Front. Endocrinol. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Berry, T.; Chan, C.; Bell, R.; Walker, J. Collective knowledge: Using a consensus conference approach to develop recommendations for physical activity and nutrition programs for persons with type 2 diabetes. Front. Endocrinol. 2012, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- US Aid. Food Security Indicators and Framework for Use in the Monitoring and Evaluation of Food Aid Programs 1999. Available online: http://pdf.usaid.gov/pdf_docs/PNACG170.pdf (accessed on 21 May 2016).
- Ross, T.; Geil, P. Healthy eating on a lean budget: Diabetes meals for less. Diabetes Spectr. 2010, 2, 120–123. [Google Scholar] [CrossRef]
- Cunningham, C.; Johnson, S.; Cowell, B.; Soroudi, N.; Isaacson, S.; Davis, N.; Wylie-Rosett, J. Menu plans in a diabetes self-management weight loss program. J. Nutr. Educ. Behav. 2006, 38, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.; Gougeon, R.; Joseph, L.; Da Costa, D.; Dasgupta, K. Nutritional education through internet-delivered menu plans among adults with type 2 diabetes mellitus: Pilot study. JMIR Res. Protoc. 2013, 2, e41–e46. [Google Scholar] [CrossRef] [PubMed]
- Soria-Contreras, D.; Bell, R.; McCargar, L.; Chan, C. Feasibility and efficacy of menu planning combined with individual counselling to improve health outcomes and dietary adherence in people with type 2 diabetes: A pilot study. Can. J. Diabetes 2014, 38, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Soria-Contreras, D.; Berry, T.R.; Chan, C.B. Using focus group interviews to develop a menu plan to improve dietary adherence in type 2 diabetes. Unpublished Work.
- Toobert, D.J.; Hampson, S.E.; Glasgow, R.E. The summary of diabetes self-care activities measure: Results from 7 studies and a revised scale. Diabetes Care 2000, 23, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Storey, K.; McCargar, L. Reliability and validity of Web-SPAN, a web-based method for assessing weight status, diet and physical activity in youth. J. Hum. Nutr. Diet. 2012, 25, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Nutrition Research Division, Health Canada. Canadian Nutrient File 2001b. Available online: http://www.hc-sc.gc.ca/fn-an/nutrition/fiche-nutri-data/index-eng.php (accessed on 21 May 2016).
- Willet, W. Nutritional Epidemiology, 2nd ed.; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Food and Nutrition Board, Institute of Medicine, National Academy of Sciences. Dietary Reference Intakes: Applications in Dietary Planning; National Academies Press: Washington, DC, USA, 2003.
- Tudor-Locke, C.; Bassett, D.R., Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sport. Med. 2004, 34, 1–8. [Google Scholar] [CrossRef]
- Black, A.E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake: Basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. 2000, 24, 1119–1130. [Google Scholar] [CrossRef]
- Garriguet, D. Diet quality in Canada. Health Rep. 2009, 2, 41–52. [Google Scholar]
- Asaad, G.; Sadegian, M.; Lau, R.; Xu, Y.; Soria-Contreras, D.C.; Bell, R.C.; Chan, B.C. The reliability and validity of the perceived dietary adherence questionnaire for people with type 2 diabetes. Nutrtients 2015, 7, 5484–5496. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A. Social Foundation of Thought and Action: A Social Cognitive Theory; Prentice Hall: Englewood Cliffs, NJ, USA, 1986. [Google Scholar]
- Health Canada. Eating Well with Canada’s Food Guide. Available online: http://www.hcsc.gc.ca/fn-an/food-guide-aliment/index-eng.php (accessed on 21 May 2016).
- The Comprehensive R Archive Network. Available online: https://cran.r-project.org/web/packages/Amelia/vignettes/amelia.pdf (accessed on 2 September 2016).
- Schwingshackl, L.; Hoffmann, G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension Score, and health outcomes: A systematic review and meta-analysis of cohort studies. J. Acad. Nutr. Diet. 2015, 5, 780–800. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.A.; Summerson, J.H.; Konen, J.C. Dietary intakes by levels of glycemic control for black and white adults with non-insulin dependent diabetes mellitus (NIDDM). J. Am. Coll. Nutr. 1995, 14, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; Noakes, M.; Clifton, P.M. Effect of energy restriction, weight loss, and diet composition on plasma lipids and glucose in patients with type 2 diabetes. Diabetes Care 1999, 22, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.J.; Tomuta, N.; Schechter, C.; Isasi, C.R.; Segal-Isaacson, C.J.; Stein, D.; Zonszein, J.; Wylie-Rosett, J. Comparative study of the effects of a 1-year dietary intervention of a low-carbohydrate diet versus a low-fat diet on weight and glycemic control in type 2 diabetes. Diabetes Care 2009, 7, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Olendzki, B.; Wedick, N.; Persuitte, G.; Culver, A.; Li, W.; Ma, Y. Challenges in sodium intake reduction and meal consumption patterns among participants with metabolic syndrome in a dietary trial. Nutr. J. 2013, 12, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, J.A.; Babyak, M.A.; Hinderliter, A.; Watkins, L.L.; Craighead, L.; Li, P.; Sherwood, A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: The ENCORE study. Arch. Intern. Med. 2010, 170, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, K.; Hajna, S.; Joseph, L.; Da Costa, D.; Christopoulos, S.; Gougeon, R. Effects of meal preparation training on body weight, glycemia, and blood pressure: Results of a phase 2 trial in type 2 diabetes. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pei, J.; Kuang, J.; Chen, H.; Chen, Z.; Li, Z.; Yang, H. Effect of lifestyle intervention in patients with type 2 diabetes: A meta-analysis. Metab. Clin. Exp. 2015, 64, 338–347. [Google Scholar] [CrossRef] [PubMed]
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar]
- UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet 1998, 352, 837–853. [Google Scholar]
- Elfhag, K.; Rössner, S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes. Rev. 2005, 6, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.; Lang, W.; Wadden, T.; Safford, M.; Knowler, W.; Bertoni, A.; Wagenknecht, L. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Heshka, S.; Kelley, D.E.; Thornton, J.; Boxt, L.; Pi-Sunyer, F.X.; Clark, J.M. Changes in adipose tissue depots and metabolic markers following a 1-year diet and exercise intervention in overweight and obese patients with type 2 diabetes. Diabetes Care 2014, 37, 3325–3332. [Google Scholar] [CrossRef] [PubMed]
- Khan, H. Clinical significance of HbA(1c) as a marker of circulating lipids in male and female type 2 diabetic patients. Acta Diabetol. 2007, 4, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Gatti, A.; Maranghi, M.; Bacci, S.; Carallo, C.; Gnasso, A.; Mandosi, E.; Filetti, S. Poor glycemic control is an independent risk factor for low HDL cholesterol in patients with type 2 diabetes. Diabetes Care 2009, 8, 1550–1552. [Google Scholar] [CrossRef] [PubMed]
- Waldman, B.; Jenkins, A.J.; Davis, T.E.; Taskinen, M.; Scott, R.; O’Connell, R.L.; Keech, A.C. HDL-C and HDL-C/ApoA-I predict long-term progression of glycemia in established type 2 diabetes. Diabetes Care 2014, 8, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yim, J.; Kim, Y.; Choue, R. Moderate diet-induced weight loss is associated with improved insulin sensitivity in middle-aged healthy obese Korean women. Nutr. Res. Pract. 2014, 4, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Rutti, S.; Ehses, J.; Sibler, R.; Prazak, R.; Rohrer, L.; Georgopoulos, S.; von Eckardstein, A. Low- and high-density lipoproteins modulate function, apoptosis, and proliferation of primary human and murine pancreatic beta-cells. Endocrinology 2009, 10, 4521–4530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dullaart, R.; Annema, W.; de Boer, J.; Tietge, U. Pancreatic β-cell function relates positively to HDL functionality in well-controlled type 2 diabetes mellitus. Atherosclerosis 2012, 2, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Drew, B.; Duffy, S.; Formosa, M.; Natoli, A.; Henstridge, D.; Penfold, S.; Kingwell, B. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation 2009, 15, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Waldman, B.; Jenkins, A.; Sullivan, D.; Ng, M.; Keechm, A. HDL as a target for glycemic control. Curr. Drug Target 2015, 16. [Google Scholar] [CrossRef]
- Maahs, D.M.; Daniels, S.R.; De Ferranti, S.D.; Dichek, H.L.; Flynn, J.; Goldstein, B.I.; Urbina, E. Cardiovascular disease risk factors in youth with diabetes mellitus: A scientific statement from the American Heart Association. Circulation 2014, 130, 1532–1558. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.B.; Bell, R.C. Food Accessibility. Unpublished Work.
- Asaad, G.; Bell, R.C.; Chan, C.B. Outcomes of the PANDA-Nutrition Arm Trial Assessed Using Per-Protocol Criteria. Unpublished work.
- Hébert, J.R.; Frongillo, E.A.; Adams, S.A.; Turner-McGrievy, G.M.; Hurley, T.G.; Miller, D.R.; Ockene, I.S. Perspective: Randomized controlled trials are not a panacea for diet-related research. Adv. Nutr. 2016, 7, 423–432. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total Cohort | Men | Women | p-Value * |
|---|---|---|---|---|
| (n = 73) | (n = 39) | (n = 34) | ||
| Demographic Variables | ||||
| Age (year ± SD) | 59.2 ± 9.7 | 59.0 ± 10.2 | 59.5 ± 9.1 | 0.846 |
| Ethnicity (%) | ||||
| White | 87.7 | 84.6 | 91.2 | 0.24 |
| Other | 12.3 | 15.4 | 8.8 | |
| Education (%) | ||||
| High school or less | 15 | 15.4 | 14.7 | 0.215 |
| More than high school | 85 | 84.6 | 85.3 | |
| Employment Status (%) | ||||
| Working | 56.2 | 56.4 | 55.9 | 0.81 |
| Other 1 | 43.8 | 43.6 | 44.1 | |
| Annual income (%) | ||||
| <$60,000 | 21.9 | 15.3 | 29.4 | 0.165 |
| >$60,000 | 78.1 | 84.7 | 70.6 | |
| Diabetes-Related Variables | ||||
| Duration of T2D (year ± SD) | 9.1 ± 8.3 | 10.8 ± 9.6 | 7.0 ± 5.8 | 0.049 |
| Diabetes treatment (%) | ||||
| Oral medication | 74 | 66.6 | 82.2 | 0.438 |
| Diet + exercise | 6.8 | 7.6 | 5.8 | |
| Insulin | 10.9 | 12.8 | 8.8 | |
| Combination 2 | 8.2 | 10.2 | 5.8 | |
| Additional medication (%) | ||||
| Anti-hypertensive drugs | 57.5 | 61.5 | 52.9 | 0.459 |
| Lipid-lowering drugs | 47.9 | 53.8 | 41.1 | 0.28 |
| A1c (% ± SD) | 8.0 ± 1.8 | 8.3 ± 1.7 | 7.7 ± 1.9 | 0.143 |
| Diabetes Self Efficacy Scale score (maximum 10) | 7.1 ± 1.5 | 7.5 ± 1.2 | 6.6 ± 1.8 | 0.012 |
| Anthropometric and Physical Activity Variables | ||||
| Weight (kg ± SD) | 96.4 ± 21.0 | 98.6 ± 20.8 | 93.8 ± 21.4 | 0.336 |
| Body mass index (kg/m2 ± SD) | 32.5 ± 6.8 | 31.3 ± 6.4 | 33.8 ± 7.1 | 0.117 |
| Waist circumference (cm ± SD) | 110.8 ± 16.8 | 112.2 ± 16.5 | 109.1 ± 17.2 | 0.336 |
| Fat mass (kg ± SD) | 40.0 ± 15.7 | 36.0 ± 14.4 | 44.6 ± 16.1 | 0.019 |
| Fat mass (% ± SD) | 40.4 ± 9.1 | 35.3 ± 7.6 | 46.2 ± 7.0 | <0.001 |
| Fat-free mass (kg ± SD) | 56.6 ± 10.5 | 62.9 ± 9.0 | 49.3 ± 6.9 | <0.001 |
| Fat-free mass (% ± SD) | 59.6 ± 9.1 | 64.7 ± 7.6 | 53.8 ± 7.0 | <0.001 |
| Physical activity (steps/day ± SD) | 5535 ± 3491 | 6722 ± 3829 | 4330 ± 2375 | 0.002 |
| Blood Pressure and Lipid Variables | ||||
| Systolic blood pressure (mmHg ± SD) | 128.5 ± 13.5 | 132.5 ± 15.0 | 124.0 ± 10.0 | 0.007 |
| Diastolic blood pressure (mmHg ± SD) | 78.6 ± 8.9 | 80.4 ± 10.2 | 76.5 ± 6.7 | 0.066 |
| Total cholesterol (mg/dL ± SD) | 328.7 ± 82.7 | 326.8 ± 77.8 | 330.9 ± 89.1 | 0.833 |
| HDL-cholesterol (mg/dL ± SD) | 57.6 ± 24.5 | 58.6 ± 25.5 | 56.5 ± 23.8 | 0.724 |
| LDL-cholesterol (mg/dL ± SD) | 243.9 ± 80.1 | 241.8 ± 76.8 | 246.3 ± 85.1 | 0.812 |
| Triglycerides (mg/dL ± SD) | 135.9 ± 73.5 | 132.0 ± 58.9 | 140.4 ± 88.2 | 0.632 |
| Nutrient Intake Variables | ||||
| Energy (kcal) | 2109 ± 721 | 2161 ± 598 | 2046 ± 845 | 0.494 |
| Energy under-reported (n (%)) 3 | 49 (67.1) | 29 (74.4) | 20 (58.8) | 0.803 |
| Energy acceptably reported (n (%)) | 18 (24.7) | 9 (23.1) | 8 (26.5) | |
| Energy over-reported (n (%)) | 6 (8.2) | 1 (2.6) | 5 (14.7) | |
| Total fat (g) | 86 ± 36 | 87 ± 35 | 84 ± 37 | 0.717 |
| Total fat (% of energy) | 36 ± 7 | 35 ± 7 | 36 ± 6 | 0.43 |
| Protein (g) | 99 ± 30 | 103 ± 30 | 94 ± 28 | 0.183 |
| Protein (% of energy) | 19 ± 4 | 19 ± 3 | 19 ± 4 | 0.812 |
| Carbohydrate (g) | 238 ± 93 | 241 ± 64 | 234 ± 118 | 0.752 |
| Carbohydrate (% of energy) | 45 ± 7 | 45 ± 6 | 45 ± 7 | 0.919 |
| Fibre (g) 4 | 22 ± 7 | 21 ± 7 | 21 ± 7 | 0.888 |
| Added sugar (g) | 50 ± 47 | 43 ± 24 | 56 ± 63 | 0.254 |
| Added sugar (% of energy) | 9 ± 5 | 8 ± 4 | 9 ± 6 | 0.123 |
| Saturated fat (g) | 28 ± 13 | 28 ± 11 | 27 ± 15 | 0.932 |
| Saturated fat (% of energy) | 12 ± 3 | 12 ± 3 | 11 ± 3 | 0.583 |
| MUFA (g) | 30 ± 13 | 29 ± 12 | 30 ± 14 | 0.889 |
| MUFA (% of energy) | 12 ± 3 | 11 ± 3 | 12 ± 3 | 0.105 |
| PUFA (g) | 15 ± 7 | 14 ± 7 | 15 ± 6 | 0.619 |
| PUFA (% of energy) | 6 ± 2 | 5 ± 2 | 7 ± 2 | 0.36 |
| Sodium (g) | 3.36 ± 1.56 | 3.57 ± 1.50 | 3.11 ± 1.60 | 0.217 |
| Sodium density (mg/1000 kcal) | 1.6 ± 0.5 | 1.6 ± 0.5 | 1.5 ± 0.4 | 0.284 |
| Diet Quality and Adherence Variables | ||||
| HEI score (maximum 100) | 68.7 ± 8.9 | 68.1 ± 8.1 | 69.3 ± 9.8 | 0.533 |
| PDAQ score (maximum 63) | 32.3 ± 11.3 | 32.9 ± 10.6 | 31.5 ± 12.1 | 0.611 |
| Outcome Variable | 3 Months | 6 Months | ||
|---|---|---|---|---|
| Mean Change | 95% CI | Mean Change | 95% CI | |
| Diabetes-related Variables 2 | ||||
| A1c (%) | −0.7 | (−1.0, −0.4) *** | −0.5 | (−0.9, −0.1) ** |
| Diabetes Self-Efficacy Scale (score) | 0.7 | (0.3, 1.0) ** | ND | ND |
| Anthropometric Variables and Physical Activity | ||||
| Weight (kg) | −1.7 | (−2.2, −1.2) *** | −1.4 | (−2.1, −0.8) *** |
| BMI (kg/m2) | −0.6 | (−0.8, −0.4) *** | −0.5 | (−0.7, −0.3) *** |
| Waist circumference (cm) | −2.4 | (−3.0, −1.8) *** | −2.4 | (−3.0, −1.8) *** |
| Fat mass (kg) | −1.2 | (−2.0, −0.4) ** | ND | ND |
| Fat mass (%) | −0.8 | (−1.5, 0.0) | ND | ND |
| Fat free mass (kg) | −0.8 | (−1.8, 0.1) | ND | ND |
| Fat free mass (%) | 0.8 | (0.1, 1.6) * | ND | ND |
| Physical activity (steps/day) | 995 | (368, 1623) ** | ND | ND |
| Blood Pressure and Lipids | ||||
| Systolic blood pressure (mm Hg) | −4.1 | (−6.8, −1.3) ** | ND | ND |
| Diastolic blood pressure (mm Hg) | −1.7 | (−3.1, −0.4) * | ND | ND |
| Total cholesterol (mg/dL) | −63.5 | (−80.1, −46.9) *** | −86.2 | (−107.3, −65.2) *** |
| HDL-cholesterol (mg/dL) | 27.5 | (20.2, 34.8) *** | 44.6 | (37.2, 52.0) *** |
| LDL-cholesterol (mg/dL) | −88.9 | (−105.3, −72.5) *** | −128.3 | (−148.5, −108.2) ** |
| Triglycerides (mg/dL) | −10.4 | (−23.1, 2.2) | −3.8 | (−20.8, 13.2) |
| Nutrient and Diet Score Variables | Mean Change | 95% CI |
|---|---|---|
| Nutrient Intake | ||
| Energy (kcal) | −178 | (−304, −51) ** |
| Total Fat (g) | −10.2 | (−17.6, −2.7) ** |
| Total Fat (%) | −1.1 | (−2.5, 0.4) |
| Protein (g) | −5.8 | (−11.1, −0.4) * |
| Protein (%) | 0.4 | (−0.4, 1.3) |
| Carbohydrate (g) | −11.8 | (−27, 3.5) |
| Carbohydrate (%) | 1.9 | (−0.2, 3.7) |
| Fiber (g) | 0.0 | (1.3, 0.0) |
| Added sugar (g) | −8.5 | (−16, −2.1) * |
| Added sugar (%) | −0.3 | (−1.4, 0.8) |
| Saturated fat (g) | −3.5 | (−6.3, −0.6) * |
| Saturated fat (%) | −0.4 | (−1.2, 0.4) |
| MUFA (g) | −2.7 | (−5.6, 2) |
| MUFA (%) | 0.1 | (−0.7, 1.0) |
| PUFA (g) | 0.1 | (−1.6, 1.8) |
| PUFA (%) | 0.8 | (0.1, 1.4) * |
| Sodium (g) | −0.57 | (−0.87, −0.28) *** |
| Sodium density (mg/kcal) | −0.14 | (−0.26, −0.03) * |
| Diet quality and adherence | ||
| Health Eating Index | ||
| Health Eating Index score (maximum 100) | 2.1 | (0.1, 4.1) * |
| Total fruits and vegetables (maximum 10) | 0.5 | (0.1, 0.9) * |
| Whole fruits (maximum 5) | 0.4 | (0.1, 0.7) * |
| Dark green/orange vegetables (maximum 5) | −0.1 | (−0.4, 0.3) |
| Total grains (maximum 5) | −0.3 | (−0.6, −0.2) * |
| Whole grains (maximum 5) | 0.3 | (−0.1, 0.7) |
| Dairy (maximum 10) | −0.2 | (−0.6, 0.3) |
| Meat/beans (maximum 10) | 0.2 | (−0.3, 0.6) |
| Unsaturated fat (maximum 10) | −0.1 | (−0.6, 0.4) |
| Saturated fat (maximum 10) | 0.9 | (0.1, 1.7) * |
| Sodium (maximum 10) | 1.1 | (0.4, 1.7) ** |
| Other (maximum 20) 1 | −0.1 | (−1.3,1.1) |
| Perceived Dietary Adherence | ||
| Perceived dietary adherence score (maximum 63) | 8.5 | (6.1, 10.8) *** |
| Model | Variables | Change in A1c (%) Per Unit Change Invariable of Interest | 95% CI |
|---|---|---|---|
| Model 1 * | Increase in PA (100 Steps) | −0.002 | −0.040 to 0.000 |
| Increase in HDL-C (10 mg/dL) | −0.054 | −0.081 to −0.027 | |
| Increase in HEI (1 unit) | −0.018 | −0.038 to 0.001 | |
| Decrease in BMI (1 kg/m2) | −0.081 | −0.030 to 0.019 | |
| Decrease in total calories (10 kcal) | 0.07 | −0.040 to 0.180 | |
| Model 2 ** | Increase in PA (100 Steps) | −0.004 | −0.011 to 0.002 |
| Increase in HDL-C (10 mg/dL) | −0.021 | −0.041 to 0.001 | |
| Increase in HEI (1 unit) | −0.019 | −0.029 to 0.002 | |
| Decrease in BMI (1 kg/m2) | −0.112 | −0.194 to −0.029 | |
| Decrease in total calories (10 kcal) | 0.033 | −0.048 to 0.114 |
| Model | Variables | Change in HEI (Score) Per Unit Change in Variable of Interest | 95% CI |
|---|---|---|---|
| Model 3 * | Decrease in total calories (10 kcal) | 2.71 | −0. 42 to 5.83 |
| Decrease in total fat (1 g) | −0.021 | −0.205 to 0.164 | |
| Decrease saturated fat (1 g) | −0.111 | −0.186 to −0.035 | |
| Decrease total sugar (1 g) | −0.002 | −0.016 to 0.012 | |
| Decrease sodium intake (10 mg) | −0.60 | −1.69 to 0.50 | |
| Model 4 ** | Decrease in total calories (10 kcal) | 2.66 | −0.60 to 5.91 |
| Decrease total fat (1 g) | −0.018 | −0.216 to 0.181 | |
| Decrease saturated fat (1 g) | −0.117 | −0.195 to −0.039 | |
| Decrease total sugar (1 g) | −0.002 | −0.016 to 0.012 | |
| Decrease sodium intake (10 mg) | −0.53 | −1.68 to 0.62 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asaad, G.; Soria-Contreras, D.C.; Bell, R.C.; Chan, C.B. Effectiveness of a Lifestyle Intervention in Patients with Type 2 Diabetes: The Physical Activity and Nutrition for Diabetes in Alberta (PANDA) Trial. Healthcare 2016, 4, 73. https://doi.org/10.3390/healthcare4040073
Asaad G, Soria-Contreras DC, Bell RC, Chan CB. Effectiveness of a Lifestyle Intervention in Patients with Type 2 Diabetes: The Physical Activity and Nutrition for Diabetes in Alberta (PANDA) Trial. Healthcare. 2016; 4(4):73. https://doi.org/10.3390/healthcare4040073
Chicago/Turabian StyleAsaad, Ghada, Diana C. Soria-Contreras, Rhonda C. Bell, and Catherine B. Chan. 2016. "Effectiveness of a Lifestyle Intervention in Patients with Type 2 Diabetes: The Physical Activity and Nutrition for Diabetes in Alberta (PANDA) Trial" Healthcare 4, no. 4: 73. https://doi.org/10.3390/healthcare4040073
APA StyleAsaad, G., Soria-Contreras, D. C., Bell, R. C., & Chan, C. B. (2016). Effectiveness of a Lifestyle Intervention in Patients with Type 2 Diabetes: The Physical Activity and Nutrition for Diabetes in Alberta (PANDA) Trial. Healthcare, 4(4), 73. https://doi.org/10.3390/healthcare4040073






