Citizen Science and Community Engagement in Tick Surveillance—A Canadian Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tick Collection—Academic Researchers
2.2. Tick Collection—Public Health
2.3. Tick Collection—Community Members
2.4. Comparison of Tick Surveillance Strategies
2.5. Tick Species Identification
2.6. DNA Extraction and Nested PCR
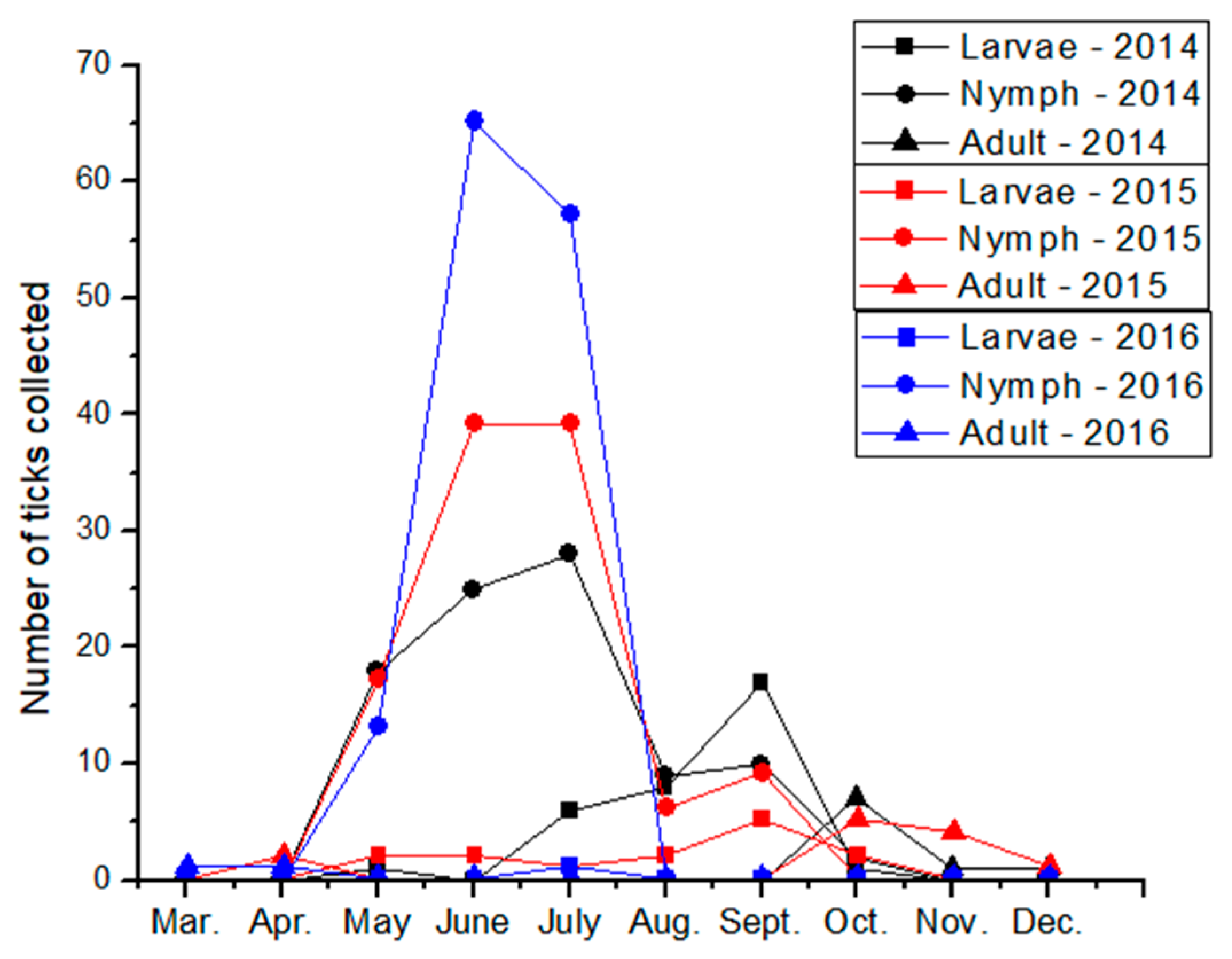
3. Results
4. Discussion
4.1. Advantages of Citizen Science and Community–Researcher Partnerships for Tick Surveillance
4.2. Disadvantages
4.3. Benefits and Applications of Citizen Science Tick Surveillance Projects
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sperling, J.L.; Sperling, F.A. Lyme borreliosis in Canada: Biological diversity and diagnostic complexity from an entomological perspective. Can. Entomol. 2009, 141, 521–549. [Google Scholar] [CrossRef]
- Borgermans, L.; Goderis, G.; Vandevoorde, J.; Devroey, D. Relevance of chronic lyme disease to family medicine as a complex multidimensional chronic disease construct: A systematic review. Int. J. Fam. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, N.; Golovchenko, M.; Grubhoffer, L.; Oliver, J.H., Jr. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick Borne Dis. 2011, 2, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Tappe, J.; Jordan, D.; Janecek, E.; Fingerle, V.; Strube, C. Revisited: Borrelia burgdorferi sensu lato infections in hard ticks (Ixodes ricinus) in the city of Hanover (Germany). Parasit. Vectors 2014, 7, 441. [Google Scholar] [CrossRef] [PubMed]
- Marcus, L.C.; Steere, A.C.; Duray, P.H.; Anderson, A.E.; Mahoney, E.B. Fatal pancarditis in a patient with coexistent lyme disease and babesiosis. Demonstration of spirochetes in the myocardium. Ann. Intern. Med 1985, 103, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Reimers, C.D.; de Koning, J.; Neubert, U.; Preac-Mursic, V.; Koster, J.G.; Muller-Felber, W.; Pongratz, D.E.; Duray, P.H. Borrelia burgdorferi myositis: Report of eight patients. J. Neurol. 1993, 240, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Muehlenbachs, A.; Bollweg, B.C.; Schulz, T.J.; Forrester, J.D.; DeLeon, C.M.; Molins, C.; Ray, G.S.; Cummings, P.M.; Ritter, J.M.; Blau, D.M.; et al. Cardiac tropism of Borrelia burgdorferi: An autopsy study of sudden cardiac death associated with lyme carditis. Am. J. Pathol. 2016, 186, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; Amrine, J.W., Jr.; Gais, R.D.; Kolanko, V.P.; Hagenbuch, B.E.; Gerencser, V.F.; Clark, S.M. Parasitization of humans in West Virginia by Ixodes cookei (acari: Ixodidae), a potential vector of lyme borreliosis. J. Med. Entomol. 1991, 28, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Dolan, M.C.; Lacombe, E.H.; Piesman, J. Vector competence of Ixodes muris (acari: Ixodidae) for Borrelia burgdorferi. J. Med. Entomol. 2000, 37, 766–768. [Google Scholar] [CrossRef] [PubMed]
- Peavey, C.A.; Lane, R.S.; Damrow, T. Vector competence of Ixodes angustus (acari: Ixodidae) for Borrelia burgdorferi sensu stricto. Exp. Appl. Acarol. 2000, 24, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.; Clark, K.; Anderson, J.; Foley, J.; Young, M. Lyme disease Bacterium, Borrelia burgdorferi sensu lato, detected in multiple tick species at Kenora, Ontario, Canada. J. Bact. Parasitol. 2017, 8. [Google Scholar] [CrossRef]
- Ogden, N.H.; Maarouf, A.; Barker, I.K.; Bigras-Poulin, M.; Lindsay, L.R.; Morshed, M.G.; O’Callaghan, C.J.; Ramay, F.; Waltner-Toews, D.; Charron, D.F. Climate change and the potential for range expansion of the Lyme disease vector Ixodes scapularis in Canada. Int. J. Parasitol. 2006, 36, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Lindsay, L.R.; Hanincova, K.; Barker, I.K.; Bigras-Poulin, M.; Charron, D.F.; Heagy, A.; Francis, C.M.; O’Callaghan, C.J.; Schwartz, I. Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl. Environ. Microbiol. 2008, 74, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Anderson, J.F.; Durden, L.A. Widespread dispersal of Borrelia burgdorferi-infected ticks collected from songbirds across Canada. J. Parasitol. 2012, 98, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Barker, I.K.; Francis, C.M.; Heagy, A.; Lindsay, L.R.; Hobson, K.A. How far north are migrant birds transporting the tick Ixodes scapularis in Canada? Insights from stable hydrogen isotope analyses of feathers. Ticks Tick Borne Dis. 2015, 6, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D. Studies abound on how far north Ixodes scapularis ticks are transported by birds. Ticks Tick Borne Dis. 2016, 7, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Bouchard, C.; Kurtenbach, K.; Margos, G.; Lindsay, L.R.; Trudel, L.; Nguon, S.; Milord, F. Active and passive surveillance and phylogenetic analysis of Borrelia burgdorferi elucidate the process of Lyme disease risk emergence in Canada. Environ. Health Perspect 2010, 118, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Mead, P.; Goel, R.; Kugeler, K. Canine serology as adjunct to human Lyme disease surveillance. Emerg. Infect. Dis. 2011, 17, 1710–1712. [Google Scholar] [CrossRef] [PubMed]
- Koffi, J.K.; Leighton, P.A.; Pelcat, Y.; Trudel, L.; Lindsay, L.R.; Milord, F.; Ogden, N.H. Passive surveillance for I. scapularis ticks: Enhanced analysis for early detection of emerging Lyme disease risk. J. Med. Entomol. 2012, 49, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Koffi, J.K.; Lindsay, L.R. Assessment of a screening test to identify lyme disease risk. In Canada Communicable Disease Report; Public Health Agency of Canada, Government of Canada: Ottawa, ON, Canada, 6 March 2014. [Google Scholar]
- Dickinson, J.L.; Shirk, J.; Bonter, D.; Bonney, R.; Crain, R.L.; Martin, J.; Phillips, T.; Purcell, K. The current state of citizen science as a tool for ecological research and public engagement. Front. Ecol. Environ. 2012, 10, 291–297. [Google Scholar] [CrossRef]
- Ranard, B.L.; Ha, Y.P.; Meisel, Z.F.; Asch, D.A.; Hill, S.S.; Becker, L.B.; Seymour, A.K.; Merchant, R.M. Crowdsourcing—Harnessing the masses to advance health and medicine, a systematic review. J. Gen. Intern. Med. 2014, 29, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, M.; Sajanti, E.; Sormunen, J.J.; Penttinen, R.; Hanninen, J.; Ruohomaki, K.; Saaksjarvi, I.; Vesterinen, E.J.; Vuorinen, I.; Hytonen, J.; et al. Crowdsourcing-based nationwide tick collection reveals the distribution of Ixodes ricinus and I. persulcatus and associated pathogens in Finland. Emerg. Microbes Infect. 2017, 6, e31. [Google Scholar] [CrossRef] [PubMed]
- Seifert, V.A.; Wilson, S.; Toivonen, S.; Clarke, B.; Prunuske, A. Community partnership designed to promote Lyme disease prevention and engagement in citizen science. J. Microbiol. Biol. Educ. 2016, 17, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marti, I.; Zurita-Milla, R.; van Vliet, A.J.H.; Takken, W. Modelling and mapping tick dynamics using volunteered observations. Int. J. Health Geogr. 2017, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Bjurman, N.K.; Bradet, G.; Lloyd, V.K. Lyme disease risk in dogs in New Brunswick. Can. Vet. J. 2016, 57, 981–984. [Google Scholar] [PubMed]
- Gabriele-Rivet, V.; Arsenault, J.; Badcock, J.; Cheng, A.; Edsall, J.; Goltz, J.; Kennedy, J.; Lindsay, L.R.; Pelcat, Y.; Ogden, N.H. Different ecological niches for ticks of public health significance in Canada. PLoS ONE 2015, 10, e0131282. [Google Scholar] [CrossRef] [PubMed]
- Keirans, J.E.; Litwak, T.R. Pictorial key to the adults of hard ticks, family ixodidae (ixodida: Ixodoidea), east of the Mississippi River. J. Med. Entomol. 1989, 26, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.; Duncan, A.M.; McIntyre, K.C.; Lloyd, V. Evidence for genetic hybridization between Ixodes scapularis and Ixodes cookei. Can. J. Zool. 2017, 95, 527–537. [Google Scholar] [CrossRef]
- Sperling, J.L.; Silva-Brandao, K.L.; Brandao, M.M.; Lloyd, V.K.; Dang, S.; Davis, C.S.; Sperling, F.A.H.; Magor, K.E. Comparison of bacterial 16S rRNA variable regions for microbiome surveys of ticks. Ticks Tick Borne Dis. 2017, 8, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Newman, G.; Wiggins, A.; Crall, A.; Graham, E.; Newman, S.; Crowston, K. The future of citizen science: Emerging technologies and shifting paradigms. Front. Ecol. Environ. 2012, 10, 298–304. [Google Scholar] [CrossRef]
- Kampen, H.; Medlock, J.M.; Vaux, A.G.; Koenraadt, C.J.; van Vliet, A.J.; Bartumeus, F.; Oltra, A.; Sousa, C.A.; Chouin, S.; Werner, D. Approaches to passive mosquito surveillance in the EU. Parasit. Vectors 2015, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, A.J.; Fraser, P.L.; Robinson, L.; Tweddle, J.C.; Sadler, J.P.; West, S.E.; Norman, S.; Batson, M.; Davies, L. The OPAL bugs count survey: Exploring the effects of urbanisation and habitat characteristics using citizen science. Urban Ecosyst. 2015, 18, 1477–1497. [Google Scholar] [CrossRef]


| Collection | Collection Type | Year | Location | Number of Sites | Number of Site Visits | Collection Method | Ixodes scapularis | Other Ticks | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Larvae | Nymph | Adult Female | Adult Male | Total | ||||||||
| Mt. Allison | academic | 2014 | NB | 66 | 1 | active | 0 | 0 | 6 | 3 | 9 | |
| Sackville Health Center | community/academic | 2014 | Sackville, NB | 1 | 1 | active | 0 | 0 | 0 | 0 | 0 | I. cookei (3 adults) |
| Recreational | community/academic | 2014 | Kejimkujik Park, NS | 1 | 3 | active | 0 | 0 | 0 | 0 | 0 | Dermacentor variabilis (6 adults) |
| NB Forestry | community/academic | 2015 | Fredericton, NB | 7 | 1 | active | 0 | 0 | 0 | 0 | 0 | D. variabilis (4 adults) |
| St. John | citizen | 2014 | Millidgeville, NB | 1 | 38 | passive | 33 | 82 | 2 | 0 | 117 | |
| 2015 | 1 | 53 | 13 | 108 | 11 | 1 | 133 | |||||
| 2016 | 1 | 26 | 1 | 137 | 2 | 0 | 140 | |||||
| Nova Scotia | citizen | 2015 | Lunenburg, NS | 2 | 6 | active | 0 | 0 | 93 | 73 | 166 | D. variabilis not enumerated |
| 2016 | 2 | 12 | 0 | 1 | 304 | 220 | 525 | |||||
| 2017 | 2 | 16 | 0 | 0 | 388 | 328 | 716 | |||||
| Hampton | citizen | 2012 | Hampton, NB | 1 | 200 a | passive | 0 | 0 | 13 | 0 | 13 | |
| 2013 | 1 | 200 a | 0 | 0 | 12 | 0 | 12 | |||||
| 2014 | 1 | 200 a | 0 | 0 | 15 | 0 | 15 | |||||
| 2015 | 1 | 200 a | 0 | 0 | 3 | 0 | 3 | |||||
| 2016 | 1 | 200 a | 0 | 0 | 5 | 0 | 5 | |||||
| Rothesay | citizen | 2014 | Rothesay, NB | 1 | 6 | passive | 0 | 0 | 3 | 3 | 6 | |
| 2016 | 1 | 8 | 0 | 0 | 5 | 9 | 14 | |||||
| Collection | Total of Ticks Recovered a | Number of Sites b | Number of Visits/Site | Average Tick/Site Visit |
|---|---|---|---|---|
| Public Health | 0 | 8 | 1 | 0 |
| Academic | 7 | 38 | 1 | 0.2 |
| Citizen (St. John) | 94 | 1 | 38 | 2.5 |
| Citizen (Hampton) | 15 | 1 | 100 c | 0.15 |
| Citizen (Rothesay) | 6 | 1 | 8 | 0.75 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, J.; Boudreau, C.R.; Patterson, J.W.; Bradet-Legris, J.; Lloyd, V.K. Citizen Science and Community Engagement in Tick Surveillance—A Canadian Case Study. Healthcare 2018, 6, 22. https://doi.org/10.3390/healthcare6010022
Lewis J, Boudreau CR, Patterson JW, Bradet-Legris J, Lloyd VK. Citizen Science and Community Engagement in Tick Surveillance—A Canadian Case Study. Healthcare. 2018; 6(1):22. https://doi.org/10.3390/healthcare6010022
Chicago/Turabian StyleLewis, Julie, Corinne R. Boudreau, James W. Patterson, Jonathan Bradet-Legris, and Vett K. Lloyd. 2018. "Citizen Science and Community Engagement in Tick Surveillance—A Canadian Case Study" Healthcare 6, no. 1: 22. https://doi.org/10.3390/healthcare6010022




