Electroanalytical Sensing of Flunitrazepam Based on Screen Printed Graphene Electrodes
Abstract
:1. Introduction

2. Experimental Section
3. Results and Discussion
3.1. Optimization of the Electrochemical Protocol



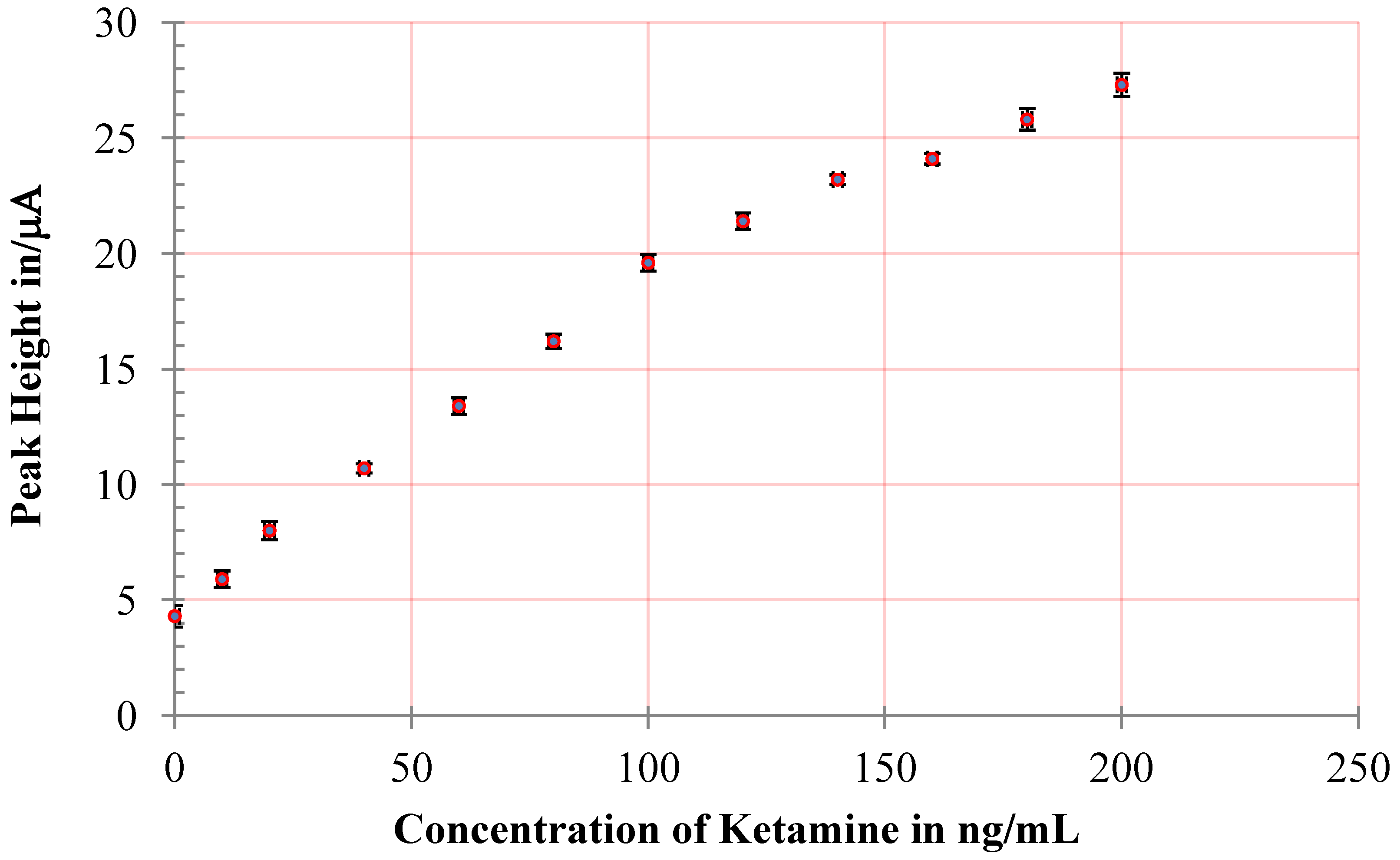
| Analytical Method | Reference | Matrix | Analytica Linear Range | Detection Limit |
|---|---|---|---|---|
| This method | Buffer, Beverage | 10–200 ng/mL | 6 ng/mL | |
| SPGE | [20,21] | Buffer, Beverage | 1–95.24 µg/mL | 0.47 µg/mL |
| LCMS-MS | [7] | Human Serum | 1–500 pg/mL | 0.2 ng/mL |
| Fluorescence spectroscopy | [29] | Beverage | 0–5 ng/mL | 1 ng/mL |
| Desorption Electrospray Ionization Mass-Spectrometry | [3] | Beverages | 25–300 ng/mL | 15 ng/mL |
| GC-MS | [11] | Oral Saliva | Not Disclosed | 0.1 pg/mL |
3.2. Electroanalytical Applications of ECL in an Alcoholic Beverage

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Goulle, J.P.; Anger, J.P. Drug-facilitated robbery or sexual assault: Problems associated with amnesia. Ther. Drug Monit. 2004, 26, 206–210. [Google Scholar] [CrossRef]
- Ghosh, P.; Reddy, M.M.K.; Ramteke, V.B.; Rao, B.S. Analysis and quantitation of diazepam in cream biscuits by high-performance thin-layer chromatography and its confirmation by mass spectrometry. Anal. Chim. Acta 2004, 508, 31. [Google Scholar] [CrossRef]
- D’Aloise, P.; Chen, H. Rapid determination of flunitrazepam in alcoholic beverages by desorption electrospray ionization-mass spectrometry. Sci. Justice 2012, 52, 2–8. [Google Scholar] [CrossRef]
- Schwartz, R.; Milteer, R.; LeBeau, M.A. Drug-facilitated sexual assault (‘date rape’). South. Med. J. 2000, 93, 558–561. [Google Scholar]
- Schwartz, R.H.; Weaver, A.B. Rohypnol, the date rape drug. Clin. Pediatr. 1998, 37, 321–322. [Google Scholar] [CrossRef]
- Ngwa, G.; Fritch, D.; Blum, K.; Newland, G. Simultaneous analysis of 14 benzodiazepines in oral fluid by solid-phase extraction and LC-MS-MS. J. Anal. Toxicol. 2007, 31, 369–376. [Google Scholar] [CrossRef]
- Moore, C.; Coulter, C.; Crompton, K.; Zumwalt, M. Determination of benzodiazepines in oral fluid using LC-MS-MS. J. Anal. Toxicol. 2007, 31, 596–600. [Google Scholar] [CrossRef]
- Kempfa, J.; Wuskeb, T.; Schubertc, R.; Weinmanna, W. Pre-analytical stability of selected benzodiazepines on a polymeric oral fluid sampling device. Forensic Sci. Int. 2009, 186, 81–85. [Google Scholar] [CrossRef]
- Saracino, M.A.; Tallarico, K.; Raggi, M.A. Liquid chromatographic analysis of oxcarbazepine and its metabolites in plasma and saliva after a novel microextraction by packed sorbent procedure. Anal. Chim. Acta 2010, 661, 222–228. [Google Scholar] [CrossRef]
- Lledo-Fernandez, C.; Banks, C. An overview of quantifying and screening drugs of abuse in biological samples: Past and present. Anal. Methods 2011, 3, 1227–1244. [Google Scholar] [CrossRef]
- Samyn, N.; de Boeck, G.; Crimele, V.; Verstaete, A.; Klintz, P. Detection of flunitrazepam and 7-aminoflunitrazepam in oral fluid after controlled administration of rohypnol. J. Anal. Toxicol. 2002, 26, 211–215. [Google Scholar] [CrossRef]
- Wang, P.H.; Lui, C.; Tsay, W.I.; Li, J.H.; Liu, R.H.; Wu, T.G.; Cheng, W.J.; Lin, D.L.; Huang, T.Y. Improved screen and confirmation test of 7-aminoflunitrazepam in urine specimens for monitoring flunitrazepam (rohypnol) exposure. J. Anal. Toxicol. 2002, 26, 411–418. [Google Scholar] [CrossRef]
- Elsohly, M.A.; Feng, S.; Salamone, S.J.; Brenneisen, R. GC-MS determination of flunitrazepam and its major metabolite in whole blood and plasma. J. Anal. Toxicol. 1999, 23, 486–489. [Google Scholar] [CrossRef]
- Lawrence, R. The Encyclopedia of Addictive Drugs; Greenwood Publishing Group: Westport, CT, USA, 2002. [Google Scholar]
- Aberl, F.; Bonenberger, J.; Berg, R.-P.; Zimmermann, R.; Sachs, H. Human Detection and Positive Identification: Methods and Technologies; SPIE: Boston, MA, USA, 1996. [Google Scholar]
- Abdul Rahman, M.Z.; Anderson, R.A.; MacDonald, M.; Williams, K.; Jacob, B.; Bonte, W. Advances in Forensic Science; Verlag Dr. Koester: Berlin, Germany, 1995; Volume 5. [Google Scholar]
- Bermejo, E.; Zapardiel, A.; Pérez, J.A.; Huerta, A.; Hernández, L. Voltammetric studies of a psychotropic drug with nitro groups. Determination of flunitrazepam in urine using HMDE. Talanta 1993, 40, 1649–1656. [Google Scholar] [CrossRef]
- Nunez Vergara, L.J.; Bollo, S.; Olea-Azar, C.; Navarrete-Encina, P.A.; Squella, J.A. Cyclic voltammetric and EPR spectroscopic studies of benzodiazepines—Loprazolam and flunitrazepam. J. Electroanal. Chem. 1997, 436, 227–238. [Google Scholar] [CrossRef]
- McGuire, N.D.; Honeychurch, K.C.; Hart, J.P. The electrochemical behaviour of nitrazepam at a screen-printed carbon electrode and its determination in beverages by adsorptive stripping voltammetry. Electroanalysis 2009, 21, 2165–2170. [Google Scholar] [CrossRef]
- Smith, J.P.; Metters, J.P.; Kampouris, D.K.; Lledo-Fernandez, C.; Sutcliffe, O.B.; Banks, C.E. Forensic electrochemistry: The electroanalytical sensing of Rohypnol[registered sign] (flunitrazepam) using screen-printed graphite electrodes without recourse for electrode or sample pre-treatment. Analyst 2013, 138, 6185–6191. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, E.; Lledo-Fernandez, C. The electroanalytical sensing of flunitrazepam (rohypnol) and 7-amino flunitrazepam in oral fluid, urine and alcoholic beverages. Univers. J. Chem. 2013, 1, 121–127. [Google Scholar]
- Pumera, M.; Ambrosi, A.; Bonanni, A.; Chng, E.L.K.; Poh, H.L. Graphene for electrochemical sensing and biosensing. TrAC Trends Anal. Chem. 2010, 29, 954–965. [Google Scholar] [CrossRef]
- Sui, Y.; Appenzeller, J. Screening and interlayer coupling in multilayer graphene field-effect transistors. Nano Lett. 2009, 9, 2973–2977. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Song, J.; Han, D.; Ivaska, A.; Niu, L. Direct electrochemistry of glucose oxidase and biosensing for glucose based on graphene. Anal. Chem. 2009, 81, 2378–2382. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S.; Wang, E. Three-dimensional Pt-on-Pd bimetallic nanodendrites supported on graphene nanosheet: Facile synthesis and used as an advanced nanoelectrocatalyst for methanol oxidation. ACS Nano 2009, 4, 547–555. [Google Scholar]
- Seger, B.; Kamat, P.V. Electrocatalytically active graphene-platinum nanocomposites. Role of 2-D carbon support in PEM fuel cells. J. Phys. Chem. C 2009, 113, 7990–7995. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor devices based on graphene materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Smith, W.F. Voltammetric Determination of Molecules of Biological Significance; Wiley: Chichester, UK, 1992. [Google Scholar]
- Leesakul, N.; Pongampai, S.; Kanatharana, P.; Sudkeaw, P.; Tantirungrotechai, Y.; Buramachai, C. A new screening method for flunitrazepam in vodka and tequila by fluorescence spectroscopy. Luminescence 2013, 28, 76–83. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Garcia-Gutierrez, E.; Lledo-Fernandez, C. Electroanalytical Sensing of Flunitrazepam Based on Screen Printed Graphene Electrodes. Chemosensors 2013, 1, 68-77. https://doi.org/10.3390/chemosensors1030068
Garcia-Gutierrez E, Lledo-Fernandez C. Electroanalytical Sensing of Flunitrazepam Based on Screen Printed Graphene Electrodes. Chemosensors. 2013; 1(3):68-77. https://doi.org/10.3390/chemosensors1030068
Chicago/Turabian StyleGarcia-Gutierrez, Enriqueta, and Carlos Lledo-Fernandez. 2013. "Electroanalytical Sensing of Flunitrazepam Based on Screen Printed Graphene Electrodes" Chemosensors 1, no. 3: 68-77. https://doi.org/10.3390/chemosensors1030068
APA StyleGarcia-Gutierrez, E., & Lledo-Fernandez, C. (2013). Electroanalytical Sensing of Flunitrazepam Based on Screen Printed Graphene Electrodes. Chemosensors, 1(3), 68-77. https://doi.org/10.3390/chemosensors1030068




