Design and Characterization of ITO-Covered Resonant Nanopillars for Dual Optical and Electrochemical Sensing
Abstract
:1. Introduction
2. Materials and Methods
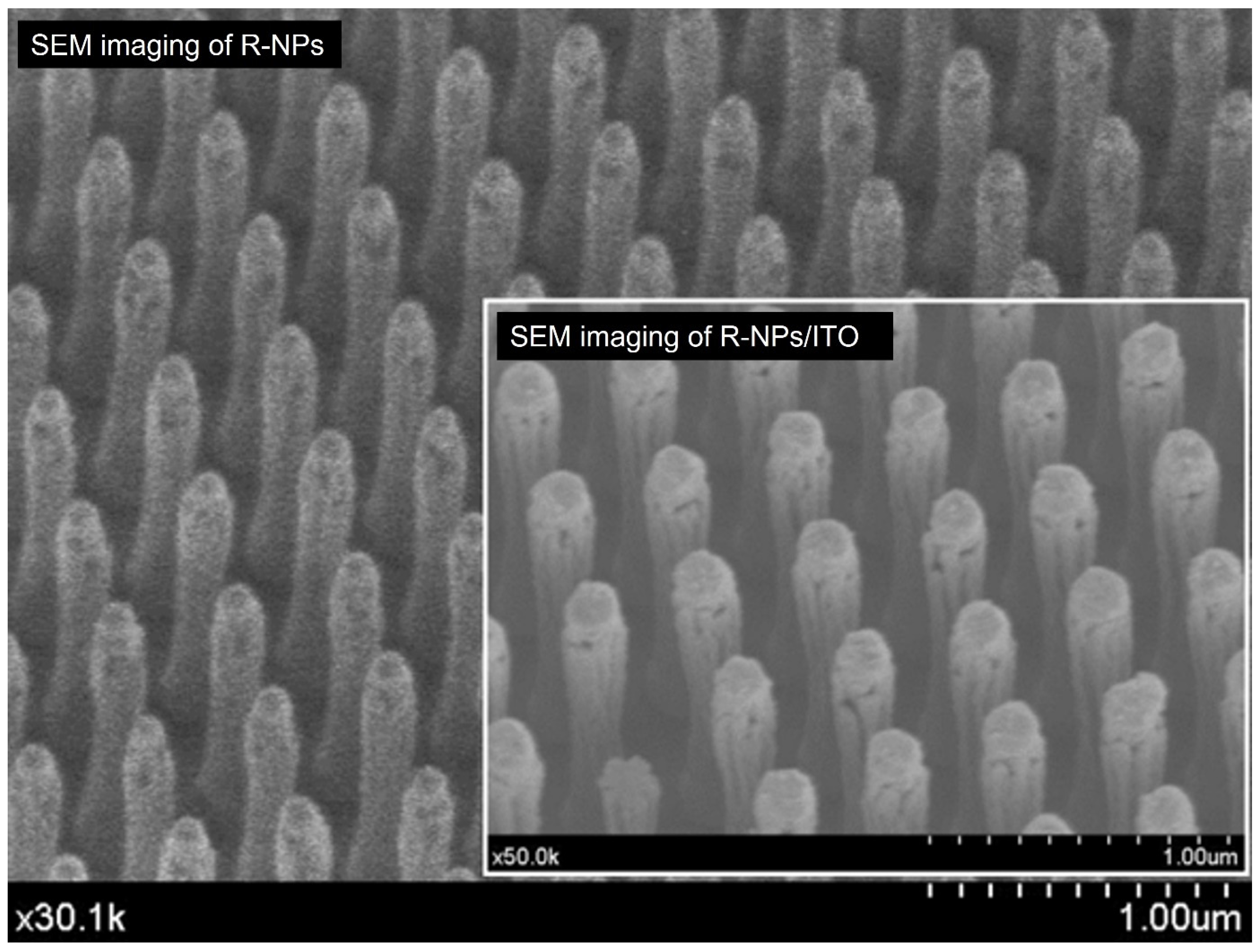
2.1. R-NPs Structure
2.2. Optical Simulations
2.3. ITO Thin Film Fabrication
2.4. Electrochemical Testing
2.5. Optical Measurement Setup
2.6. Biological Detection Setup
3. Results and Discussion
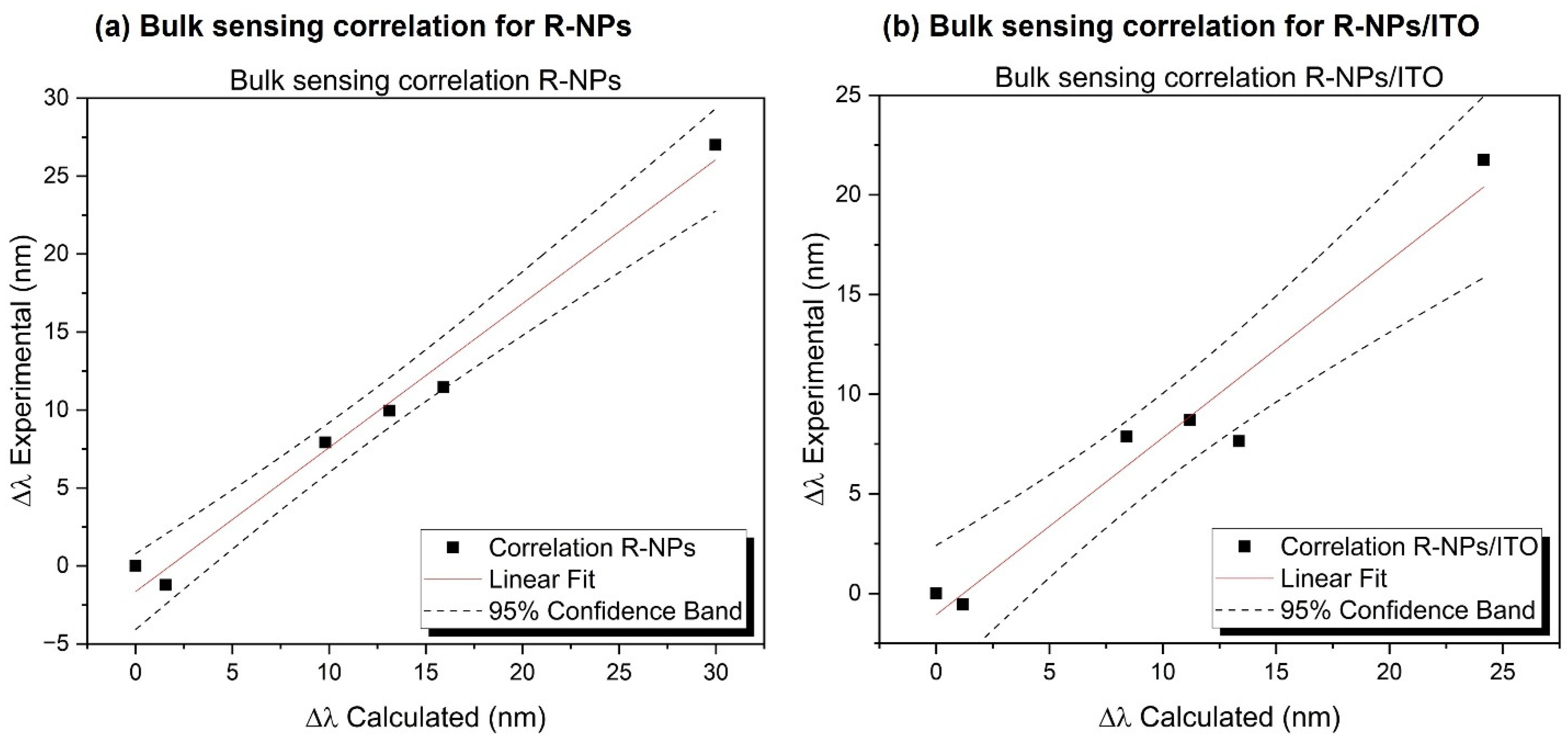
3.1. Bulk Sensing Comparison between R-NPs and R-NPs/ITO and Simulations
3.2. Electrochemical Response of R-NPs/ITO
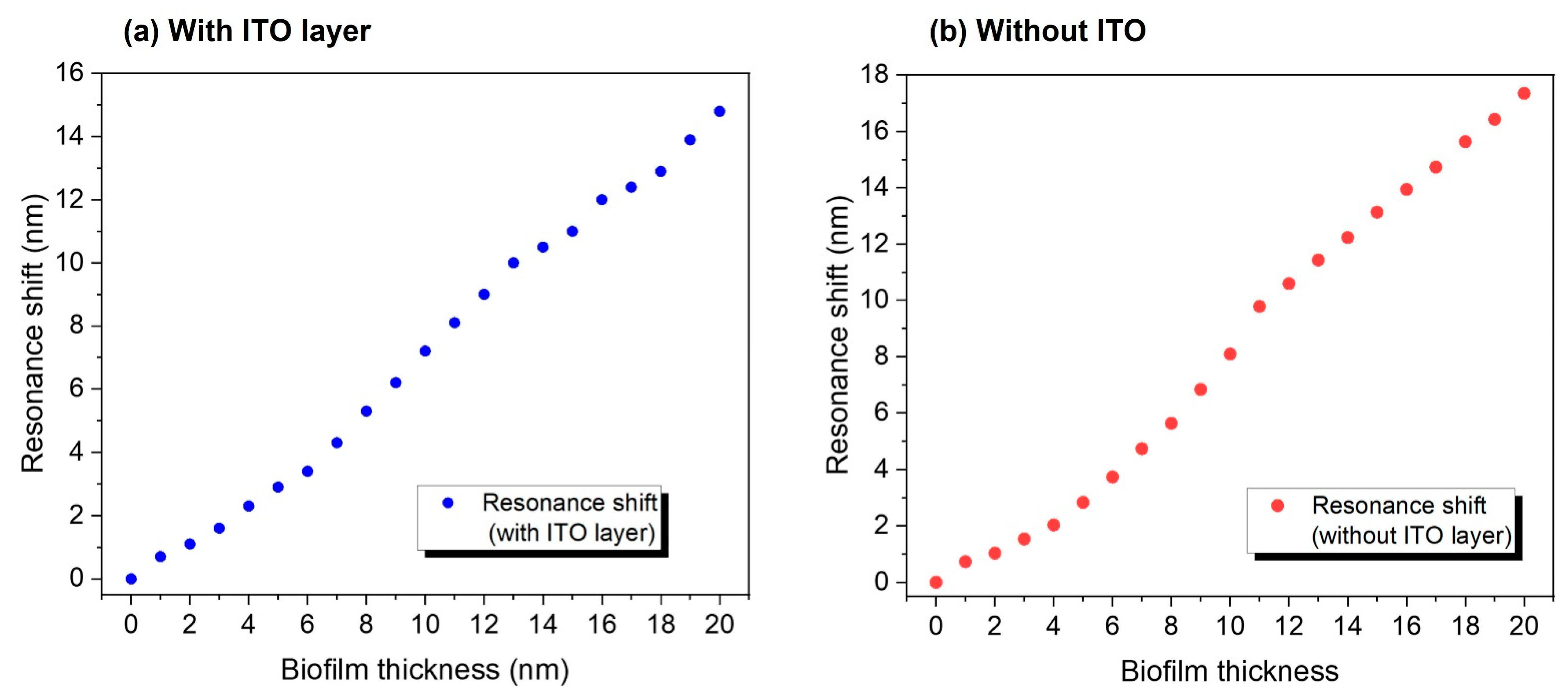
3.3. Biological Detection Performed with R-NPs/ITO Arrays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casquel, R.; Holgado, M.; Laguna, M.F.; Hernández, A.L.; Santamaría, B.; Lavín, Á.; Tramarin, L.; Herreros, P. Engineering Vertically Interrogated Interferometric Sensors for Optical Label-Free Biosensing. Anal. Bioanal. Chem. 2020, 412, 3285–3297. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.F.S.; Castro-Camus, E.; Ottaway, D.J.; López-Higuera, J.M.; Feng, X.; Jin, W.; Jeong, Y.; Picqué, N.; Tong, L.; Reinhard, B.M.; et al. Roadmap on Optical Sensors. J. Opt. (UK) 2017, 19, 083001. [Google Scholar] [CrossRef]
- Peltomaa, R.; Glahn-Martínez, B.; Benito-Peña, E.; Moreno-Bondi, M.C. Optical Biosensors for Label-Free Detection of Small Molecules. Sensors (Basel) 2018, 18, 4126. [Google Scholar] [CrossRef] [PubMed]
- Qazi, H.H.; bin Mohammad, A.B.; Akram, M. Recent Progress in Optical Chemical Sensors. Sensors 2012, 12, 16522–16556. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qiu, C.; Ji, X.; Wang, S.; Shao, G. Plasmon Lattice Resonances Induced by an All-Dielectric Periodic Array of Si Nanopillars on SiO2nanopillars. Nanotechnology 2021, 32, 505206. [Google Scholar] [CrossRef]
- Bezares, F.J.; Long, J.P.; Glembocki, O.J.; Guo, J.; Rendell, R.W.; Kasica, R.; Shirey, L.; Owrutsky, J.C.; Caldwell, J.D. Mie Resonance-Enhanced Light Absorption in Periodic Silicon Nanopillar Arrays. Opt. Express 2013, 21, 27587. [Google Scholar] [CrossRef]
- Kugel, V.; Ji, H.F. Nanopillars for Sensing. J. Nanosci. Nanotechnol. 2014, 14, 6469–6477. [Google Scholar] [CrossRef]
- Hernández, A.L.; Casquel, R.; Holgado, M.; Cornago, I.; Sanza, F.J.; Santamaría, B.; Maigler, M.; Fernández, F.; Lavín, A.; Laguna, M.F. Arrays of Resonant Nanopillars for Biochemical Sensing. Opt. Lett. 2015, 40, 2370. [Google Scholar] [CrossRef]
- Quintero, S.; Casquel, R.; Laguna, M.F.; Holgado, M. Optical Vapor Sensors Based on Periodic Resonant Nanopillar Structures. ACS Omega 2020, 5, 25913–25918. [Google Scholar] [CrossRef]
- Szyszka, B.; Dewald, W.; Gurram, S.K.; Pflug, A.; Schulz, C.; Siemers, M.; Sittinger, V.; Ulrich, S. Recent Developments in the Field of Transparent Conductive Oxide Films for Spectral Selective Coatings, Electronics and Photovoltaics. Curr. Appl. Phys. 2012, 12, S2–S11. [Google Scholar] [CrossRef]
- Minami, T. Present Status of Transparent Conducting Oxide Thin-Film Development for Indium-Tin-Oxide (ITO) Substitutes. Thin Solid Film. 2008, 516, 5822–5828. [Google Scholar] [CrossRef]
- Lopez-Santos, C.; Puerto, D.; Siegel, J.; Macias-Montero, M.; Florian, C.; Gil-Rostra, J.; López-Flores, V.; Borras, A.; González-Elipe, A.R.; Solis, J. Anisotropic Resistivity Surfaces Produced in ITO Films by Laser-Induced Nanoscale Self-Organization. Adv. Opt. Mater. 2021, 9, 2001086. [Google Scholar] [CrossRef]
- Kim, H.; Horwitz, J.S.; Kushto, G.; Piqué, A.; Kafafi, Z.H.; Gilmore, C.M.; Chrisey, D.B. Effect of Film Thickness on the Properties of Indium Tin Oxide Thin Films. J. Appl. Phys. 2000, 88, 6021–6025. [Google Scholar] [CrossRef]
- Paine, D.C.; Whitson, T.; Janiac, D.; Beresford, R.; Yang, C.O.; Lewis, B. A Study of Low Temperature Crystallization of Amorphous Thin Film Indium-Tin-Oxide. J. Appl. Phys. 1999, 85, 8445–8450. [Google Scholar] [CrossRef]
- del Villar, I.; Zamarreño, C.R.; Hernaez, M.; Arregui, F.J.; Matias, I.R. Lossy Mode Resonance Generation with Indium-Tin-Oxide-Coated Optical Fibers for Sensing Applications. J. Lightwave Technol. 2010, 28, 111–117. [Google Scholar] [CrossRef]
- Zamarreño, C.R.; Hernaez, M.; del Villar, I.; Matias, I.R.; Arregui, F.J. Tunable Humidity Sensor Based on ITO-Coated Optical Fiber. Sens. Actuators B Chem. 2010, 146, 414–417. [Google Scholar] [CrossRef]
- Zamarreño, C.R.; Hernáez, M.; del Villar, I.; Matías, I.R.; Arregui, F.J. ITO Coated Optical Fiber Refractometers Based on Resonances in the Infrared Region. IEEE Sens. J. 2010, 10, 365–366. [Google Scholar] [CrossRef]
- Socorro, A.B.; Soltani, S.; del Villar, I.; Corres, J.M.; Armani, A.M. Temperature Sensor Based on a Hybrid ITO-Silica Resonant Cavity. Opt. Express 2015, 23, 1930. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.; Cerruti, M.; Efremenko, A.; Losego, M.; Aspnes, D.E.; Maria, J.P.; Franzen, S. Dependence of Plasmon Polaritons on the Thickness of Indium Tin Oxide Thin Films. J. Appl. Phys. 2008, 103, 093108. [Google Scholar] [CrossRef]
- Mitsubayashi, K.; Wakabayashi, Y.; Tanimoto, S.; Murotomi, D.; Endo, T. Optical-Transparent and Flexible Glucose Sensor with ITO Electrode. Biosens. Bioelectron. 2003, 19, 67–71. [Google Scholar] [CrossRef]
- Ding, Y.; Deng, D.; Zhou, X.; Zhen, W.; Gao, M.; Zhang, Y. Barcode Encryption Based on Negative and Positive Goos-Hänchen Shifts in a Graphene-ITO/TiO2/ITO Sandwich Structure. Opt. Express 2021, 29, 41164. [Google Scholar] [CrossRef]
- Rooney, M.B.; Coomber, D.C.; Bond, A.M. Achievement of Near-Reversible Behavior for the [Fe(CN) 6 ]3−/4− Redox Couple Using Cyclic Voltammetry at Glassy Carbon, Gold, and Platinum Macrodisk Electrodes in the Absence of Added Supporting Electrolyte. Anal. Chem. 2000, 72, 3486–3491. [Google Scholar] [CrossRef] [PubMed]
- Agrisuelas, J.; Giménez-Romero, D.; García-Jareño, J.J.; Vicente, F. Vis/NIR Spectroelectrochemical Analysis of Poly-(Azure A) on ITO Electrode. Electrochem. Commun. 2006, 8, 549–553. [Google Scholar] [CrossRef]
- Śmietana, M.; Koba, M.; Sezemsky, P.; Szot-Karpińska, K.; Burnat, D.; Stranak, V.; Niedziółka-Jönsson, J.; Bogdanowicz, R. Simultaneous Optical and Electrochemical Label-Free Biosensing with ITO-Coated Lossy-Mode Resonance Sensor. Biosens. Bioelectron. 2020, 154, 112050. [Google Scholar] [CrossRef] [PubMed]
- Cornago, I.; Hernández, A.L.; Casquel, R.; Holgado, M.; Laguna, M.F.; Sanza, F.J.; Bravo, J. Bulk Sensing Performance Comparison between Silicon Dioxide and Resonant High Aspect Ratio Nanopillars Arrays Fabricated by Means of Interference Lithography. Opt. Mater. Express 2016, 6, 2264. [Google Scholar] [CrossRef]
- Available online: https://www.synopsys.com/photonic-solutions/rsoft-photonic-device-tools/rsoft-products.html (accessed on 25 September 2022).
- Tsai, M.C.; Chen, P.Y. Voltammetric Study and Electrochemical Detection of Hexavalent Chromium at Gold Nanoparticle-Electrodeposited Indium Tinoxide (ITO) Electrodes in Acidic Media. Talanta 2008, 76, 533–539. [Google Scholar] [CrossRef]
- JCGM. Evaluation of Measurement Data-Guide to the Expression of Uncertainty in Measurement; JCGM: Sèvres, France, 2008. [Google Scholar]
- Alonso, R.; Jiménez-Meneses, P.; García-Rupérez, J.; Bañuls, M.J.; Maquieira, Á. Thiol-Ene Click Chemistry towards Easy Microarraying of Half-Antibodies. Chem. Commun. 2018, 54, 6144–6147. [Google Scholar] [CrossRef]
- Parolo, C.; Sena-Torralba, A.; Bergua, J.F.; Calucho, E.; Fuentes-Chust, C.; Hu, L.; Rivas, L.; Álvarez-Diduk, R.; Nguyen, E.P.; Cinti, S.; et al. Tutorial: Design and Fabrication of Nanoparticle-Based Lateral-Flow Immunoassays. Nat. Protoc. 2020, 15, 3788–3816. [Google Scholar] [CrossRef]
- Barranco, A.; Borras, A.; Gonzalez-Elipe, A.R.; Palmero, A. Perspectives on Oblique Angle Deposition of Thin Films: From Fundamentals to Devices. Prog. Mater. Sci. 2016, 76, 59–153. [Google Scholar] [CrossRef]
- Spindel, S.; Sapsford, K.E. Evaluation of Optical Detection Platforms for Multiplexed Detection of Proteins and the Need for Point-of-Care Biosensors for Clinical Use. Sensors 2014, 14, 22313–22341. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.T.; Lee, Y.C.; Lai, Y.H.; Lim, J.C.; Huang, N.T.; Lin, C.T.; Huang, J.J. Review of Integrated Optical Biosensors for Point-Of-Care Applications. Biosensors 2020, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Tokel, O.; Inci, F.; Demirci, U. Advances in Plasmonic Technologies for Point of Care Applications. Chem. Rev. 2014, 114, 5728–5752. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Liquid | Refractive Index [n] |
|---|---|
| Methanol | 1.328 |
| Deionized water | 1.333 |
| Ethanol | 1.361 |
| Acetic acid | 1.372 |
| 2-propanol | 1.380 |
| Cyclohexane | 1.426 |
| Resonance Position | Resonance Shift | ||||||
|---|---|---|---|---|---|---|---|
| α-Aβ-1-16 | Aβ-40 | Au-NPs with α-Aβ-1-40 | PeptideIncubation | Recognition with Au-NPs | |||
| Biological detection | [nm] | 501.99 | 503.04 | 503.98 | ∆nm | 1.05 | 0.94 |
| [cm−1] | 19,905 | 19,863 | 19,829 | ∆cm−1 | −42 | −34 | |
| Negative control | [nm] | 501.15 | 500.90 | 501.16 | ∆nm | −0.25 | 0.26 |
| [cm−1] | 19,942 | 19,953 | 19,942 | ∆cm−1 | 11 | −11 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tramarin, L.; Casquel, R.; Gil-Rostra, J.; González-Martínez, M.Á.; Herrero-Labrador, R.; Murillo, A.M.M.; Laguna, M.F.; Bañuls, M.-J.; González-Elipe, A.R.; Holgado, M. Design and Characterization of ITO-Covered Resonant Nanopillars for Dual Optical and Electrochemical Sensing. Chemosensors 2022, 10, 393. https://doi.org/10.3390/chemosensors10100393
Tramarin L, Casquel R, Gil-Rostra J, González-Martínez MÁ, Herrero-Labrador R, Murillo AMM, Laguna MF, Bañuls M-J, González-Elipe AR, Holgado M. Design and Characterization of ITO-Covered Resonant Nanopillars for Dual Optical and Electrochemical Sensing. Chemosensors. 2022; 10(10):393. https://doi.org/10.3390/chemosensors10100393
Chicago/Turabian StyleTramarin, Luca, Rafael Casquel, Jorge Gil-Rostra, Miguel Ángel González-Martínez, Raquel Herrero-Labrador, Ana María M. Murillo, María Fe Laguna, María-José Bañuls, Agustín R. González-Elipe, and Miguel Holgado. 2022. "Design and Characterization of ITO-Covered Resonant Nanopillars for Dual Optical and Electrochemical Sensing" Chemosensors 10, no. 10: 393. https://doi.org/10.3390/chemosensors10100393







