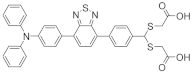
Mercury Ion Chemosensor Derived from Barbiturate Acid with Aggregation-Induced Emission Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Synthesis and Characterization of Compounds [24,25]
3. Results
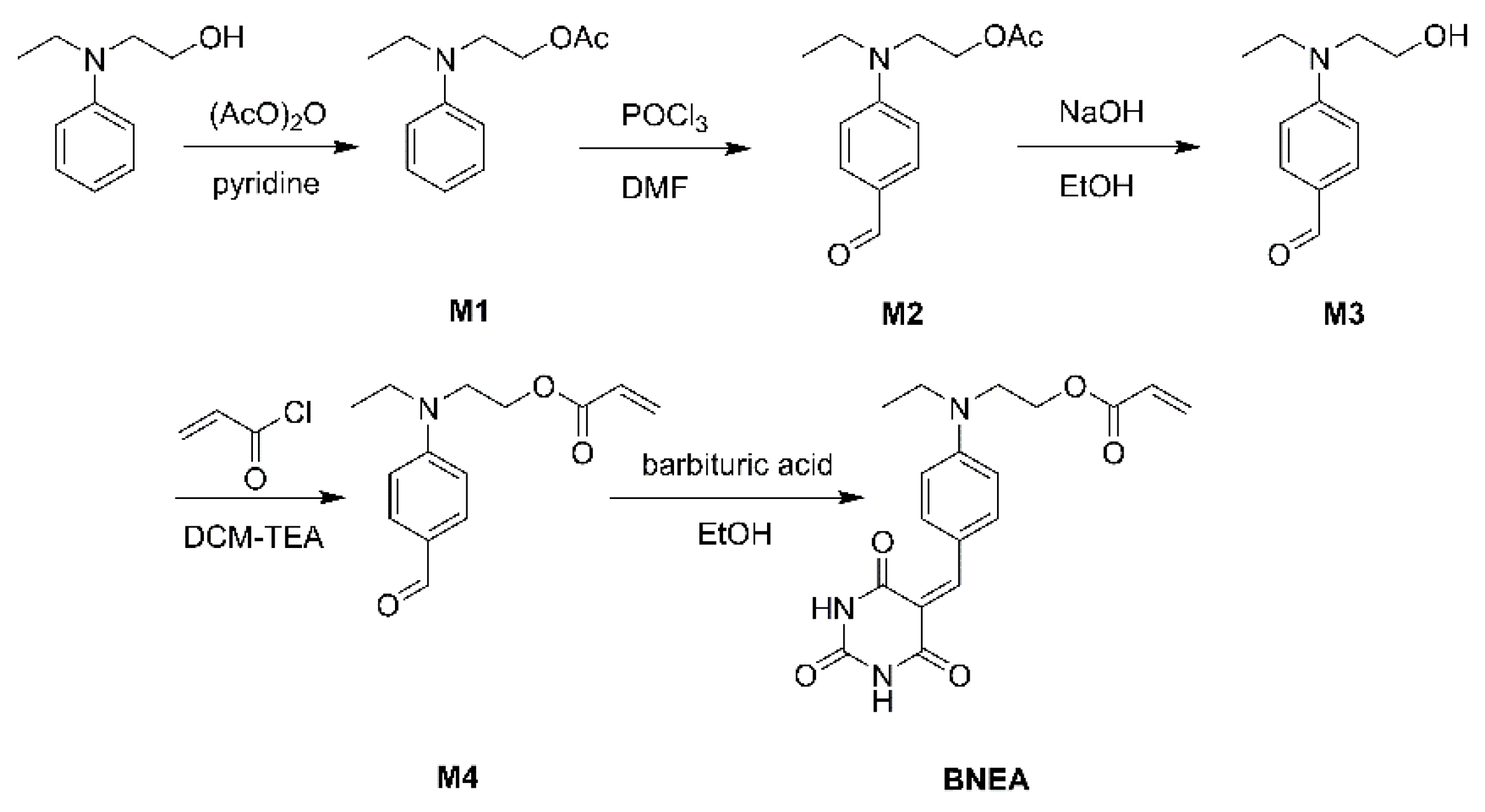
3.1. Synthesis of Monomers
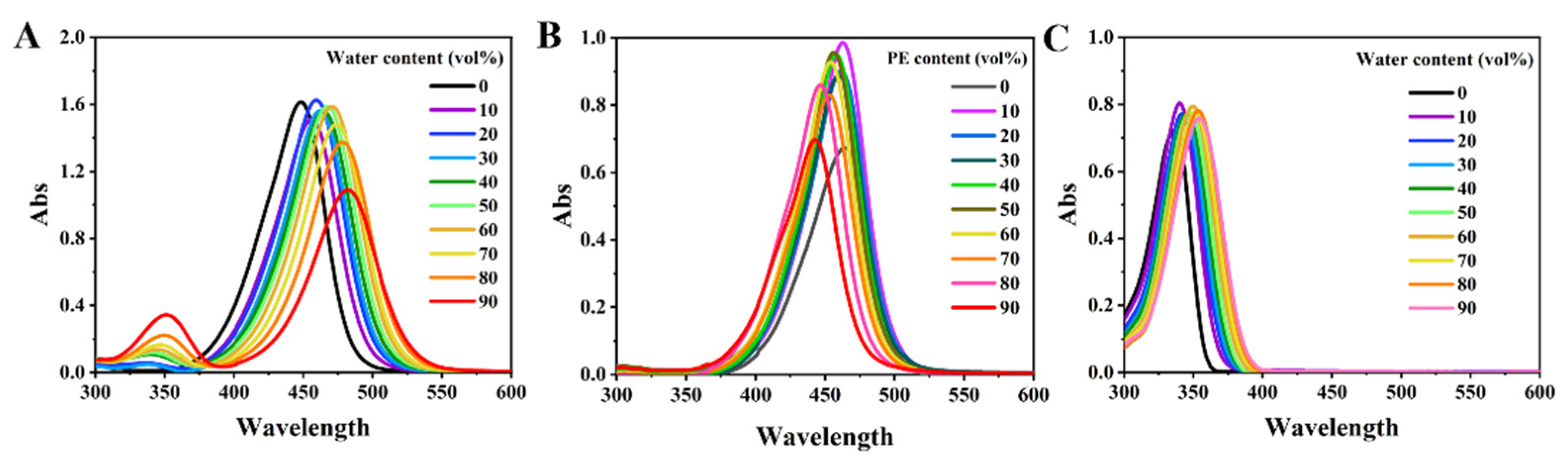
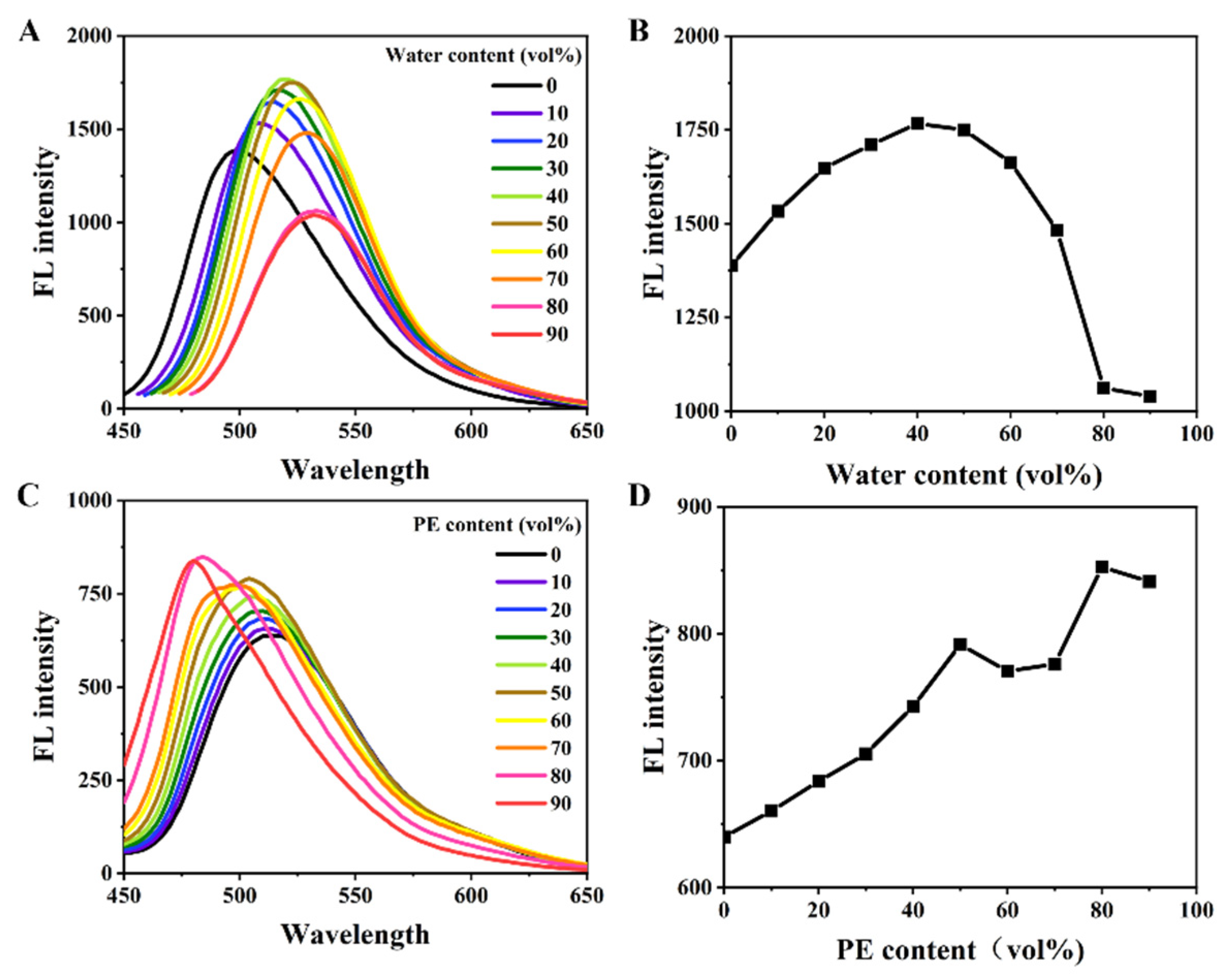
3.2. Aggregation-Induced Emission Phenomenon
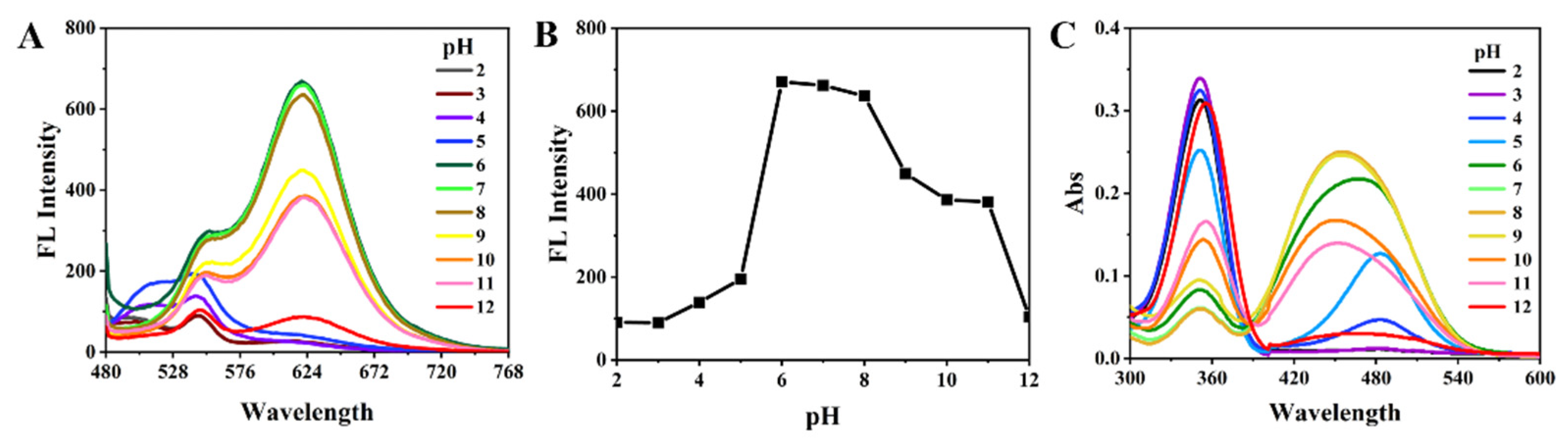
3.3. Response of BNEA to pH
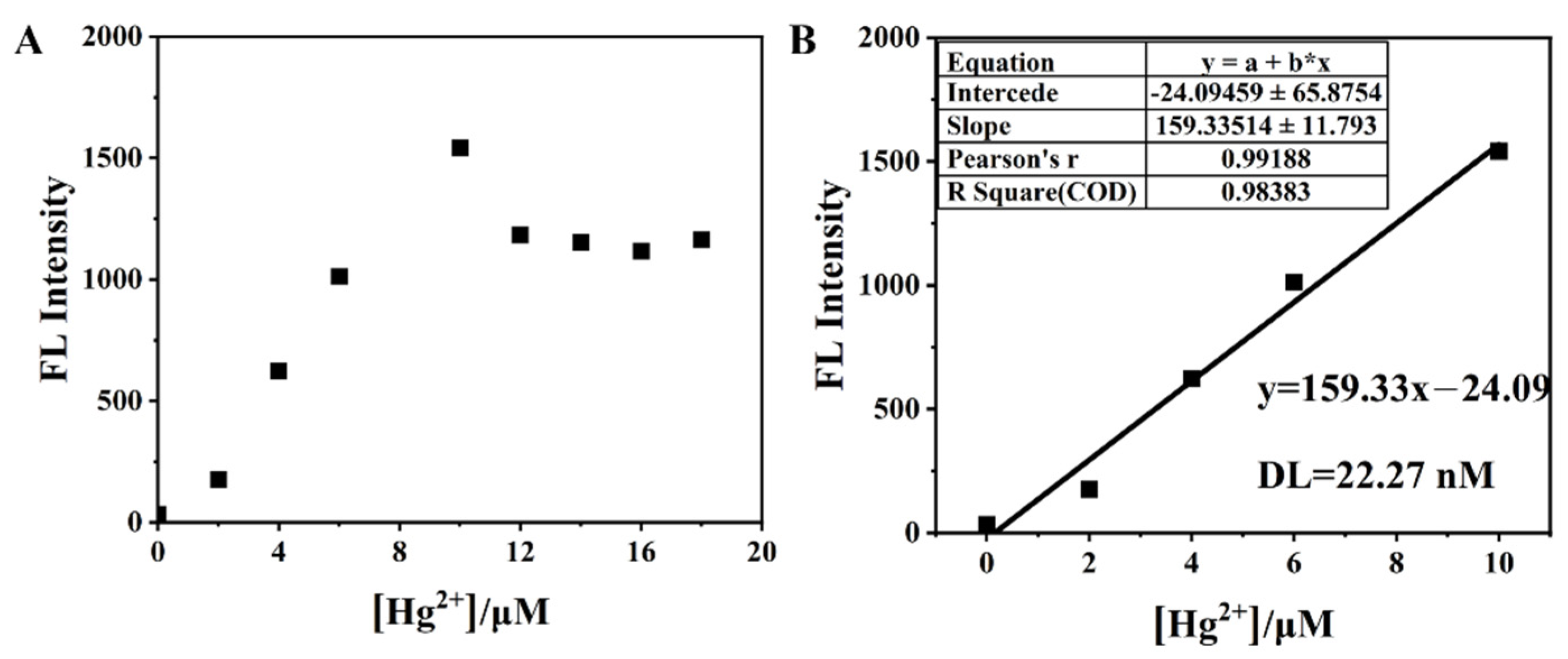
3.4. Effect of Hg2+ on BNEA Fluorescence in 99% Aqueous Solution (VH2O:VDMSO = 99:1)
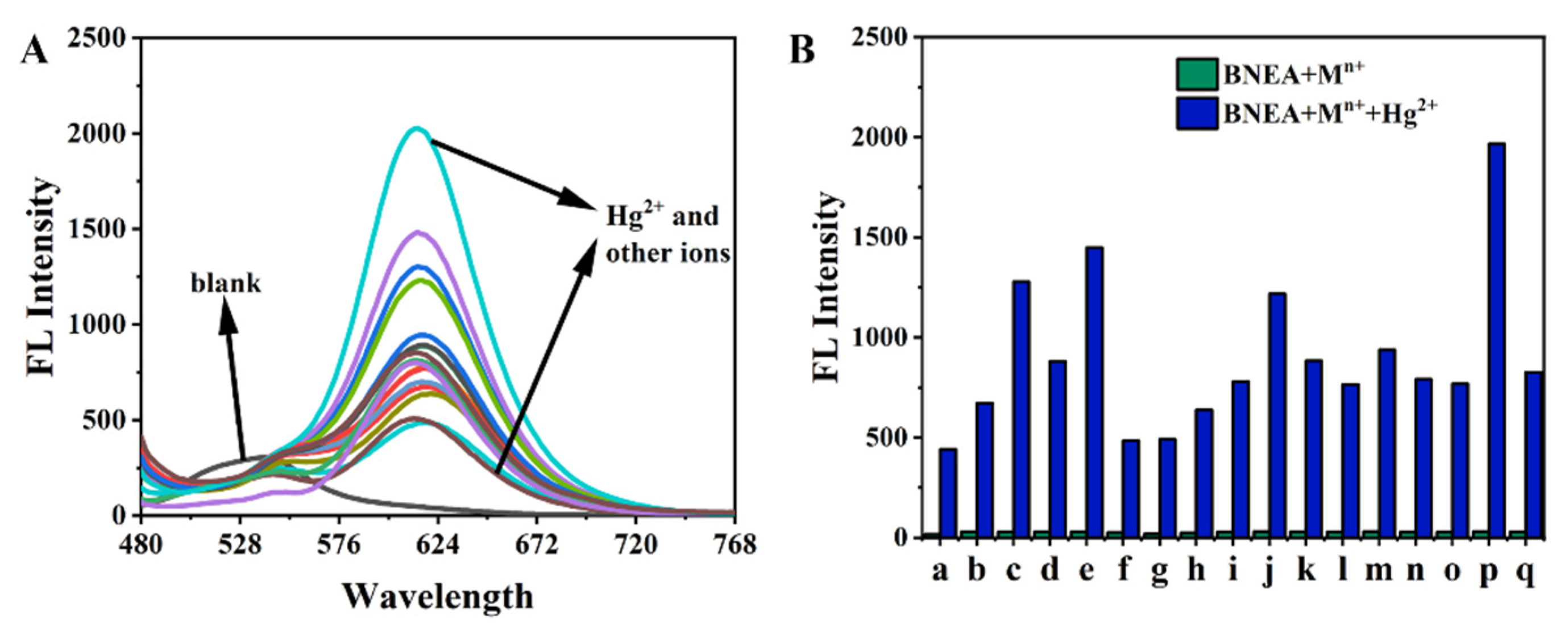
3.5. Anti-Interference Ability of BNEA for Cations in Water
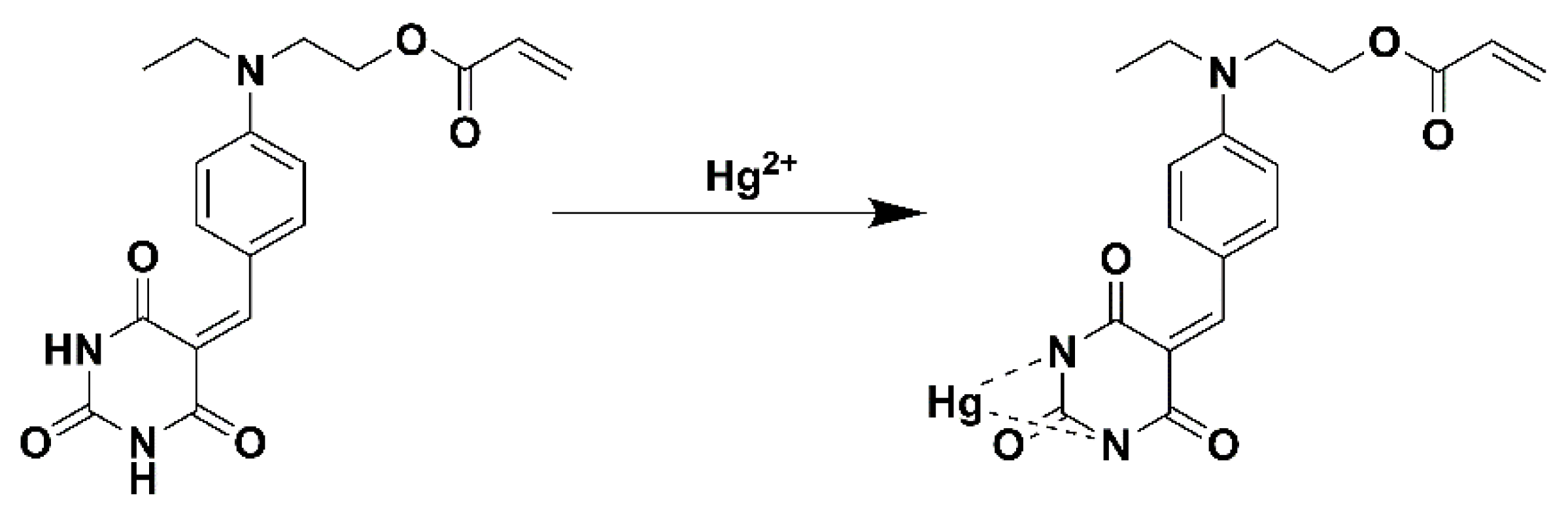
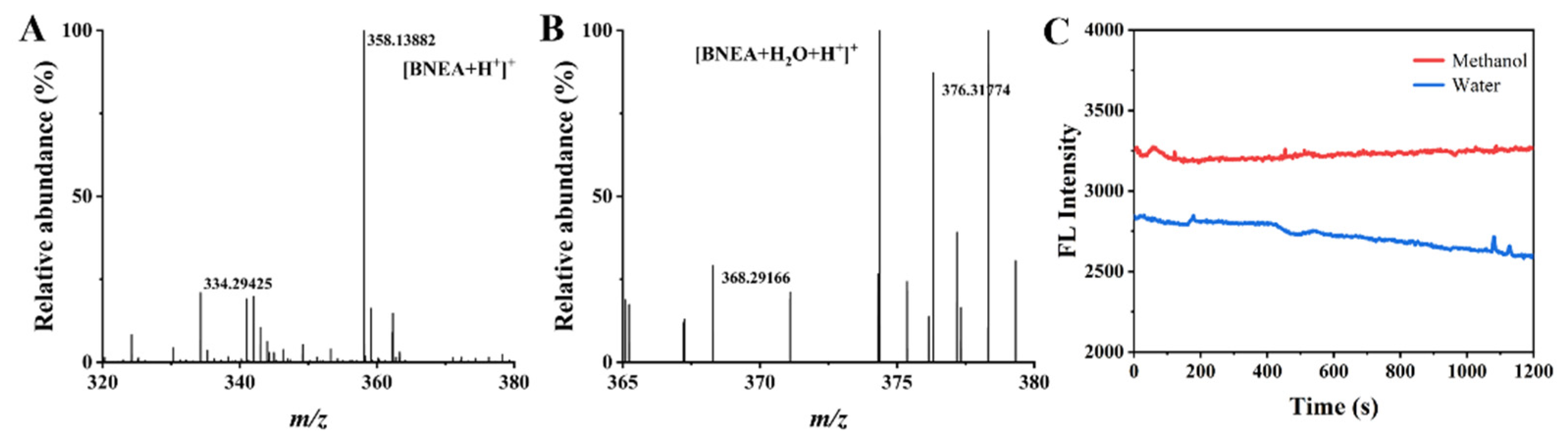
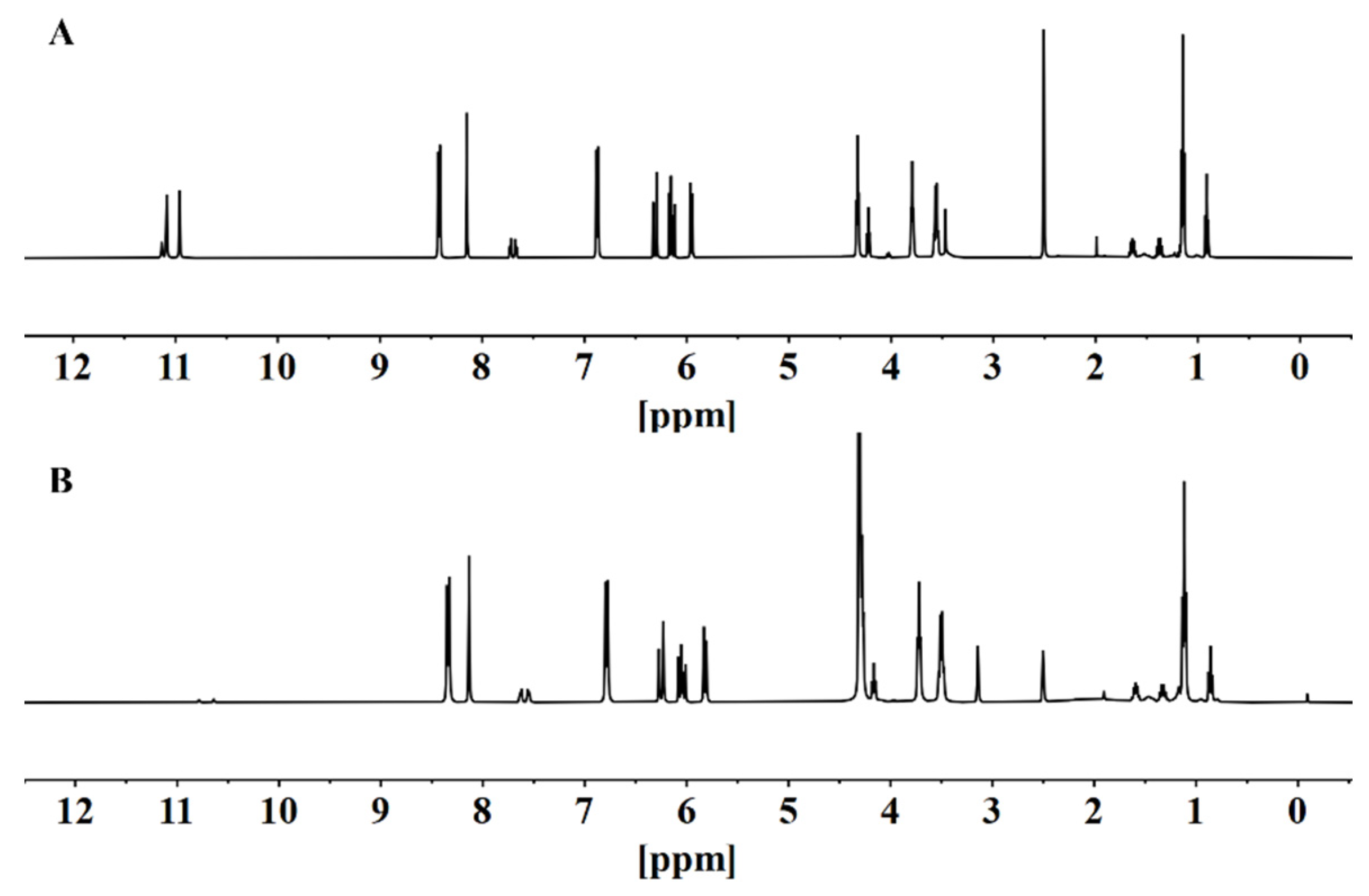
3.6. Complexation Mechanism of Hg2+ and BNEA
3.7. Detection of BNEA in Real Water Samples
3.8. Comparison with other AIE Probes Targeting Metal Ions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, P.; Xue, S.; Chen, B.; Liao, F. A novel peptide-based fluorescent probe for highly selective detection of mercury (II) ions in real water samples and living cells based on aggregation-induced emission effect. Anal. Bioanal. Chem. 2022, 414, 4717–4726. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Jiang, L.; Shao, Q.; Liu, X.; Marks, R.S.; Ma, J.; Chen, X. Colorimetric detection of mercury ions based on plasmonic nanoparticles. Small 2013, 9, 1467–1481. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, H.; Hirata, A.; Wu, H.; Xue, Q.-K.; Chen, M. Nanoporous gold based optical sensor for sub-ppt detection of mercury ions. ACS Nano 2013, 7, 4595–4600. [Google Scholar] [CrossRef]
- Su, Y.; Su, L.; Liu, B.; Lin, Y.; Tang, D. Self-Powered Photoelectrochemical Assay for Hg2+ Detection Based on g-C3N4-CdS-CuO Composites and Redox Cycle Signal Amplification Strategy. Chemosensors 2022, 10, 286. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Su, M.; Rong, X.; Wang, X.; Wang, K.; Li, X.; Zhu, H.; Yu, M.; Sheng, W.; et al. A highly selective barbiturate-based fluorescent probe for detecting Hg2+ in cells and zebrafish as well as in real water samples. J. Photochem. Photobiol. A Chem. 2022, 425, 113706. [Google Scholar] [CrossRef]
- Liu, J.; Tai, W.; Wang, D.; Su, J.; Yu, L. Cholesteric Liquid Crystal Photonic Hydrogel Films Immobilized with Urease Used for the Detection of Hg2+. Chemosensors 2022, 10, 140. [Google Scholar] [CrossRef]
- Lv, H.; Sun, H. A novel coumarin-benzopyrylium based near-infrared fluorescent probe for Hg(2+) and its practical applications. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2022, 267, 120527. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef]
- Yang, T.; Qin, J.; Zhang, J.; Guo, L.; Yang, M.; Wu, X.; You, M.; Peng, H.J.C. Recent Progresses in NIR-II Luminescent Bio/Chemo Sensors Based on Lanthanide Nanocrystals. Chemosensors 2022, 10, 206. [Google Scholar] [CrossRef]
- Yue, Y.; Huo, F.; Yin, C.; Escobedo, J.O.; Strongin, R.M.J.A. Recent progress in chromogenic and fluorogenic chemosensors for hypochlorous acid. Analyst 2016, 141, 1859–1873. [Google Scholar] [CrossRef] [Green Version]
- Mei, J.; Hong, Y.; Lam, J.W.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-induced emission: The whole is more brilliant than the parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, J.; Zhang, G.; Liu, S. Highly selective fluorogenic multianalyte biosensors constructed via enzyme-catalyzed coupling and aggregation-induced emission. J. Am. Chem. Soc. 2014, 136, 9890–9893. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Lam, J.W.; Qin, W.; Li, J.; Xie, N.; Tang, B.Z. Molecular luminogens based on restriction of intramolecular motions through host-guest inclusion for cell imaging. Chem. Commun. 2014, 50, 1725–1727. [Google Scholar] [CrossRef]
- Zhang, K.-R.; Hu, M.; Luo, J.; Ye, F.; Zhou, T.-T.; Yuan, Y.-X.; Gao, M.-L.; Zheng, Y.-S. Pseudo-crown ether having AIE and PET effects from a TPE-CD conjugate for highly selective detection of mercury ions. Chin. Chem. Lett. 2022, 33, 1505–1510. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.J.C.C. Aggregation-induced emission of 1-methyl-1, 2, 3, 4, 5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; He, J.; Zhang, J.; Zhou, H.; Gao, C. A novel red-emitting fluorescent probe for the highly selective detection of Hg2+ ion with AIE mechanism. Chem. Phys. 2020, 539, 110944. [Google Scholar] [CrossRef]
- Liu, L.; Ma, J.; Pan, J.; Li, D.; Wang, H.; Yang, H. The preparation of novel triphenylamine-based AIE-effect fluorescent probe for selectively detecting mercury(ii) ion in aqueous solution. New J. Chem. 2021, 45, 5049–5059. [Google Scholar] [CrossRef]
- Shan, Y.; Yao, W.; Liang, Z.; Zhu, L.; Yang, S.; Ruan, Z. Reaction-based AIEE-active conjugated polymer as fluorescent turn on probe for mercury ions with good sensing performance. Dye. Pigment. 2018, 156, 1–7. [Google Scholar] [CrossRef]
- Ma, J.; Xiao, Y.; Zhang, C.; Zhang, M.; Wang, Q.; Zheng, W.; Zhang, S. Preparation a novel pyrene-based AIE-active ratiometric turn-on fluorescent probe for highly selective and sensitive detection of Hg2+. Mater. Sci. Eng. B 2020, 259, 114582. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, C.; Xiao, Y.; Zhang, M.; Wang, Q.; Zheng, W.; Zhang, S. Preparation 2-(anthracen-9-yl)-1,3-dithiolane as a novel dual-channel AIE-active fluorescent probe for mercury (II) ion with excellent performance. J. Photochem. Photobiol. A Chem. 2019, 378, 142–146. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, S.; Lei, Q.; Chen, F.; Zheng, F.; Zhong, S.; Huang, X.; Feng, B.; Feng, X.; Zeng, W. The exquisite integration of ESIPT, PET and AIE for constructing fluorescent probe for Hg(II) detection and poisoning. Chin. Chem. Lett. 2022, 33, 1861–1864. [Google Scholar] [CrossRef]
- Pannipara, M.; Al-Sehemi, A.G.; Irfan, A.; Assiri, M.; Kalam, A.; Al-Ammari, Y.S. AIE active multianalyte fluorescent probe for the detection of Cu(2+), Ni(2+) and Hg(2+) ions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 201, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, X.; Chen, Q.; Jiang, G.; Wang, H.; Wang, J. New switch on fluorescent probe with AIE characteristics for selective and reversible detection of mercury ion in aqueous solution. Anal. Biochem. 2019, 585, 113403. [Google Scholar] [CrossRef]
- He, M.; Twieg, R.J.; Gubler, U.; Wright, D.; Moerner, W.J.C.O.M. Synthesis and photorefractive properties of multifunctional glasses. Chem. Mater. 2003, 15, 1156–1164. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Yue, Y.; Chao, J.; Huo, F.; Yin, C. Based on morpholine as luminescence mechanism regulation and organelle targeting dual function Cys NIR specific biological imaging probe. Sens. Actuators B Chem. 2020, 320, 128348. [Google Scholar] [CrossRef]
- Singh, R.B.; Mahanta, S.; Kar, S.; Guchhait, N. Spectroscopic and theoretical evidence for the photoinduced twisted intramolecular charge transfer state formation in N,N-dimethylaminonaphthyl-(acrylo)-nitrile. J. Lumin. 2008, 128, 1421–1430. [Google Scholar] [CrossRef]
- Yun, D.; Chae, J.B.; So, H.; Lee, H.; Kim, K.-T.; Kim, C. Sensing of zinc ions and sulfide using a highly practical and water-soluble fluorescent sensor: Applications in test kits and zebrafish. New J. Chem. 2020, 44, 442–449. [Google Scholar] [CrossRef]
- Li, R.-Y.; Wei, Z.-L.; Wang, L.; Zhang, Y.; Ru, J.-X. A new salamo-based fluorescence probe to visually detect aluminum(III) ion and bio-imaging in zebrafish. Microchem. J. 2021, 162, 105720. [Google Scholar] [CrossRef]
- Yin, Z.-Y.; Hu, J.-H.; Gui, K.; Fu, Q.-Q.; Yao, Y.; Zhou, F.-L.; Ma, L.-L.; Zhang, Z.-P. AIE based colorimetric and “turn-on” fluorescence Schiff base sensor for detecting Fe3+ in an aqueous media and its application. J. Photochem. Photobiol. A Chem. 2020, 396, 112542. [Google Scholar] [CrossRef]
- Zou, Q.; Du, J.; Gu, C.; Zhang, D.; Tao, F.; Cui, Y. A new dibenzothiophene-based dual-channel chemosensor for cyanide with aggregation induced emission. J. Photochem. Photobiol. A Chem. 2021, 405, 112993. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Gao, L.; Tao, F.; Wu, S.; Cui, Y. Mercury Ion Chemosensor Derived from Barbiturate Acid with Aggregation-Induced Emission Effect. Chemosensors 2022, 10, 422. https://doi.org/10.3390/chemosensors10100422
Guo X, Gao L, Tao F, Wu S, Cui Y. Mercury Ion Chemosensor Derived from Barbiturate Acid with Aggregation-Induced Emission Effect. Chemosensors. 2022; 10(10):422. https://doi.org/10.3390/chemosensors10100422
Chicago/Turabian StyleGuo, Xuezu, Lanlan Gao, Furong Tao, Shining Wu, and Yuezhi Cui. 2022. "Mercury Ion Chemosensor Derived from Barbiturate Acid with Aggregation-Induced Emission Effect" Chemosensors 10, no. 10: 422. https://doi.org/10.3390/chemosensors10100422