Design and Evaluation of Low-Power Co3O4 Gas Sensing Element as a Part of Cyber Physical Systems
Abstract
:1. Introduction
2. Design and Evaluation of CPS
2.1. Preparation of Co3O4 Powder and Sensing Film
2.2. Characterization of Materials
2.3. Gas Sensing Measurements
2.4. Measurement Section
2.5. MCU Performance
2.6. Data Post-Processing and Noise Mitigation
3. Results and Discussion
3.1. Structural and Morphological Properties of Powder and Sensing Elements of Co3O4
3.2. System Evaluation with Sensing Element of Co3O4 Nanoparticles
3.3. Stability of Co3O4 Sensing Element on Different Setups
3.4. Power Consumption and Quantitative Analysis
4. Sensing Mechanism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, E.A. Cyber Physical Systems: Design Challenges. In Proceedings of the 2008 11th IEEE International Symposium on Object and Component-Oriented Real-Time Distributed Computing (ISORC), Orlando, FL, USA, 5–7 May 2008; pp. 363–369. [Google Scholar] [CrossRef] [Green Version]
- Bagula, A.; Ajayi, O.; Maluleke, H. Cyber Physical Systems Dependability Using CPS-IOT Monitoring. Sensors 2021, 21, 2761. [Google Scholar] [CrossRef] [PubMed]
- Ionascu, M.; Castell, N.; Boncalo, O.; Schneider, P.; Darie, M.; Marcu, M. Calibration of CO, NO2, and O3 Using Airify: A Low-Cost Sensor Cluster for Air Quality Monitoring. Sensors 2021, 21, 7977. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.; Lorite, G.S.; Rautio, A.R.; Juhász, K.L.; Kukovecz, Á.; Kónya, Z.; Kordas, K.; Toth, G. Portable Cyber-Physical System for Indoor and Outdoor Gas Sensing. Sens. Actuators B Chem. 2017, 252, 983–990. [Google Scholar] [CrossRef] [Green Version]
- Sinha, N.; Pujitha, K.E.; Alex, J.S.R. Xively Based Sensing and Monitoring System for IoT. In Proceedings of the 2015 International Conference on Computer Communication and Informatics (ICCCI), Coimbatore, India, 8–10 January 2015; pp. 8–13. [Google Scholar] [CrossRef]
- Heyasa, B.B.L.; Galarpe, V.R.K.R. Preliminary Development and Testing of Microcontroller-MQ2 Gas Sensorfor University Air Quality Monitoring. IOSR J. Electr. Electron. Eng. 2017, 12, 47–53. [Google Scholar] [CrossRef]
- Mujawar, T.H.; Bachuwar, V.D.; Kasbe, M.S.; Shaligram, A.D.; Deshmukh, L.P. Development of Wireless Sensor Network System for LPG Gas Leakage Detection System. Int. J. Sci. Eng. Res. 2015, 6, 558–563. [Google Scholar] [CrossRef]
- Dewi, L.; Somantri, Y. Wireless Sensor Network on LPG Gas Leak Detection and Automatic Gas Regulator System Using Arduino. IOP Conf. Ser. Mater. Sci. Eng. 2018, 384, 12064. [Google Scholar] [CrossRef]
- Sakayo, N.M.; Mutuku, J.N.; Ngaruiya, J.M. Design and Calibration of a Microcontroller Based MQ-4 Gas Sensor for Domestic Cooking Gas System. Int. J. Appl. Phys. 2019, 6, 31–40. [Google Scholar] [CrossRef]
- Wu, J.; Gao, H.; Yao, S.; Chen, L.; Gao, Y.; Zhang, H. Degradation of Crystal Violet by Catalytic Ozonation Using Fe/Activated Carbon Catalyst. Sep. Purif. Technol. 2015, 147, 179–185. [Google Scholar] [CrossRef]
- Magara, Y.; Itoh, M.; Morioka, T. Application of Ozone to Water Treatment and Power Consumption of Ozone Generating Systems. Prog. Nucl. Energy 1995, 29, 175–182. [Google Scholar] [CrossRef]
- Kumarage, G.W.C.; Comini, E. Low-Dimensional Nanostructures Based on Cobalt Oxide (Co3O4) in Chemical-Gas Sensing. Chemosensors 2021, 9, 197. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.Y.; Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Co3O4-Loaded ZnO Nanofibers for Excellent Hydrogen Sensing. Int. J. Hydrog. Energy 2019, 44, 27499–27510. [Google Scholar] [CrossRef]
- Yoon, J.W.; Choi, J.K.; Lee, J.H. Design of a Highly Sensitive and Selective C2H5OH Sensor Using P-Type Co3O4 Nanofibers. Sens. Actuators B Chem. 2012, 161, 570–577. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, Z.; Ye, Z.; Zhu, L. Gas Sensors Based on Ultrathin Porous Co3O4 Nanosheets to Detect Acetone at Low Temperature. RSC Adv. 2015, 5, 59976–59982. [Google Scholar] [CrossRef]
- Akamatsu, T.; Itoh, T.; Masuda, Y.; Shin, W.; Matsubara, I.; Kida, M. Gas Sensor Properties of Nanopore-Bearing Co3O4 Particles Containing Pt or Pd Particles. J. Asian Ceram. Soc. 2020, 8, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.; El-Safty, S.A. Meso- and Macroporous Co3O4 Nanorods for Effective VOC Gas Sensors. J. Phys. Chem. C 2011, 115, 8466–8474. [Google Scholar] [CrossRef]
- Liu, L.; Li, T.; Yi, Z.; Chi, F.; Lin, Z.; Zhang, X.; Xu, K. Conductometric Ozone Sensor Based on Mesoporous Ultrafine Co3O4 Nanobricks. Sens. Actuators B Chem. 2019, 297, 126815. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Kheel, H.; Lee, C. Oxidizing Gas Sensing Properties of the N-ZnO/p-Co3O4 Composite Nanoparticle Network Sensor. Sens. Actuators B Chem. 2016, 222, 1193–1200. [Google Scholar] [CrossRef]
- Akamatsu, T.; Itoh, T.; Izu, N.; Shin, W.; Sato, K. Sensing Properties of Pd-Loaded Co3O4 Film for a Ppb-Level NO Gas Sensor. Sensors 2015, 15, 8109–8120. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gao, J.; Fu, P.; Zhe, C.; Wang, S.; Lin, Z. The Preparation and Ozone-Sensing Performance of Co3O4 Nanobricks. J. Mater. Sci. Mater. Electron. 2019, 30, 9678–9682. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Blinov, I.; Brinzari, V.; Stetter, J.R. Effect of Air Humidity on Gas Response of SnO2 Thin Film Ozone Sensors. Sens. Actuators B Chem. 2007, 122, 519–526. [Google Scholar] [CrossRef]
- Mills, S.; Lee, B.; Misra, V. Room Temperature Ozone and Humidity Response Evolution of Atomic Layer Deposited SnO2 Sensors. In Proceedings of the IEEE Sensors, Glasgow, UK, 1–3 December 2017. [Google Scholar] [CrossRef]
- Bârsan, N.; Stetter, J.R.; Findlay, M.; Göpel, W. High-Performance Gas Sensing of CO: Comparative Tests Semiconducting (SnO2-Based) and for Amperometric Gas Sensors. Anal. Chem. 1999, 71, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, D.D.M.; Sadaiyandi, K.; Mahendran, M.; Sagadevan, S. Precipitation Method and Characterization of Cobalt Oxide Nanoparticles. Appl. Phys. A Mater. Sci. Process. 2017, 123, 1–6. [Google Scholar] [CrossRef]
- Mayedwa, N.; Mongwaketsi, N.; Khamlich, S.; Kaviyarasu, K.; Matinise, N.; Maaza, M. Green Synthesis of Nickel Oxide, Palladium and Palladium Oxide Synthesized via Aspalathus Linearis Natural Extracts: Physical Properties & Mechanism of Formation. Appl. Surf. Sci. 2018, 446, 266–272. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceramics 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Theory of Power Laws for Semiconductor Gas Sensors. Sens. Actuators B Chem. 2008, 128, 566–573. [Google Scholar] [CrossRef]
- Hua, Z.; Qiu, Z.; Li, Y.; Zeng, Y.; Wu, Y.; Tian, X.; Wang, M.; Li, E. A Theoretical Investigation of the Power-Law Response of Metal Oxide Semiconductor Gas Sensors ΙI: Size and Shape Effects. Sens. Actuators B Chem. 2018, 255, 3541–3549. [Google Scholar] [CrossRef]
- Choi, S.D.; Min, B.K. Co3O4-Based Isobutane Sensor Operating at Low Temperatures. Sens. Actuators B Chem. 2001, 77, 330–334. [Google Scholar] [CrossRef]
- Joshi, N.; Da Silva, L.F.; Jadhav, H.; M’Peko, J.C.; Millan Torres, B.B.; Aguir, K.; Mastelaro, V.R.; Oliveira, O.N. One-Step Approach for Preparing Ozone Gas Sensors Based on Hierarchical NiCo2O4 Structures. RSC Adv. 2016, 6, 92655–92662. [Google Scholar] [CrossRef] [Green Version]
- Onofre, Y.J.; Catto, A.C.; Bernardini, S.; Fiorido, T.; Aguir, K.; Longo, E.; Mastelaro, V.R.; da Silva, L.F.; de Godoy, M.P.F. Highly Selective Ozone Gas Sensor Based on Nanocrystalline Zn0.95Co0.05O Thin Film Obtained via Spray Pyrolysis Technique. Appl. Surf. Sci. 2019, 478, 347–354. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Mastelaro, V.R.; Escanhoela, C.A. In-Depth Understanding of the Relation between CuAlO2 Particle Size and Morphology for Ozone Gas Sensor Detection at a Nanoscale Level. ACS Appl. Mater. Interfaces 2014, 6, 21739–21749. [Google Scholar] [CrossRef]
- Petromichelaki, E.; Gagaoudakis, E.; Moschovis, K.; Tsetseris, L.; Anthopoulos, T.D.; Kiriakidis, G.; Binas, V. Highly Sensitive and Room Temperature Detection of Ultra-Low Concentrations of O3 Using Self-Powered Sensing Elements of Cu2O Nanocubes. Nanoscale Adv. 2019, 1, 2009–2017. [Google Scholar] [CrossRef] [Green Version]
- Paralikis, A.; Gagaoudakis, E.; Kampitakis, V.; Aperathitis, E.; Kiriakidis, G.; Binas, V. Study on the Ozone Gas Sensing Properties of Rf-Sputtered Al-Doped Nio Films. Appl. Sci. 2021, 11, 3104. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Xuan, L.; Zhu, Z.; Pan, Q.; Shi, K.; Zhang, G. Novel Co3O4 Nanocrystalline Chain Material as a High Performance Gas Sensor at Room Temperature. J. Alloys Compd. 2018, 768, 190–197. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas Sensing Mechanisms of Metal Oxide Semiconductors: A Focus Review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.H. Highly Sensitive and Selective Gas Sensors Using P-Type Oxide Semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]

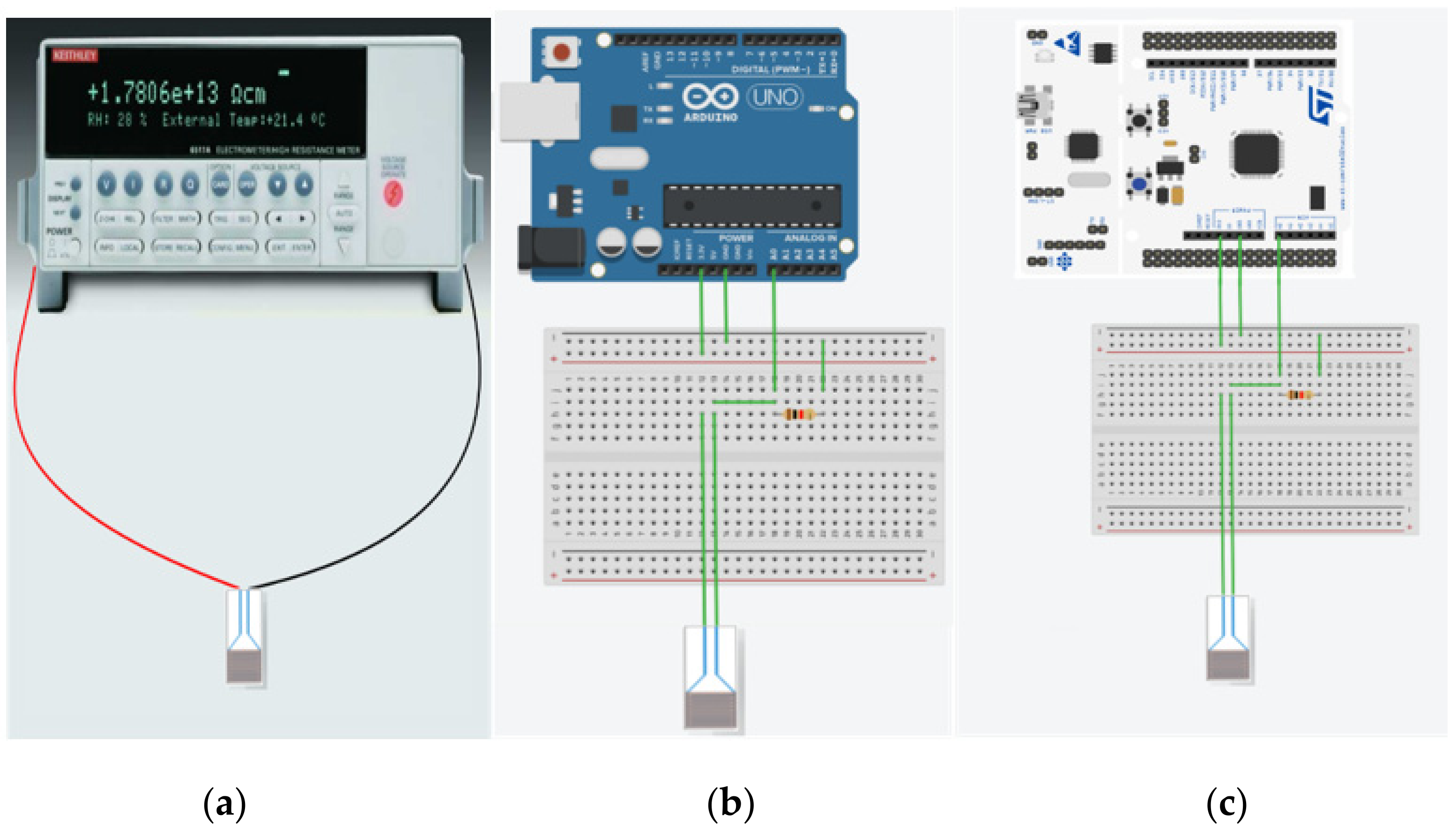
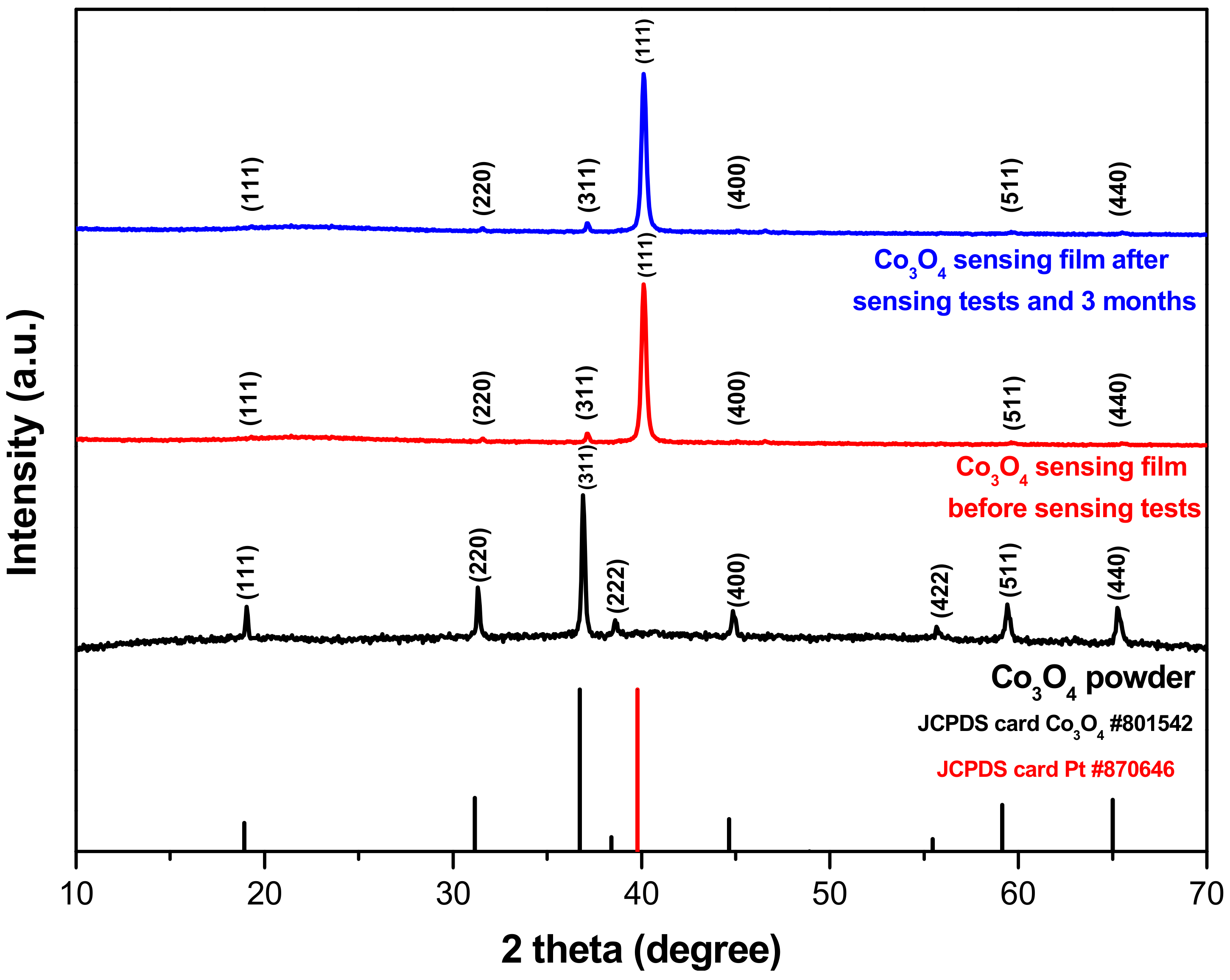
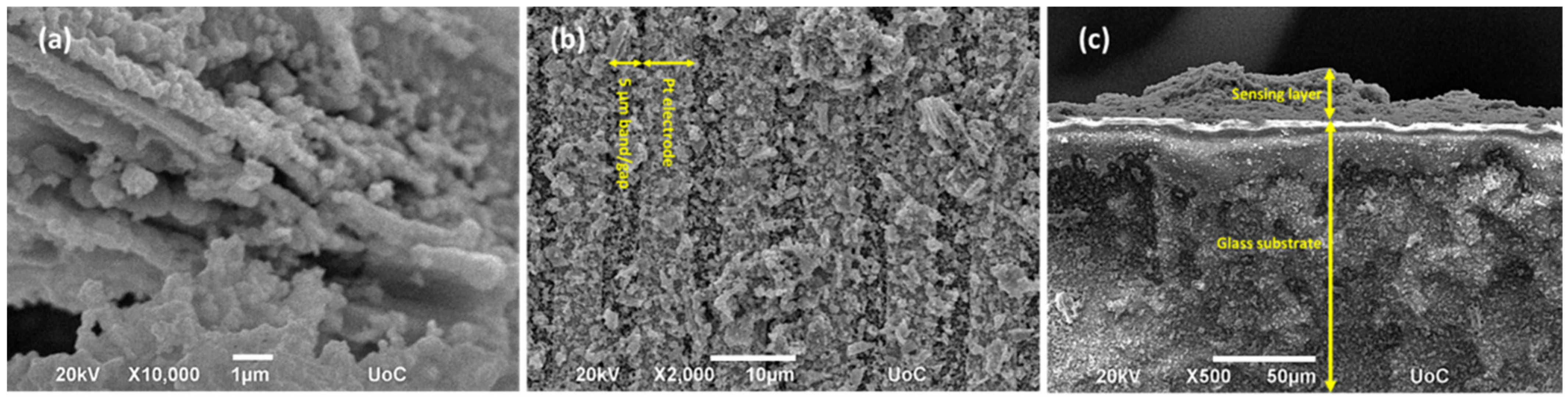

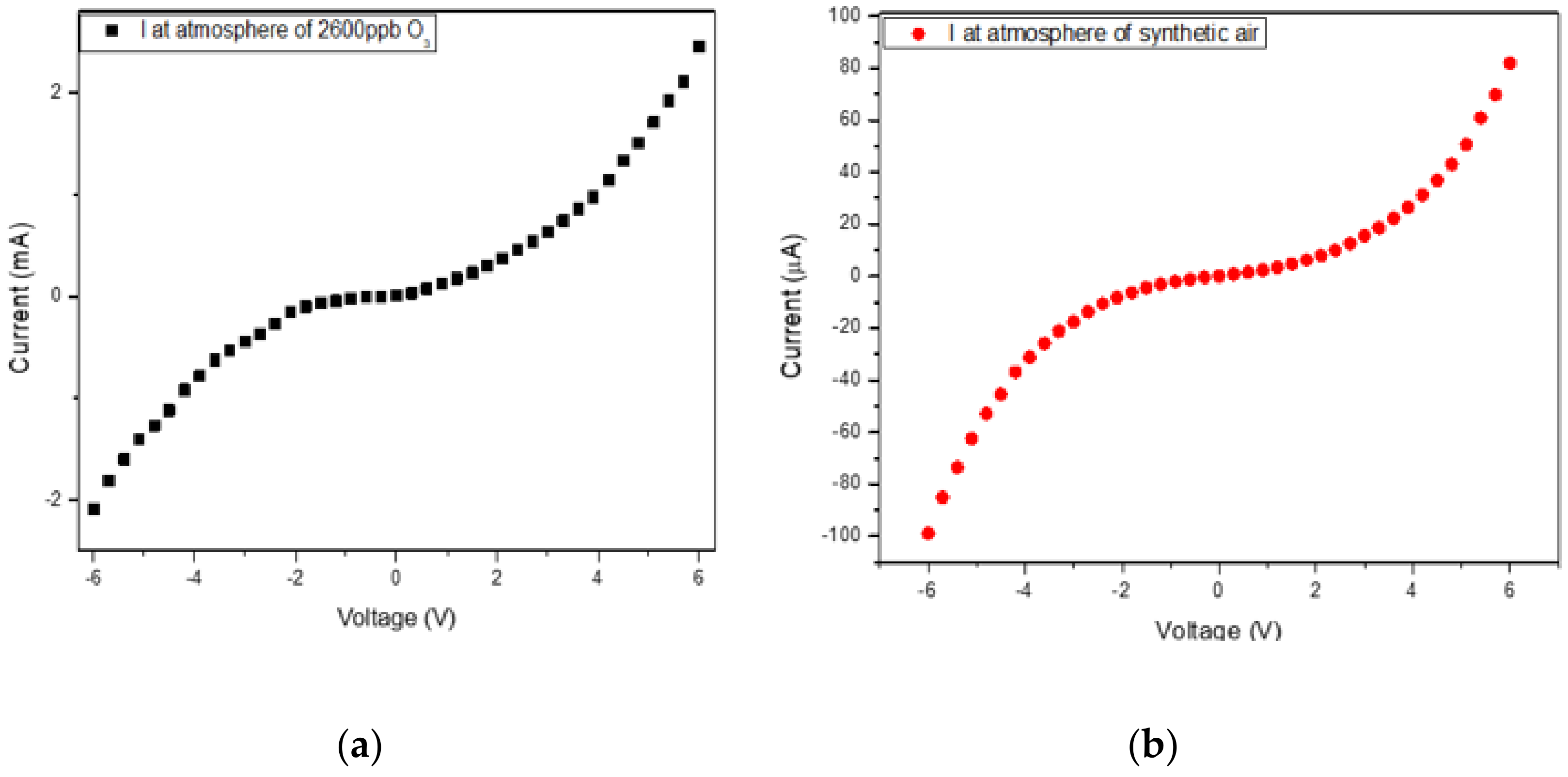



| Setup | Current Resolution (μA) | Low Detection Limit (ppb) | O3 Resolution (ppb) | Sensitivity (μA/ppm) |
|---|---|---|---|---|
| Lab | 0.001 | 80.50 | 0.0101 | 102.72 |
| Arduino | 4.8 | 109.73 | 8.1258 | 92.81 |
| STM32 | 0.8 | 85.90 | 0.7628 | 75.84 |
| Sensor | Voltage Supply (V) | Min Consumption (μW) | Μax Consumption (μW) | Pre-Heat Needed | Lower Detection Limit (ppb) | Sensitivity (μA/ppm) | O3 Resolution (ppb) |
|---|---|---|---|---|---|---|---|
| Co3O4 Keithley Setup | 3.3 | 127.71 | 744.81 | No | 80.50 | 102.72 | 0.0101 |
| Winsen MQ131 | 5 | 900,000 | - | Yes | 10 | - | - |
| Spec 3SP_O3_20 | - | 10 | 50 | No | <20 | 0.06 ± 0.1 | <20 |
| Spec ULPSM-O3 968-046 | 3.3 | 15 | 45 | No | 100 | - | <100 |
| Winsen ME3-O3 | - | - | - | No | 200 | 0.60 ± 0.15 | 200 |
| Winsen ME2-O3 | - | - | - | No | <20 ppb | ≥0.030 | <020 |
| Sensor | Voltage Supply (V) | Min Consumption (μW) | Μax Consumption (μW) | Pre-Heat Needed | Lower Detection Limit (ppb) | Sensitivity (μA/ppm) | O3 Resolution (ppb) |
|---|---|---|---|---|---|---|---|
| Co3O4 Arduino Uno Setup | 3.3 | 15 | 637 | No | 109.73 | 92.81 | 8.1258 |
| Co3O4 STM32 Setup | 3.3 | 2.6 | 390 | No | 85.90 | 75.84 | 0.7628 |
| Spec DGS-O3 968-042 | 3.3 | 100 | 14,000 | No | 20 | - | 20 |
| Sensing Material | Voltage Supply (V) | Operating Temperature (°C) | O3 Detection Limit (ppb) | O3 Response | Response, Recovery Time | Ref. |
|---|---|---|---|---|---|---|
| Co3O4 Keithley Setup | 3.3 | 25 | 120 | 13% a | 10 min | Present work |
| Co3O4 | - | 85 | 1000 | 3.13 b | 15 s, - | [18] |
| Co3O4 | - | 85 | 2000 | - | 25 s, 45 s | [21] |
| NiCo2O4 | 1 | 200 | 28 | ~0.3% c | 32 s, 60 s | [31] |
| Zn0.95Co0.05O | 1 | 250 | 20 | 0.4 b | 46 s, 62 s | [32] |
| CuAlO2 | 1 | 250 | 200 | 1.4 b | 25 s, 39 s | [33] |
| Cu2O | 10 | 25 | 10 | 28% a | 30 s, 24 s | [34] |
| NiO:Al | 10 | 80 | 10 | 2.55 c | 189.6 s, 243.6 s | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moschogiannaki, M.; Vardakis, G.; Gagaoudakis, E.; Papadakis, S.; Binas, V. Design and Evaluation of Low-Power Co3O4 Gas Sensing Element as a Part of Cyber Physical Systems. Chemosensors 2022, 10, 266. https://doi.org/10.3390/chemosensors10070266
Moschogiannaki M, Vardakis G, Gagaoudakis E, Papadakis S, Binas V. Design and Evaluation of Low-Power Co3O4 Gas Sensing Element as a Part of Cyber Physical Systems. Chemosensors. 2022; 10(7):266. https://doi.org/10.3390/chemosensors10070266
Chicago/Turabian StyleMoschogiannaki, Marilena, George Vardakis, Emmanouil Gagaoudakis, Stefanos Papadakis, and Vassilios Binas. 2022. "Design and Evaluation of Low-Power Co3O4 Gas Sensing Element as a Part of Cyber Physical Systems" Chemosensors 10, no. 7: 266. https://doi.org/10.3390/chemosensors10070266








