“JUMPing into Diabetes Control”: A Group-Setting Self-Empowerment Lifestyle Intervention among Diabetes Patients
Abstract
1. Introduction
2. Methods
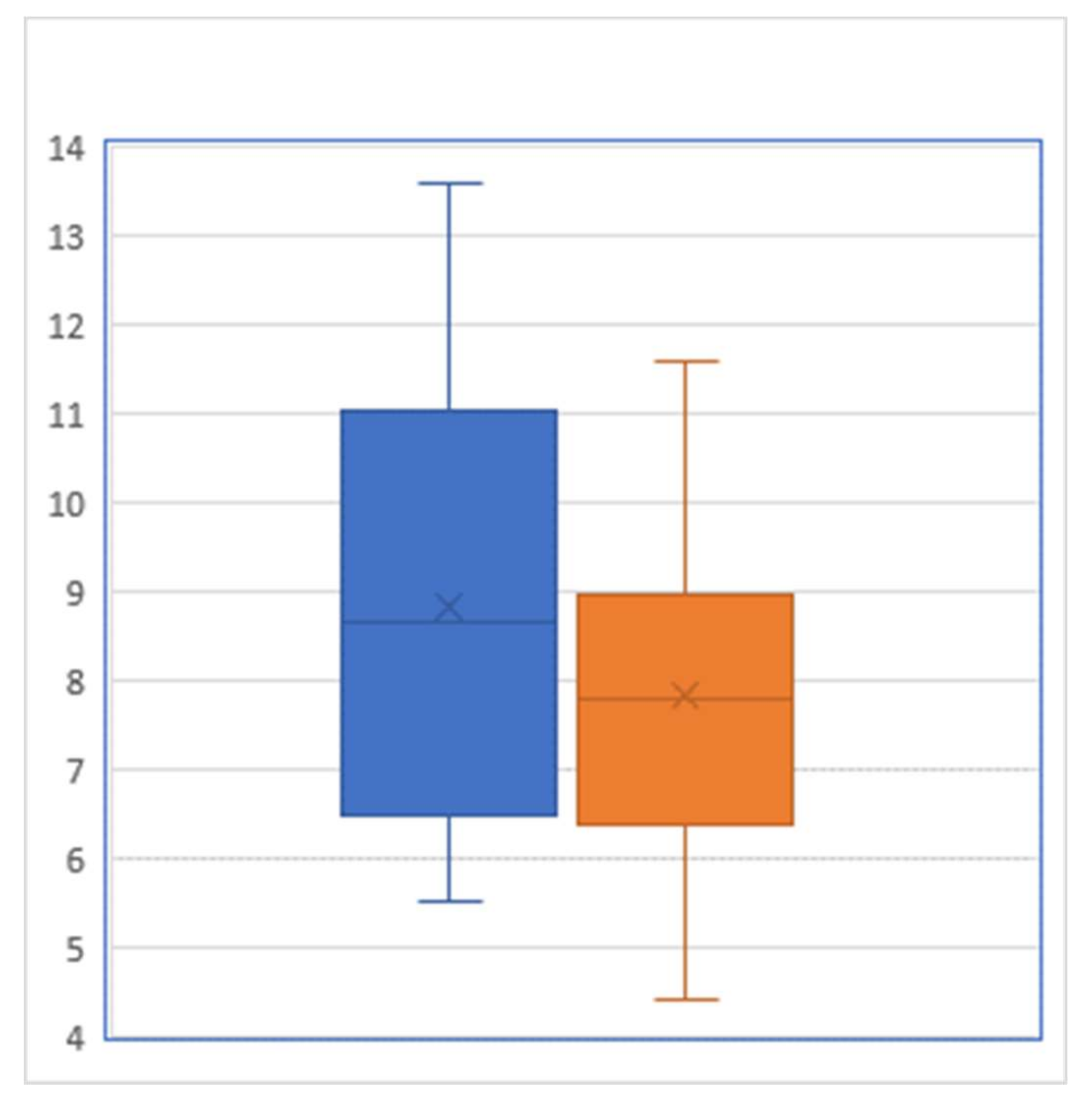
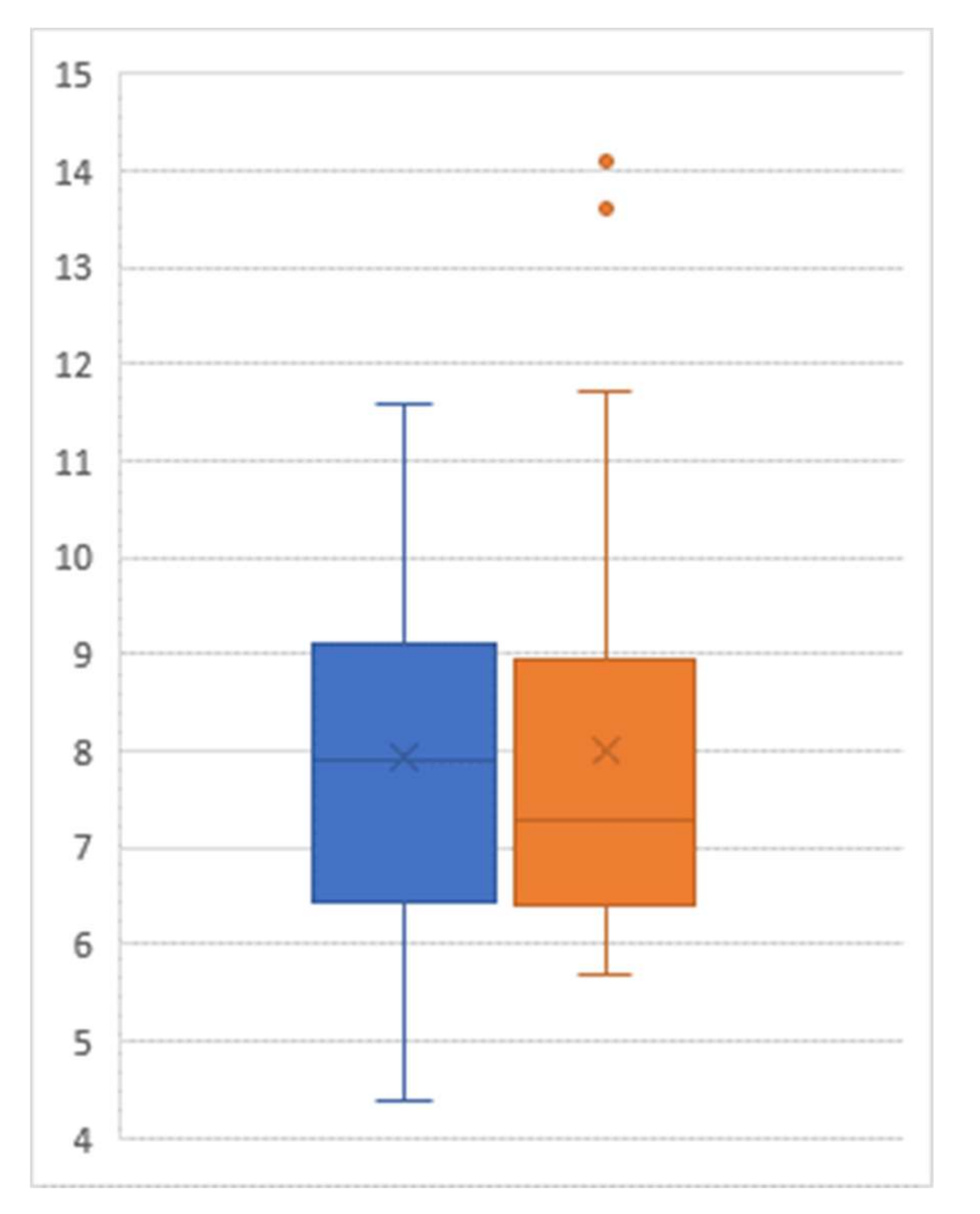
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. 2014. Available online: https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf (accessed on 24 October 2016).
- International Diabetes Federation. IDF Diabetes, 7th ed. 2015. Available online: http://www.diabetesatlas.org (accessed on 2 November 2016).
- Zhang, Q.; Wang, Y.; Huang, E.S. Changes in racial/ethnic disparities in the prevalence of Type 2 diabetes by obesity level among US adults. Ethn. Health 2009, 14, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Agency for Healthcare Research and Quality. Diabetes Disparities among Racial and Ethnic Minorities: Fact Sheet. 2001. Available online: http://archive.ahrq.gov/research/findings/factsheets/diabetes/diabdisp/diabdisp.html (accessed on 24 October 2016).
- Link, C.L.; McKinlay, J.B. Disparities in the Prevalence of Diabetes: Is it Race/Ethnicity or Socioeconomic Status? Results from the Boston Area Community Health (BACH) Survey. Ethn. Dis. 2009, 19, 288–292. [Google Scholar] [PubMed]
- Signorello, L.B.; Schlundt, D.G.; Cohen, S.; Steinwandel, M.D.; Buchowski, M.; McLaughlin, J.K.; Hargreaves, M.K.; Blot, W.J. Comparing Diabetes Prevalence Between African Americans and Whites of Similar Socioeconomic Status. Am. J. Public Health 2007, 97, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Formosa, C.; Muscat, R. Improving Diabetes Knowledge and Self-Care Practices. J. Am. Podiatr. Med Assoc. 2016, 106, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Powell, P.W.; Corathers, S.D.; Raymond, J.; Streisand, R. New approaches to providing individualized diabetes care in the 21st century. Curr. Diabetes Rev. 2015, 11, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Edelman, D.; Gierisch, J.; McDuffie, J.R.; Oddone, E.; Williams, J.W. Shared Medical Appointments for Patients with Diabetes Mellitus: A Systematic Review. J. Gen. Intern. Med. 2014, 30, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Noordman, J.; Mejino, A.; Van Dulmen, S. Shared medical appointments for children and adolescents with type I diabetes: Perspectives and experiences of patients, parents, and health care providers. Adolesc. Health Med. Ther. 2012, 3, 75–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Floyd, B.D.; Block, J.M.; Buckingham, B.B.; Ly, T.; Foster, N.; Wright, R.; Mueller, C.; Hood, K.K.; Shah, A. Stabilization of glycemic control and improved quality of life using a shared medical appointment model in adolescents with type 1 diabetes in suboptimal control. Pediatr. Diabetes 2016, 18, 204–212. [Google Scholar] [CrossRef]
- Rijswijk, C.; Zantinge, E.; Seesing, F.; Raats, I.; Van Dulmen, S. Shared and individual medical appointments for children and adolescents with type 1 diabetes; differences in topics discussed? Patient Educ. Couns. 2010, 79, 351–355. [Google Scholar] [CrossRef]
- Davis, L.M.; Vitagliano, C.P. Shared Medical Appointments for Adolescents with Type 1 Diabetes Mellitus: Important Learning Communities. J. Pediatr. Nurs. 2015, 30, 632–634. [Google Scholar] [CrossRef]
- Christie, D.; Channon, S. The potential for motivational interviewing to improve outcomes in the management of diabetes and obesity in paediatric and adult populations: A clinical review. Diabetes, Obes. Metab. 2013, 16, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.M.; Creedy, D.K.; Lin, H.-S.; Wollin, J. Effects of motivational interviewing intervention on self-management, psychological and glycemic outcomes in type 2 diabetes: A randomized controlled trial. Int. J. Nurs. Stud. 2012, 49, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Heisler, M.; Vijan, S.; Makki, F.; Piette, J.D. Diabetes control with reciprocal peer support versus nurse care management: A randomized trial. Ann. Intern. Med. 2010, 153, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Johansson, T.; Keller, S.; Winkler, H.; Ostermann, T.; Weitgasser, R.; Sönnichsen, A. Effectiveness of a Peer Support Programme versus Usual Care in Disease Management of Diabetes Mellitus Type 2 regarding Improvement of Metabolic Control: A Cluster-Randomised Controlled Trial. J. Diabetes Res. 2015, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, S.; Sun, K.; Fisher, E.B.; Sun, X.; Information, P.E.K.F.C. How to achieve better effect of peer support among adults with type 2 diabetes: A meta-analysis of randomized clinical trials. Patient Educ. Couns. 2016, 99, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, Q.; Qi, X.; Wu, N.; Tang, W.; Xiong, H. Effectiveness of peer support for improving glycaemic control in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. BMC Public Health 2015, 15, 471. [Google Scholar]
- Dale, J.; Williams, S.M.; Bowyer, V. What is the effect of peer support on diabetes outcomes in adults? A systematic review. Diabet. Med. 2012, 29, 1361–1377. [Google Scholar] [CrossRef] [PubMed]
- Due-Christensen, M.; Zoffmann, V.; Hommel, E.; Lau, M. Can sharing experiences in groups reduce the burden of living with diabetes, regardless of glycaemic control? Diabet. Med. 2012, 29, 251–256. [Google Scholar] [CrossRef]
- Lorig, K.; Ritter, P.L.; Villa, F.J.; Armas, J. Community-Based Peer-Led Diabetes Self-management. Diabetes Educ. 2009, 35, 641–651. [Google Scholar] [CrossRef]
- Funnell, M.M. Peer-based behavioural strategies to improve chronic disease self-management and clinical outcomes: Evidence, logistics, evaluation considerations and needs for future research. Fam. Pr. 2009, 27, i17–i22. [Google Scholar] [CrossRef]
- Joensen, L.E.; Filges, T.; Willaing, I. Patient perspectives on peer support for adults with type 1 diabetes: A need for diabetes-specific social capital. Patient Prefer. Adherence 2016, 10, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Zlatković, J.; Todorović, N.; Bošković, M.; Pajovic, S.B.; Demajo, M.; Filipovic, D.; Martinovic, J. Different susceptibility of prefrontal cortex and hippocampus to oxidative stress following chronic social isolation stress. Mol. Cell. Biochem. 2014, 393, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Novick, G. CenteringPregnancy and the Current State of Prenatal Care. J. Midwifery Women’s Health 2004, 49, 405–411. [Google Scholar] [CrossRef]
- Parikh, L.I.; Jelin, A.C.; Iqbal, S.N.; Belna, S.L.; Fries, M.H.; Patel, M.; Desale, S.; Ramsey, P. Glycemic control, compliance, and satisfaction for diabetic gravidas in Centering® group care. J. Matern. Neonatal Med. 2016, 30, 1–21. [Google Scholar] [CrossRef]
- Schellinger, M.M.; Abernathy, M.P.; Amerman, B.; May, C.; Foxlow, L.A.; Carter, A.L.; Barbour, K.; Luebbehusen, E.; Ayo, K.; Bastawros, D.; et al. Improved Outcomes for Hispanic Women with Gestational Diabetes Using the Centering Pregnancy© Group Prenatal Care Model. Matern. Child Health J. 2016, 21, 297–305. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; ISBN 9789241565257. Available online: https://apps.who.int/iris/handle/10665/204871 (accessed on 4 April 2020).
- Genuth, S.; Eastman, R.; Kahn, R.; Klein, A.P.; Lachin, J.M.; Lebovitz, H.; Nathan, D.; Vinicor, F. Implications of the United Kingdom prospective diabetes study. Diabetes Care 2003, 26, 28–32. [Google Scholar]
- Umpierre, D.; Ribeiro, P.A.; Kramer, C.K.; Leitão, C.B.; Zucatti, A.T.; Azevedo, M.J.; Gross, J.L.; Ribeiro, J.P.; Schaan, B.D. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: A systematic review and meta-analysis. JAMA 2011, 305, 1790–1799. [Google Scholar] [CrossRef]
- Ackermann, R.T.; Finch, E.A.; Brizendine, E.; Zhou, H.; Marrero, D.G. Translating the Diabetes Prevention Program into the Community. Am. J. Prev. Med. 2008, 35, 357–363. [Google Scholar] [CrossRef]
- Anderson, R.; Funnell, M.M.; Fitzgerald, J.T.; Marrero, D.G. The Diabetes Empowerment Scale: A measure of psychosocial self-efficacy. Diabetes Care 2000, 23, 739–743. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Camara, A.; Baldé, N.; Enoru, S.; Bangoura, J.; Sobngwi, E.; Bonnet, F. Prevalence of anxiety and depression among diabetic African patients in Guinea: Association with HbA1c levels. Diabetes Metab. 2015, 41, 62–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stange, K.C.; Nutting, P.A.; Miller, W.L.; Jaén, C.R.; Crabtree, B.; Flocke, S.A.; Gill, J.M. Defining and Measuring the Patient-Centered Medical Home. J. Gen. Intern. Med. 2010, 25, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Kirsh, S.R.; Watts, S.; Pascuzzi, K.; O’Day, M.E.; Davidson, D.; Strauss, G.; Kern, E.O.; Aron, D.C. Shared medical appointments based on the chronic care model: A quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual. Saf. Health Care 2007, 16, 349–353. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henry, S.; Shi, L.; Alexander, V.; O’Neal, R.; Carey, S.; Spitler, H.D.; Leonard, D.; Chastain, G.; Hassan, L.; Jindal, M. “JUMPing into Diabetes Control”: A Group-Setting Self-Empowerment Lifestyle Intervention among Diabetes Patients. Healthcare 2020, 8, 90. https://doi.org/10.3390/healthcare8020090
Henry S, Shi L, Alexander V, O’Neal R, Carey S, Spitler HD, Leonard D, Chastain G, Hassan L, Jindal M. “JUMPing into Diabetes Control”: A Group-Setting Self-Empowerment Lifestyle Intervention among Diabetes Patients. Healthcare. 2020; 8(2):90. https://doi.org/10.3390/healthcare8020090
Chicago/Turabian StyleHenry, Sheena, Lu Shi, Virginia Alexander, Richard O’Neal, Stephen Carey, Hugh D. Spitler, Deborah Leonard, Gail Chastain, Lauren Hassan, and Meenu Jindal. 2020. "“JUMPing into Diabetes Control”: A Group-Setting Self-Empowerment Lifestyle Intervention among Diabetes Patients" Healthcare 8, no. 2: 90. https://doi.org/10.3390/healthcare8020090
APA StyleHenry, S., Shi, L., Alexander, V., O’Neal, R., Carey, S., Spitler, H. D., Leonard, D., Chastain, G., Hassan, L., & Jindal, M. (2020). “JUMPing into Diabetes Control”: A Group-Setting Self-Empowerment Lifestyle Intervention among Diabetes Patients. Healthcare, 8(2), 90. https://doi.org/10.3390/healthcare8020090





