Signal Amplification for Detection of Nilutamide in Three-Dimensional Electrochemical Sensor Using Copper Metal–Organic Framework Decorated Carbon Nanofibers
Abstract
:1. Introduction
2. Experimental Part
2.1. Chemicals and Reagents
2.2. Synthesis Procedure
2.2.1. Synthesis of Cu-MOF
2.2.2. Acid Treatment of Carbon Nanofiber (CNF)
2.2.3. Preparation of Cu-MOF/f-CNF Composite
2.3. Electrode Fabrication
3. Results and Discussion
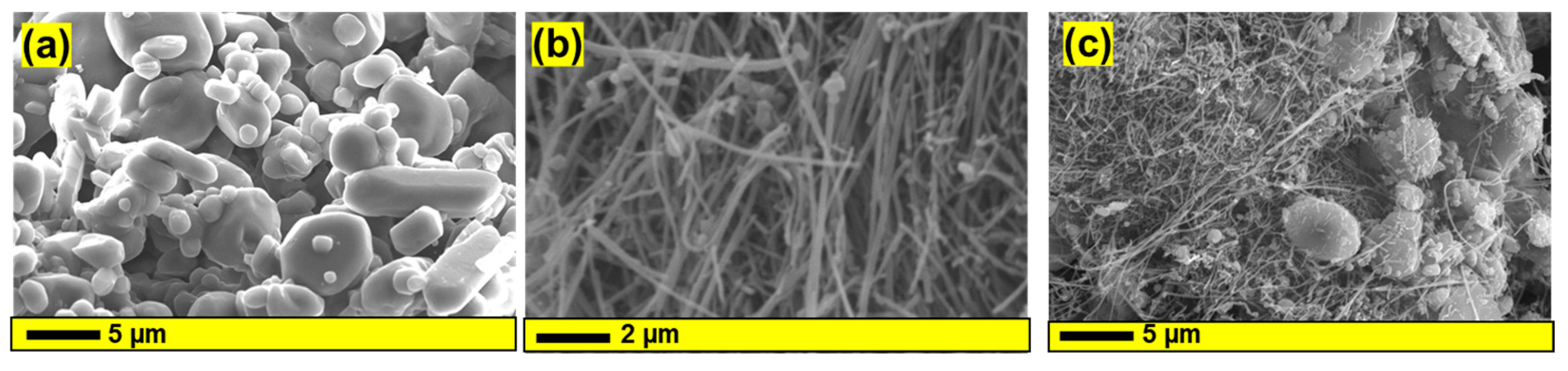
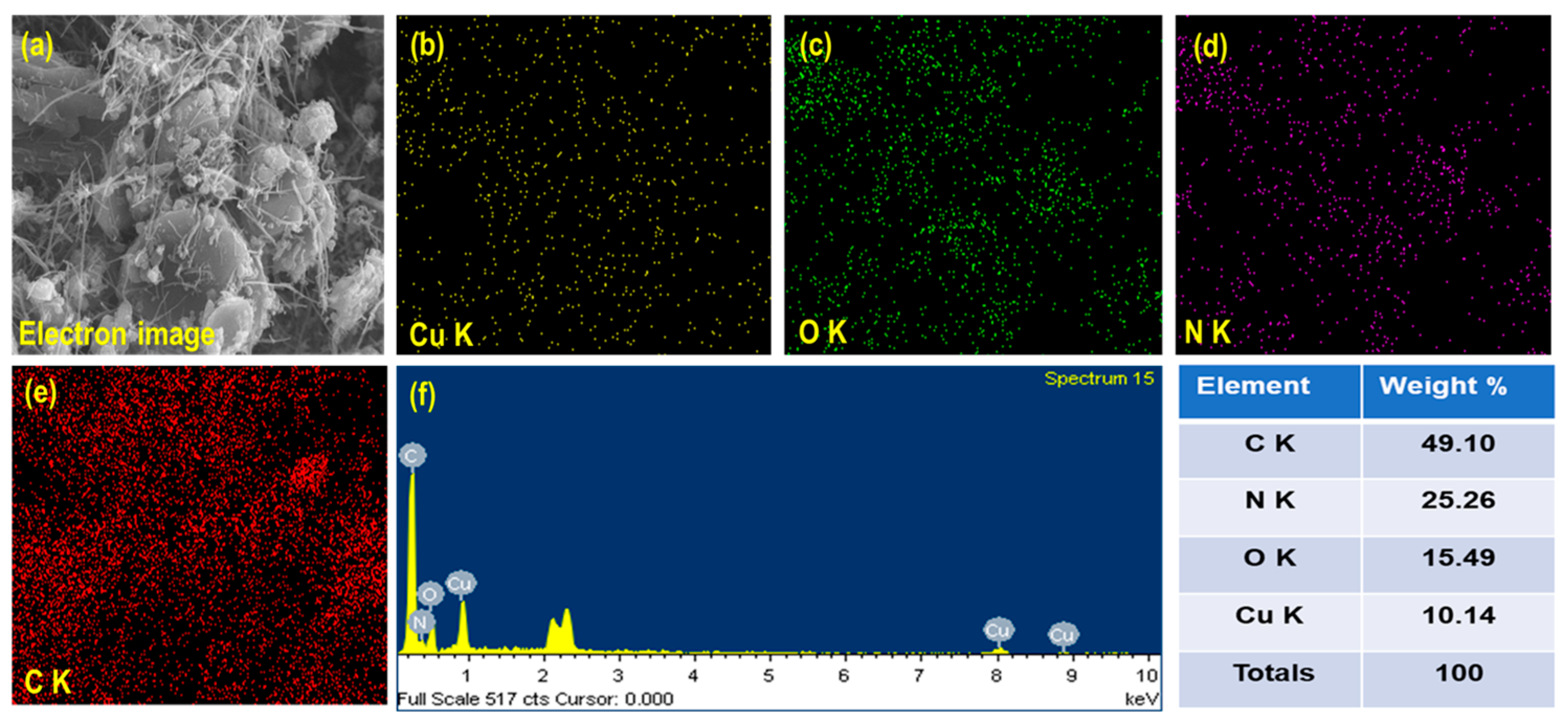
3.1. Structural and Morphological Investigation
3.2. Impedance Spectroscopy
3.3. Electrochemical Surface Area Analysis
3.4. Electrochemical Detection of Nlu
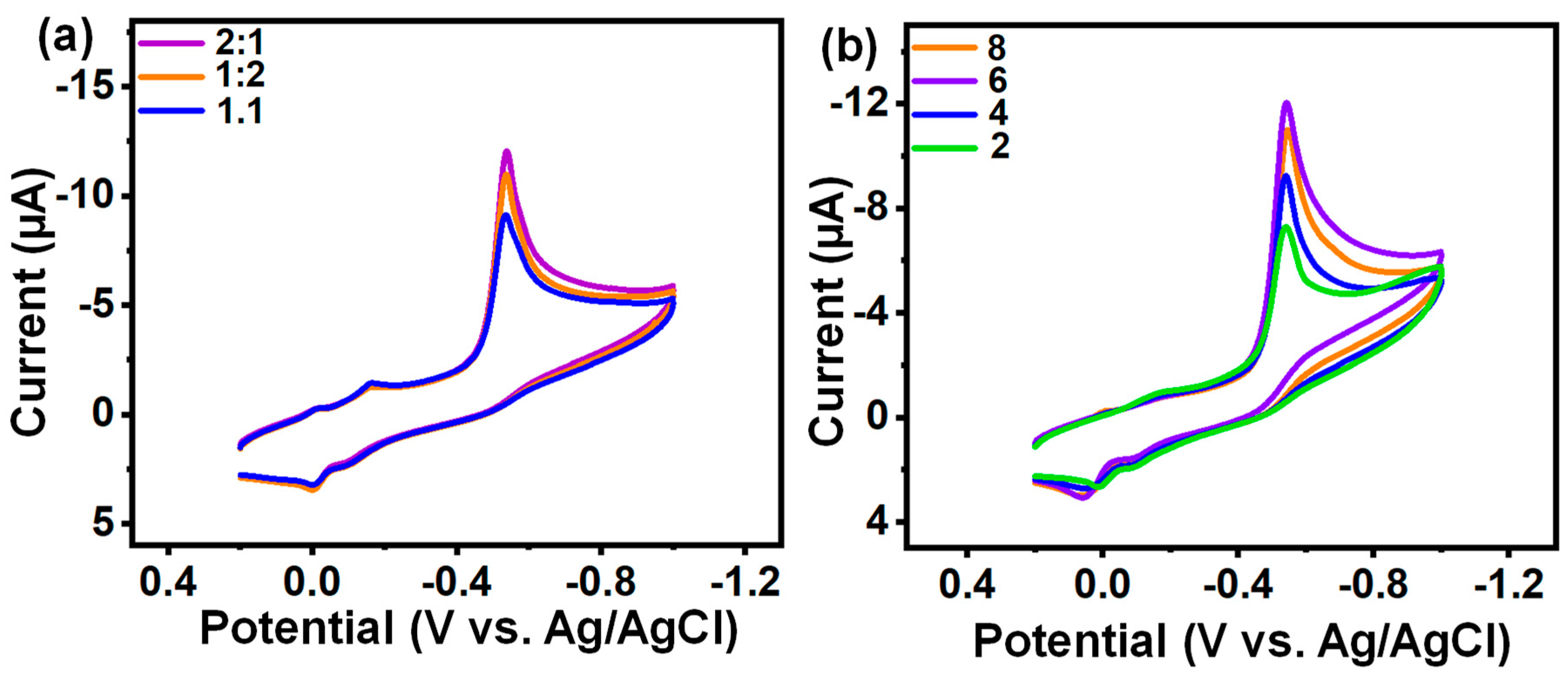
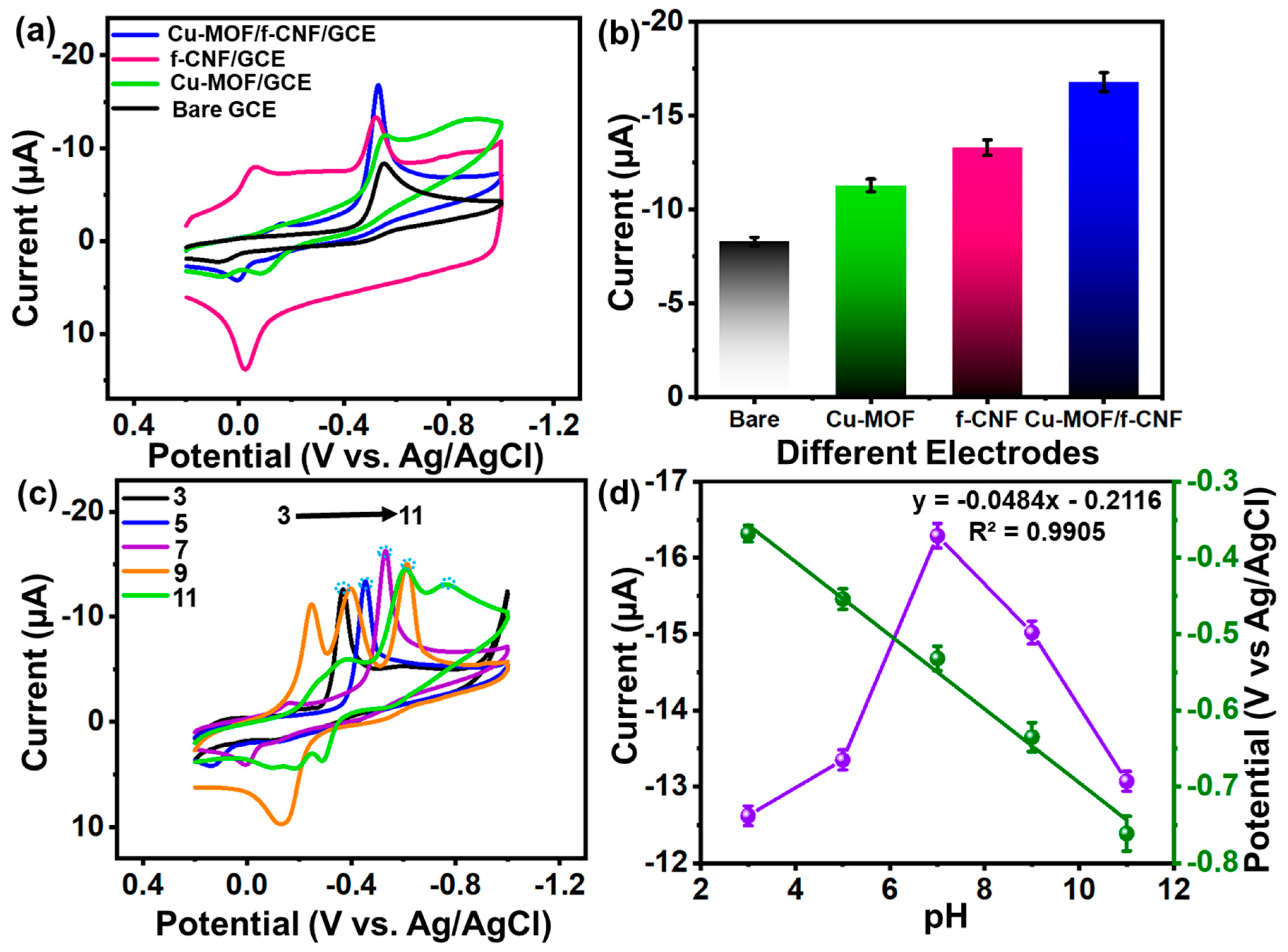
3.4.1. Optimization Analysis
3.4.2. Electrochemical Behavior of Electrode
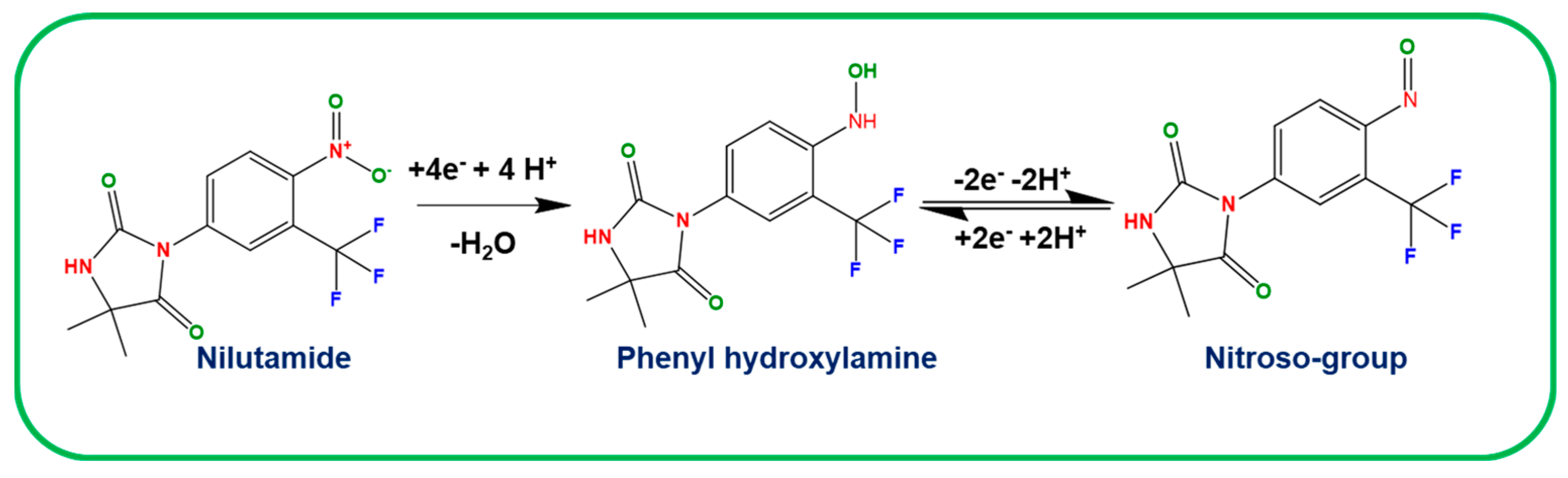
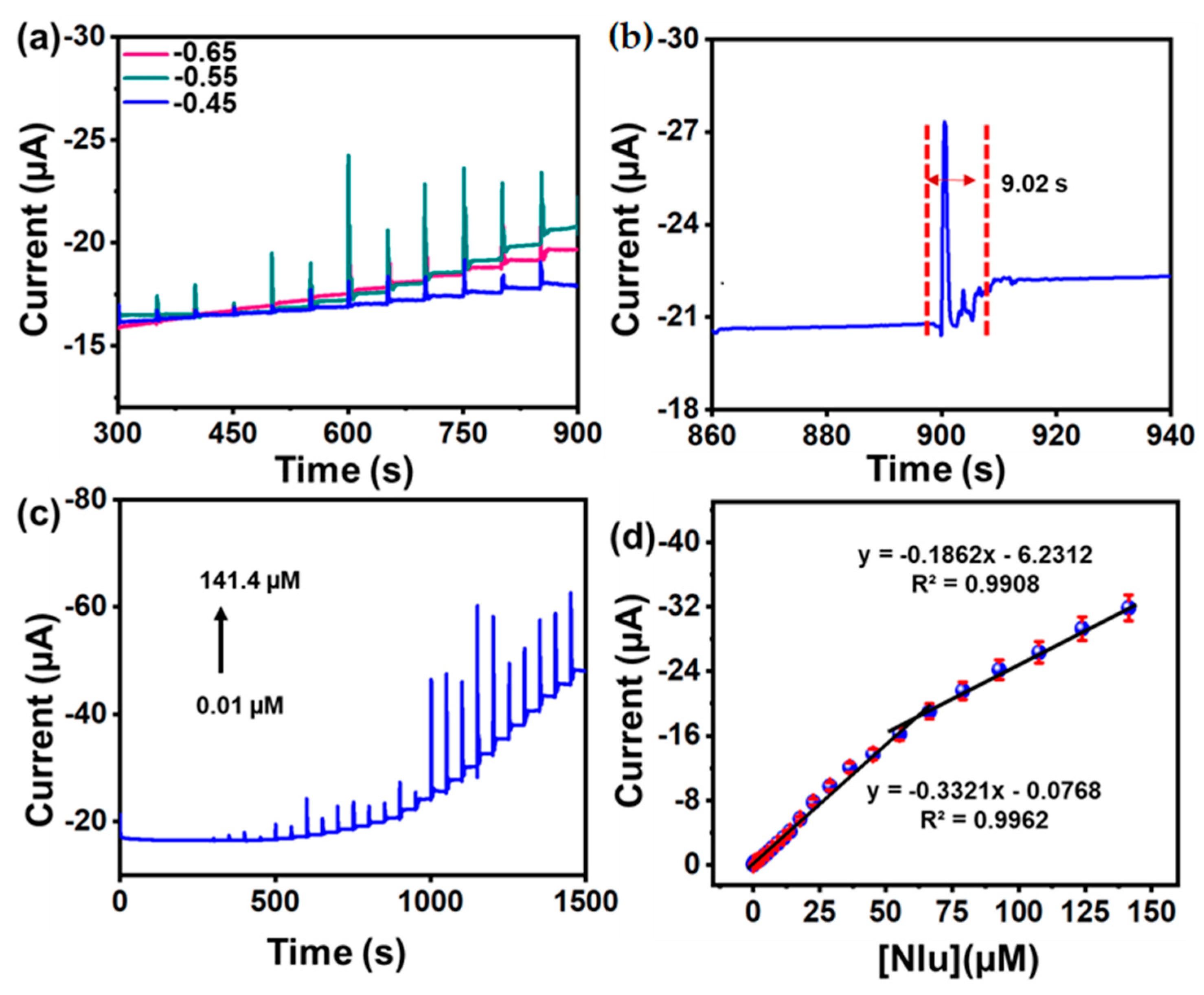
3.4.3. Amperometric (i-t) Determination of Nlu
3.4.4. Effect of Anti-Interference, Reproducible Nature, and Operational and Storage Stability of Modified Electrode
3.4.5. Real-Time Sample Analysis for Nlu
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haidyrah, A.S.; Sundaresan, P.; Venkatesh, K.; Ramaraj, S.K.; Thirumalraj, B. Fabrication of functionalized carbon nanofibers/carbon black composite for electrochemical investigation of antibacterial drug nitrofurantoin. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127112. [Google Scholar] [CrossRef]
- Turner, A.P.F. Perspective-an age of sensors. ECS Sens. Plus 2022, 1, 011601. [Google Scholar] [CrossRef]
- Moonla, C.; Lee, D.H.; Rokaya, D.; Rasitanon, N.; Kathayat, G.; Lee, W.-Y.; Kim, J.; Jeerapan, I. Lab-in-a-mouth and advanced point-of-care sensing systems: Detecting bioinformation from the oral cavity and saliva. ECS Sens. Plus 2022, 1, 021603. [Google Scholar] [CrossRef]
- Hwa, K.; Ganguly, A.; Santhan, A.; Sharma, T. Construction of three-dimensional/one-dimensional heterostructure of flower-like Sr nanoflowers on Se microrods decorated on reduced graphene oxide: An efficient electrocatalyst for oxidation of promethazine hydrochloride. Mater. Today Chem. 2022, 23, 100654. [Google Scholar] [CrossRef]
- Hortobagyi, G. Anthracyclines in the treatment of cancer: An overview. Drugs 1997, 54, 1–7. [Google Scholar] [PubMed]
- Znaleziona, J.; Maier, V.; Petr, J.; Chrastina, J.; Ševčík, J. MEKC determination of nilutamide in human serum using sweeping in high salt sample matrix. Chromatographia 2011, 74, 151–155. [Google Scholar] [CrossRef]
- Janknegt, R.; Abbou, C.; Bartoletti, R.; Bernstein-Hahn, L.; Bracken, B.; Brisset, J.; Da Silva, F.C.; Chisholm, G.; Crawford, E.; Debruyne, F. Orchiectomy and nilutamide or placebo as treatment of metastatic prostatic cancer in a multinational double-blind randomized trial. J. Urol. 1993, 149, 77–82. [Google Scholar] [CrossRef]
- Álvarez-Lueje, A.; Pena, C.; Núñez-Vergara, L.J.; Squella, J. Electrochemical study of flutamide, an anticancer drug, and its polarographic, UV spectrophotometric and HPLC determination in tablets. Electroanalysis 1998, 10, 1043–1051. [Google Scholar] [CrossRef]
- Madan, R.A.; Gulley, J.L.; Schlom, J.; Steinberg, S.M.; Liewehr, D.J.; Dahut, W.L.; Arlen, P.M. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin. Cancer Res. 2008, 14, 4526–4531. [Google Scholar] [CrossRef]
- Harris, M.G.; Coleman, S.G.; Faulds, D.; Chrisp, P. Nilutamide: A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in prostate cancer. Drugs Aging 1993, 3, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Sarat, P.; Ramachandran, D. Two new UV-visible spectrophotometric methods for the determination of nilutamide in pure and their tablets using 2, 3-Dichloro-5, 6-Dicyano-1, 4-benzoquinone (DDQ) and Para Chloranilic acid (PCA). Int. J. Pharm. Pharm. Res. 2015, 2, 161–171. [Google Scholar]
- Payen, O.; Top, S.; Vessières, A.; Brulé, E.; Plamont, M.-A.; McGlinchey, M.J.; Müller-Bunz, H.; Jaouen, G. Synthesis and structure–activity relationships of the first ferrocenyl-aryl-hydantoin derivatives of the nonsteroidal antiandrogen nilutamide. J. Med. Chem. 2008, 51, 1791–1799. [Google Scholar] [CrossRef]
- Arlen, P.M.; Gulley, J.L.; Todd, N.; Lieberman, R.; Steinberg, S.M.; Morin, S.; Bastian, A.; Marte, J.; Tsang, K.-Y.; Beetham, P. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J. Urol. 2005, 174, 539–546. [Google Scholar] [CrossRef]
- Temerk, Y.; Ibrahim, H.; Farhan, N. Square wave adsorptive stripping voltammetric determination of anticancer drug nilutamide in biological fluids using cationic surfactant cetyltrimethylammonium bromide. Anal. Methods 2015, 7, 9137–9144. [Google Scholar] [CrossRef]
- Sundaresan, P.; Yamuna, A.; Chen, S.-M. Sonochemical synthesis of samarium tungstate nanoparticles for the electrochemical detection of nilutamide. Ultrason. Sonochem. 2020, 67, 105146. [Google Scholar] [CrossRef]
- Carvalho, M.M.; Ribeiro, D.S.; Santos, J.L.; Prior, J.A. pH-sensitive spectrophotometric control of nilutamide in an automatic micro-flow system. New J. Chem. 2014, 38, 2856–2864. [Google Scholar] [CrossRef]
- Sriram, B.; Baby, J.N.; Hsu, Y.-F.; Wang, S.-F.; George, M.; Veerakumar, P.; Lin, K.-C. Electrochemical sensor-based barium zirconate on sulphur-doped graphitic carbon nitride for the simultaneous determination of nitrofurantoin (antibacterial agent) and nilutamide (anticancer drug). J. Electroanal. Chem. 2021, 901, 115782. [Google Scholar] [CrossRef]
- Sharma, T.S.K.; Hwa, K.-Y. Rational design and preparation of copper vanadate anchored on sulfur doped reduced graphene oxide nanocomposite for electrochemical sensing of antiandrogen drug nilutamide using flexible electrodes. J. Hazard. Mater. 2021, 410, 124659. [Google Scholar] [CrossRef] [PubMed]
- Sriram, B.; Kogularasu, S.; Wang, S.-F.; Sheu, J.-K. Deep Eutectic Solvent-Mediated Synthesis of Spinel Zinc Chromite Nanoparticles: A Simple Label-Free Electrochemical Sensor for Dopamine and Ascorbic Acid. ACS Appl. Nano Mater. 2023, 6, 17593–17602. [Google Scholar] [CrossRef]
- Dey, B.; Ahmad, M.W.; Kim, B.H.; Kamal, T.; Yang, D.-J.; Patra, C.N.; Hossain, S.; Choudhury, A. Manganese cobalt-MOF@carbon nanofiber-based non-enzymatic histamine sensor for the determination of food freshness. Anal. Bioanal. Chem. 2023, 415, 3487–3501. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, J.; Zhang, F.; Wang, Z.; Liu, Q. An electrochemical sensor based on copper-based metal-organic frameworks-graphene composites for determination of dihydroxybenzene isomers in water. Talanta 2018, 181, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-Y.A.; Hsieh, Y.-T. Copper-based metal organic framework (MOF), HKUST-1, as an efficient adsorbent to remove p-nitrophenol from water. J. Taiwan Inst. Chem. Eng. 2015, 50, 223–228. [Google Scholar]
- Micheroni, D.; Lan, G.; Lin, W. Efficient electrocatalytic proton reduction with carbon nanotube-supported metal–organic frameworks. J. Am. Chem. Soc. 2018, 140, 15591–15595. [Google Scholar] [CrossRef]
- Dey, B.; Sarkhel, G.; Choudhury, A. Facile synthesis of copper MOF/carbon nanofiber nanocomposite paper for electrochemical detection of toxic 4-nitrophenol. J. Macromol. Sci. Part A 2023, 60, 150–160. [Google Scholar] [CrossRef]
- Mariyappan, V.; Chen, S.-M.; Jeyapragasam, T.; Devi, J.M. Designing and construction of a cobalt-metal-organic framework/heteroatoms co-doped reduced graphene oxide mesoporous nanocomposite based efficient electrocatalyst for chlorogenic acid detection. J. Alloys Compd. 2022, 898, 163028. [Google Scholar] [CrossRef]
- Ramaraj, S.; Mani, S.; Chen, S.-M.; Kokulnathan, T.; Lou, B.-S.; Ali, M.A.; Hatamleh, A.; Al-Hemaid, F.M. Synthesis and application of bismuth ferrite nanosheets supported functionalized carbon nanofiber for enhanced electrochemical detection of toxic organic compound in water samples. J. Colloid Interface Sci. 2018, 514, 59–69. [Google Scholar] [CrossRef]
- Sukanya, R.; Chen, S.-M. Amorphous cobalt boride nanosheets anchored surface-functionalized carbon nanofiber: An bifunctional and efficient catalyst for electrochemical sensing and oxygen evolution reaction. J. Colloid Interface Sci. 2020, 580, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Kokulnathan, T.; Sharma, T.S.K.; Chen, S.-M.; Han-Yu, Y. Synthesis and characterization of zirconium dioxide anchored carbon nanofiber composite for enhanced electrochemical determination of chloramphenicol in food samples. J. Electrochem. Soc. 2018, 165, B281. [Google Scholar] [CrossRef]
- Song, J.; Lin, X.; Ee, L.Y.; Li, S.F.Y.; Huang, M. A review on electrospinning as versatile supports for diverse nanofibers and their applications in environmental sensing. Adv. Fiber Mater. 2023, 5, 429–460. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, K.; Yu, D.-G.; Zheng, X.; Huang, C. Advances in biosensing and environmental monitoring based on electrospun nanofibers. Adv. Fiber Mater. 2022, 4, 404–435. [Google Scholar] [CrossRef]
- Ahmad, M.W.; Dey, B.; Kim, B.-H.; Sarkhel, G.; Yang, D.-J.; Hossain, S.S.; Kamal, T.; Choudhury, A. Bimetallic copper-cobalt MOFs anchored carbon nanofibers hybrid mat based electrode for simultaneous determination of dopamine and tyramine. Microchem. J. 2023, 193, 109074. [Google Scholar] [CrossRef]
- Elanthamilan, E.; Ganeshkumar, A.; Wang, S.-F.; Rajaram, R. In situ synthesis of a non-toxic cobalt–benzimidazole metal–organic framework decorated reduced graphene oxide composite for asymmetric supercapacitor applications. New J. Chem. 2023, 47, 4832–4844. [Google Scholar] [CrossRef]
- Potter, M.E.; Ross, C.P.; Gianolio, D.; Rios, R.; Raja, R. Cobalt-containing zeolitic imidazole frameworks for C–H activation using visible-light redox photocatalysis. Catal. Sci. Technol. 2020, 10, 7262–7269. [Google Scholar] [CrossRef]
- Priscillal, I.J.D.; Wang, S.-F. Synchronously activated strontium aluminate nanoflakes anchored functionalized carbon nanofiber nanocomposite for sensitive amperometric detection of food additive: Propyl gallate. Food Chem. 2022, 389, 133119. [Google Scholar] [CrossRef]
- Priscillal, I.J.D.; Wang, S.J. Coral reef-like zinc niobate nanostructures decorated functionalized carbon nanofiber as electrode modifier for detection of oxidative stress biomarker: 3-nitro-L-tyrosine. Mater. Today Chem. 2022, 25, 100970. [Google Scholar] [CrossRef]
- Patil, Y.A.; Shankarling, G.S. Deep eutectic solvent-mediated, energy-efficient synthesis of copper terephthalate metal-organic framework and its application in degradation of an azo dye. Chem. Eng. J. Adv. 2020, 3, 100032. [Google Scholar] [CrossRef]
- Elanthamilan, E.; Wang, S.-F. Construction of 3D flower-like SnS particles anchored Ni-metal-organic framework composite: A novel positive electrode material for high-performance asymmetric supercapacitors. J. Energy Storage 2023, 71, 108144. [Google Scholar] [CrossRef]
- Brown, A.P.; Anson, F.C. Electron transfer kinetics with both reactant and product attached to the electrode surface. J. Electroanal. Chem. Interfacial Electrochem. 1978, 92, 133–145. [Google Scholar] [CrossRef]
- Hambly, B.P.; Sheppard, J.B.; Pendley, B.D.; Lindner, E. Voltammetric Determination of Diffusion Coefficients in Polymer Membranes: Guidelines to Minimize Errors. Electroanalysis 2018, 30, 681–689. [Google Scholar] [CrossRef]
- Karthik, R.; Sasikumar, R.; Chen, S.-M.; Kumar, J.V.; Elangovan, A.; Muthuraj, V.; Muthukrishnan, P.; Al-Hemaid, F.M.; Ali, M.A.; Elshikh, M.S. A highly sensitive and selective electrochemical determination of non-steroidal prostate anti-cancer drug nilutamide based on f-MWCNT in tablet and human blood serum sample. J. Colloid Interface Sci. 2017, 487, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Hwa, K.-Y.; Santhan, A.; Ganguly, A.; Sharma, T.S.K. Two dimensional architectures of graphitic carbon nitride with the substitution of heteroatoms for bifunctional electrochemical detection of nilutamide. Chemosphere 2023, 320, 138068. [Google Scholar] [CrossRef] [PubMed]
- Rajaji, U.; Raghu, M.; Kumar, K.Y.; Almutairi, T.M.; Mohammed, A.A.; Juang, R.-S.; Liu, T.-Y. A sonochemical synthesis of SrTiO3 supported N-doped graphene oxide as a highly efficient electrocatalyst for electrochemical reduction of a chemotherapeutic drug. Ultrason. Sonochem. 2023, 93, 106293. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Baby, J.N.; Hsu, Y.-F.; Wang, S.-F.; George, M. Design of Neodymium Vanadate Nanoparticles Decorated on Carbon–Boron Core-Shell Microspheres Matrix: An Electrochemical Detection of Nilutamide. J. Electrochem. Soc. 2022, 169, 087508. [Google Scholar] [CrossRef]
- Devi, R.K.; Ganesan, M.; Chen, T.-W.; Chen, S.-M.; Lou, B.-S.; Ali, M.A.; Al-Hemaid, F.M.; Li, R.-H. Gadolinium vanadate nanosheets entrapped with 1D-halloysite nanotubes-based nanocomposite for the determination of prostate anticancer drug nilutamide. J. Electroanal. Chem. 2022, 923, 116817. [Google Scholar] [CrossRef]
- Keerthana, S.; Rajapriya, A.; Viswanathan, C.; Ponpandian, N. Hybrid nanostructures of WS2 nanoflowers on N, B co-doped rGO for sensitive amperometric detection of Nilutamide. Mater. Today Chem. 2022, 26, 101052. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Karthik, R.; Chen, S.-M.; Kumar, J.V.; Sakthinathan, S. A cerium vanadate interconnected with a carbon nanofiber heterostructure for electrochemical determination of the prostate cancer drug nilutamide. Microchim. Acta 2019, 186, 579. [Google Scholar] [CrossRef]
- Akhter, S.; Zain, N.K.M.; Shalauddin, M.; Singh, V.K.; Misnon, I.I.; Sharma, R.K.; Das, S.; Basirun, W.J.; Johan, M.R.; Jose, R. Tri-metallic Co-Ni-Cu based metal organic framework nanostructures for the detection of an anticancer drug nilutamide. Sens. Actuators A Phys. 2021, 325, 112711. [Google Scholar] [CrossRef]





| Electrodes | Methods | Linear Range (µM) | LOD (µM) | Reference |
|---|---|---|---|---|
| a S/P/g-C3N4 | i-t | 0.01–158.3 | 0.0024 | [41] |
| b SrTiO3/NGO/LIGE | j DPV | 0.02–892 | 0.0012 | [42] |
| c NdVO4/B-C | i-t | 0.001–344 | 0.0002 | [43] |
| d GdV/HNT | DPV | 0.5–478 | 0.0018 | [44] |
| e WS2/N, B-rGO | i-t | 0.1–250 | 0.003 | [45] |
| f BZO/SCN | DPV | 0.09–189.61 | 0.006 | [17] |
| g CeV/CNF | DPV | 0.01–540 | 2.0 | [46] |
| h Co-Ni-Cu-MOF/NF | DPV | 0.5–900 | 0.00048 | [47] |
| SNPs | DPV | 0.05–918 | 0.0026 | [10] |
| Cu-MOF/f-CNF | i-t | 0.01–141.4 | 0.002 | This work |
| Real Samples | Added (nM) | Found (nM) | Recovery (%) |
|---|---|---|---|
| 80 | 79.85 | 99.1 | |
| River water | 100 | 99.79 | 98.8 |
| 120 | 119.78 | 98.9 | |
| 100 | 99.59 | 97.7 | |
| Human urine | 120 | 119.65 | 98.2 |
| 140 | 139.43 | 97.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elanthamilan, E.; Wang, S.-F. Signal Amplification for Detection of Nilutamide in Three-Dimensional Electrochemical Sensor Using Copper Metal–Organic Framework Decorated Carbon Nanofibers. Chemosensors 2023, 11, 544. https://doi.org/10.3390/chemosensors11100544
Elanthamilan E, Wang S-F. Signal Amplification for Detection of Nilutamide in Three-Dimensional Electrochemical Sensor Using Copper Metal–Organic Framework Decorated Carbon Nanofibers. Chemosensors. 2023; 11(10):544. https://doi.org/10.3390/chemosensors11100544
Chicago/Turabian StyleElanthamilan, Elaiyappillai, and Sea-Fue Wang. 2023. "Signal Amplification for Detection of Nilutamide in Three-Dimensional Electrochemical Sensor Using Copper Metal–Organic Framework Decorated Carbon Nanofibers" Chemosensors 11, no. 10: 544. https://doi.org/10.3390/chemosensors11100544





