Quantitative Analysis of Meteorite Elements Based on the Multidimensional Scaling–Back Propagation Neural Network Algorithm Combined with Raman Mapping-Assisted Micro-Laser Induced Breakdown Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Description and Preparation
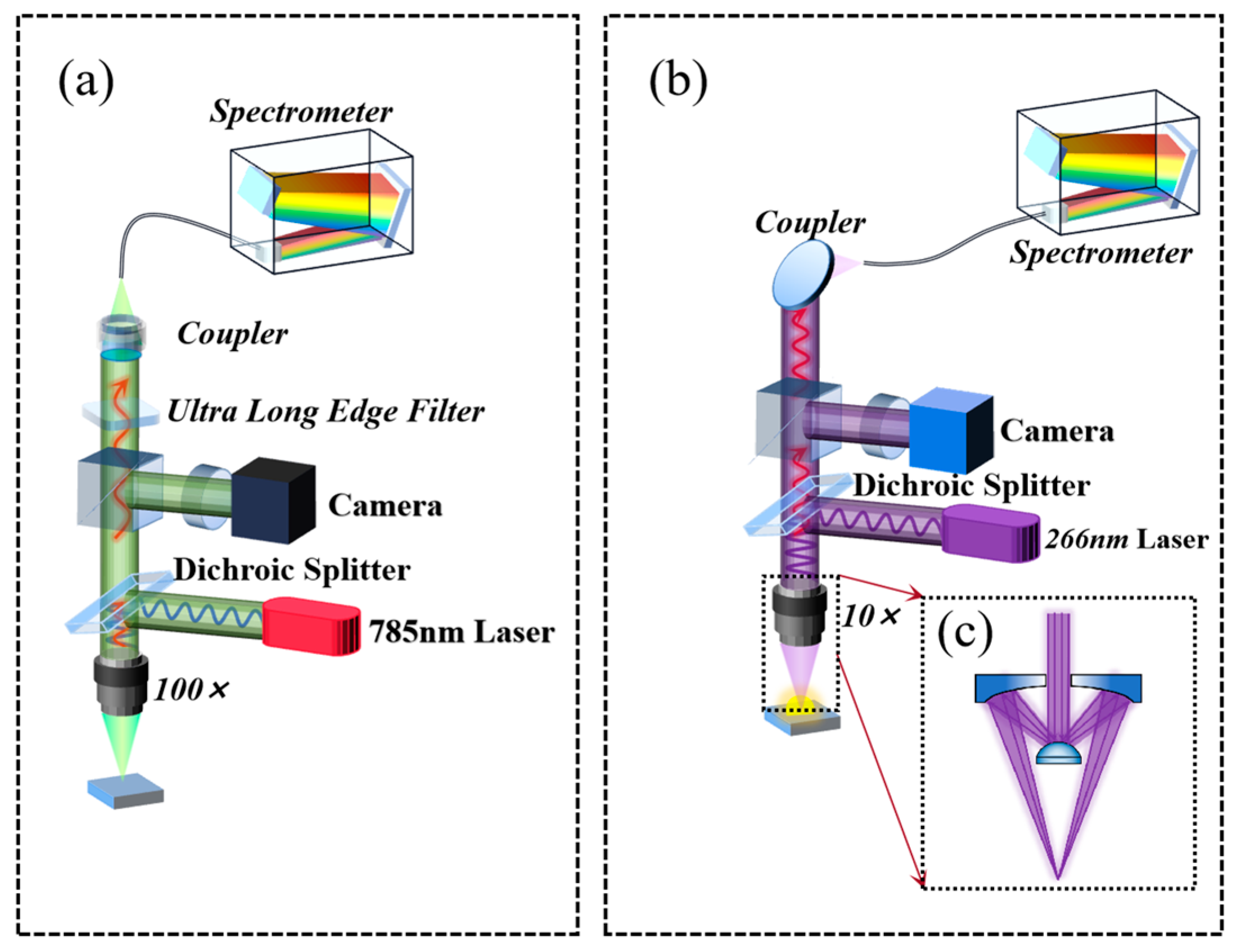
2.2. Raman Spectroscopy Setup
2.3. Laser-Induced Breakdown Spectroscopy (LIBS) Setup
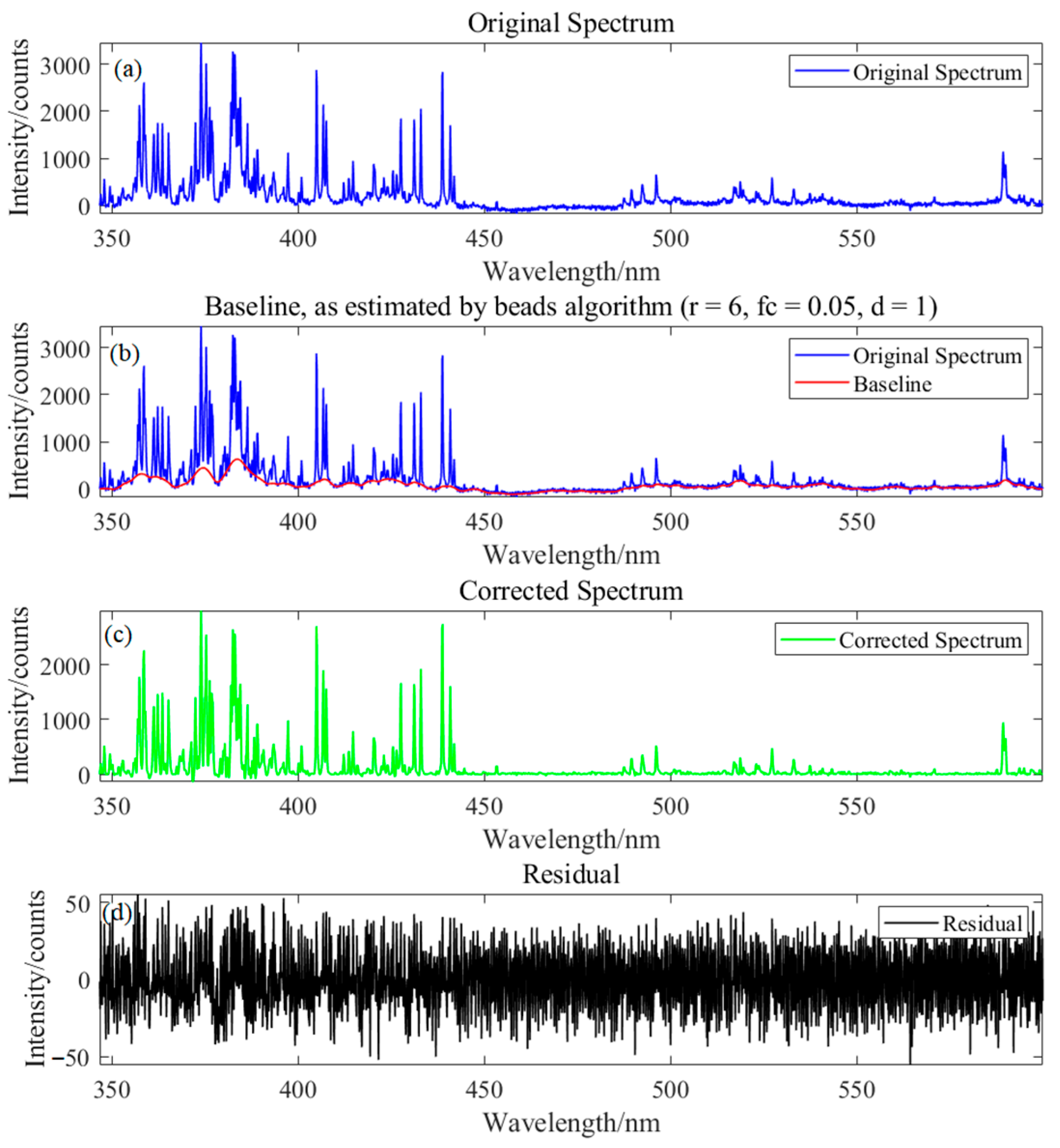
2.4. Spectral Data Pre-Processing
2.5. Chemometric Analysis
2.6. Methods and Steps of Data Analysis
3. Results
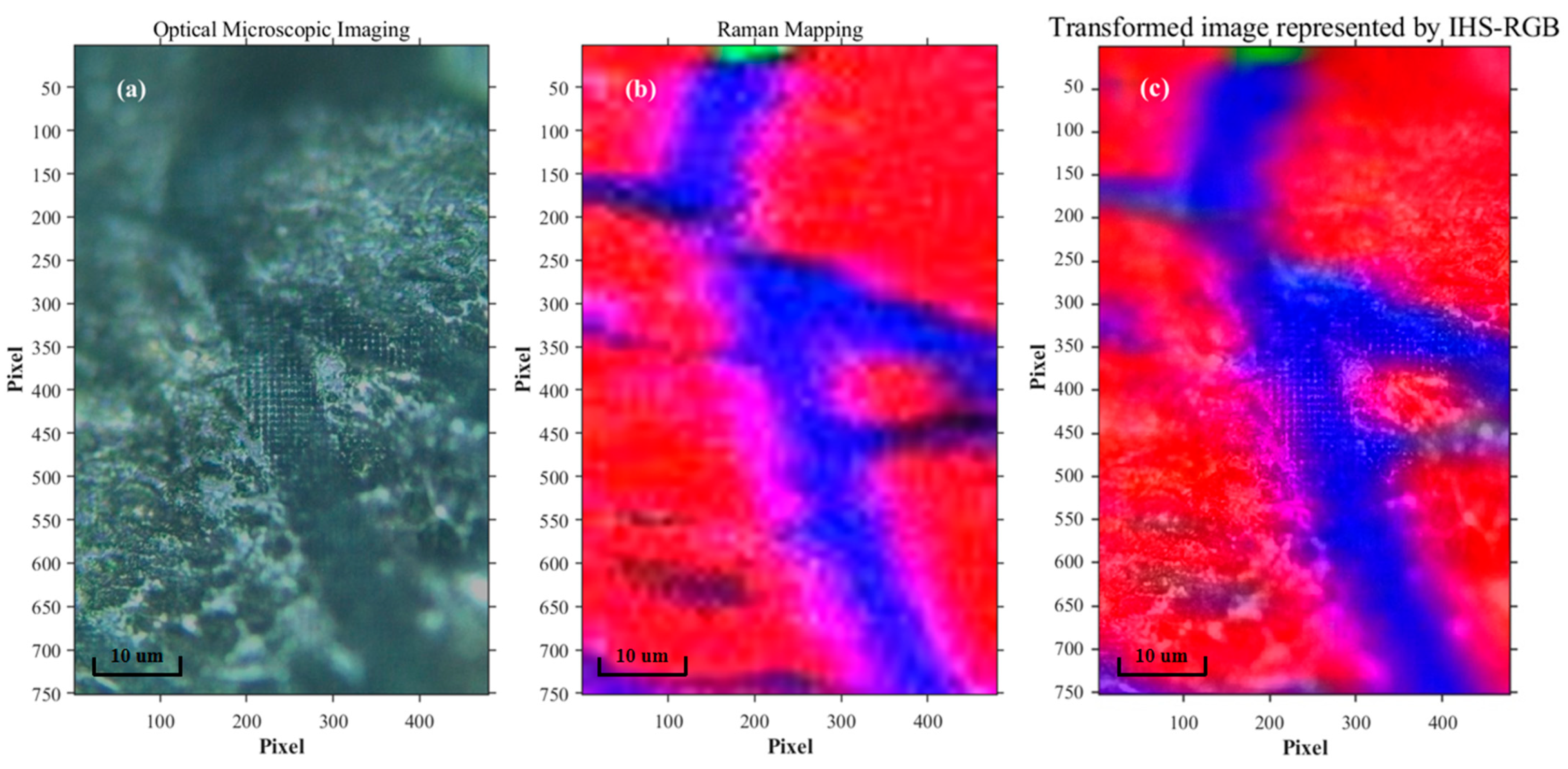
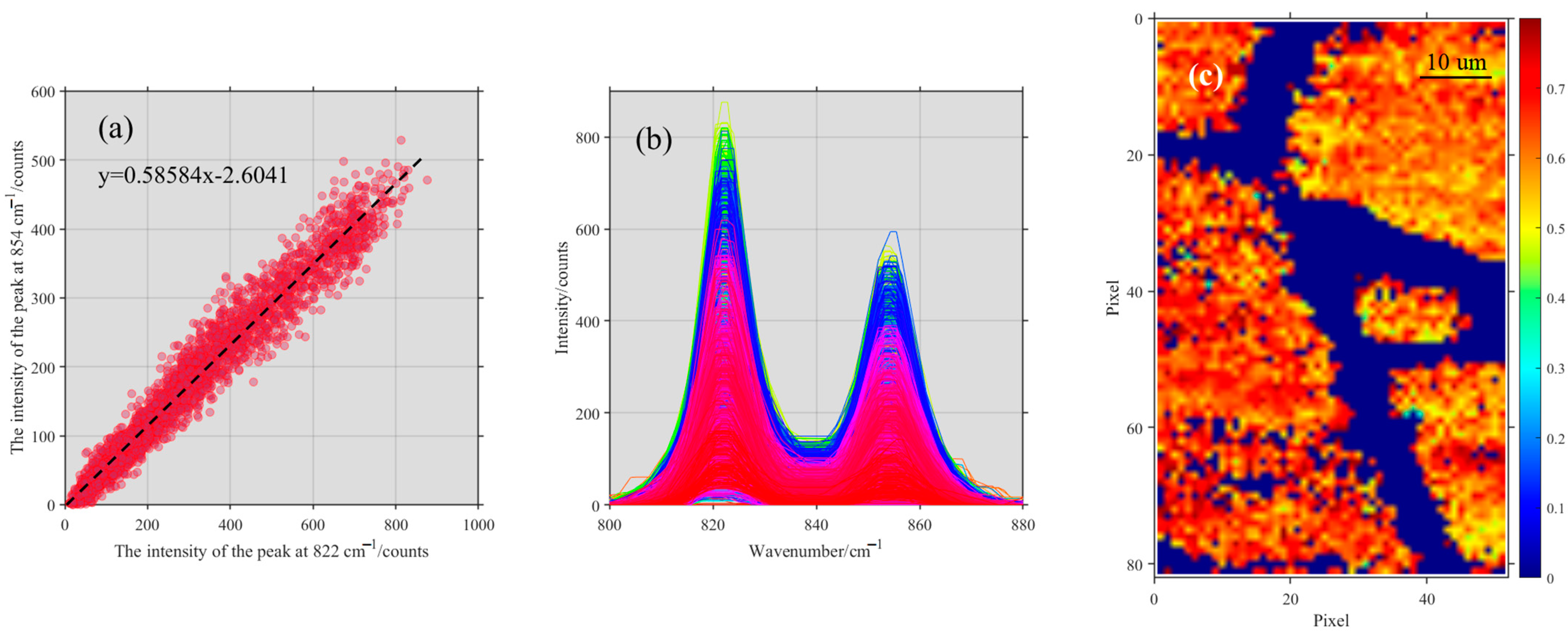
3.1. The Fusion of Raman Mapping and Microscopic Image
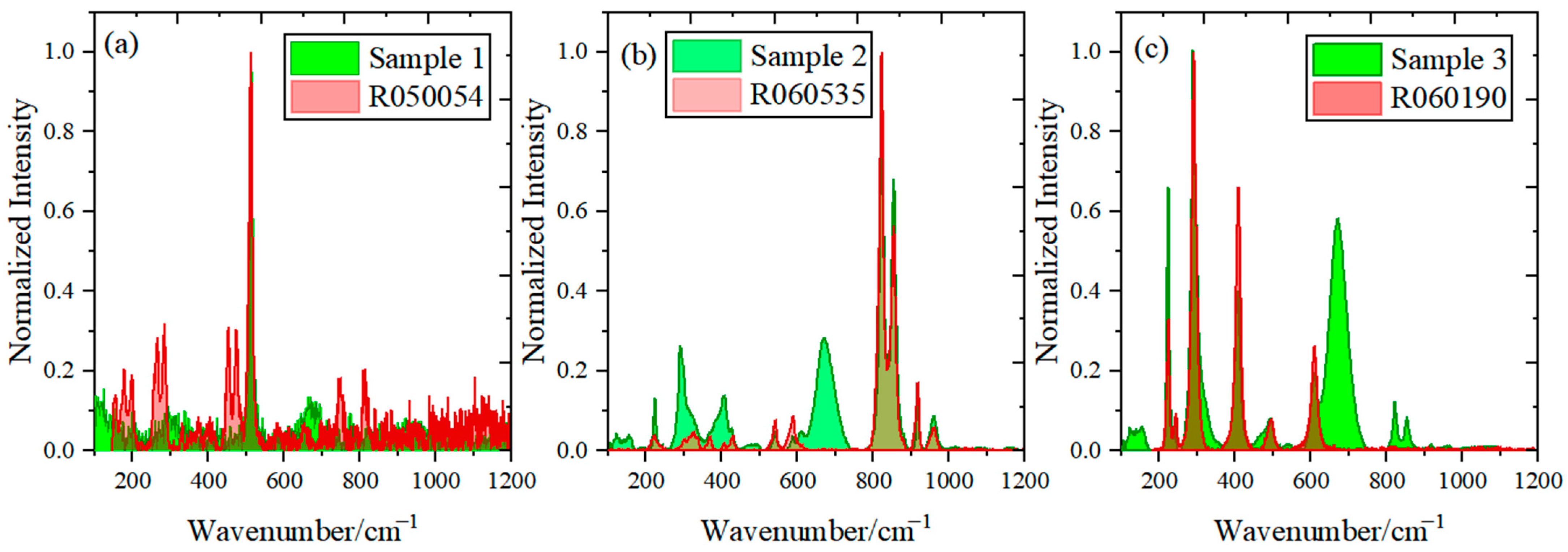
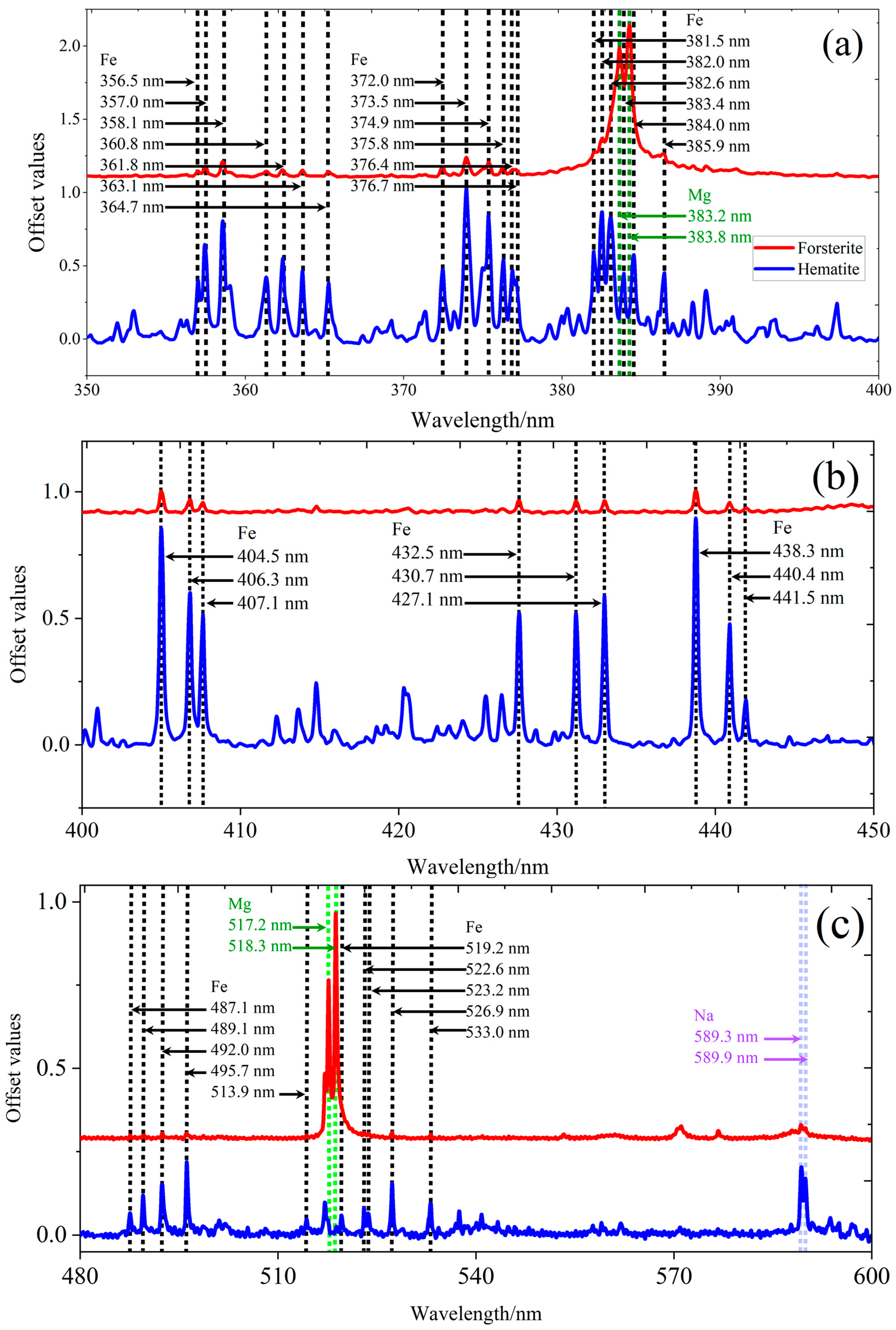
3.2. Micro-LIBS
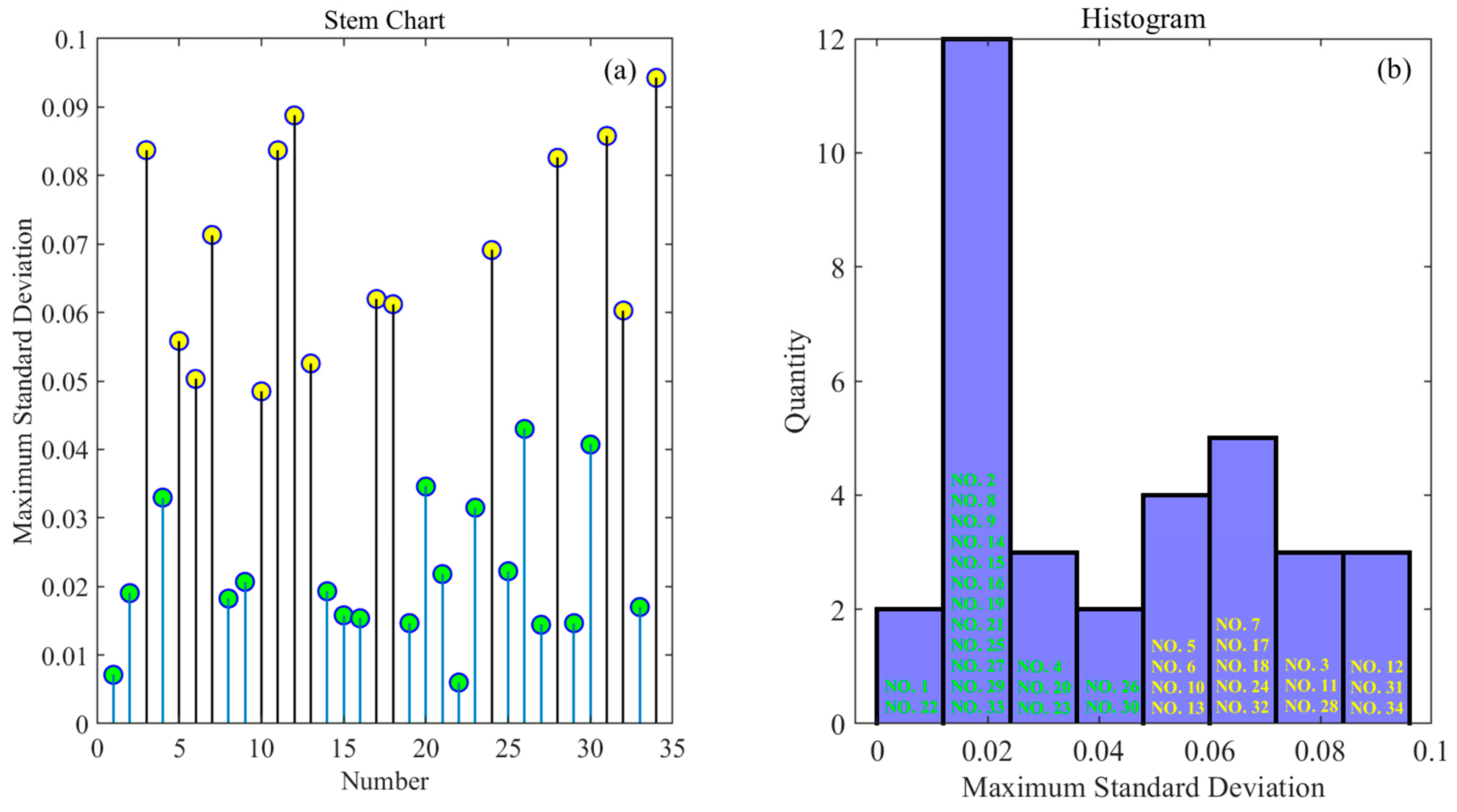
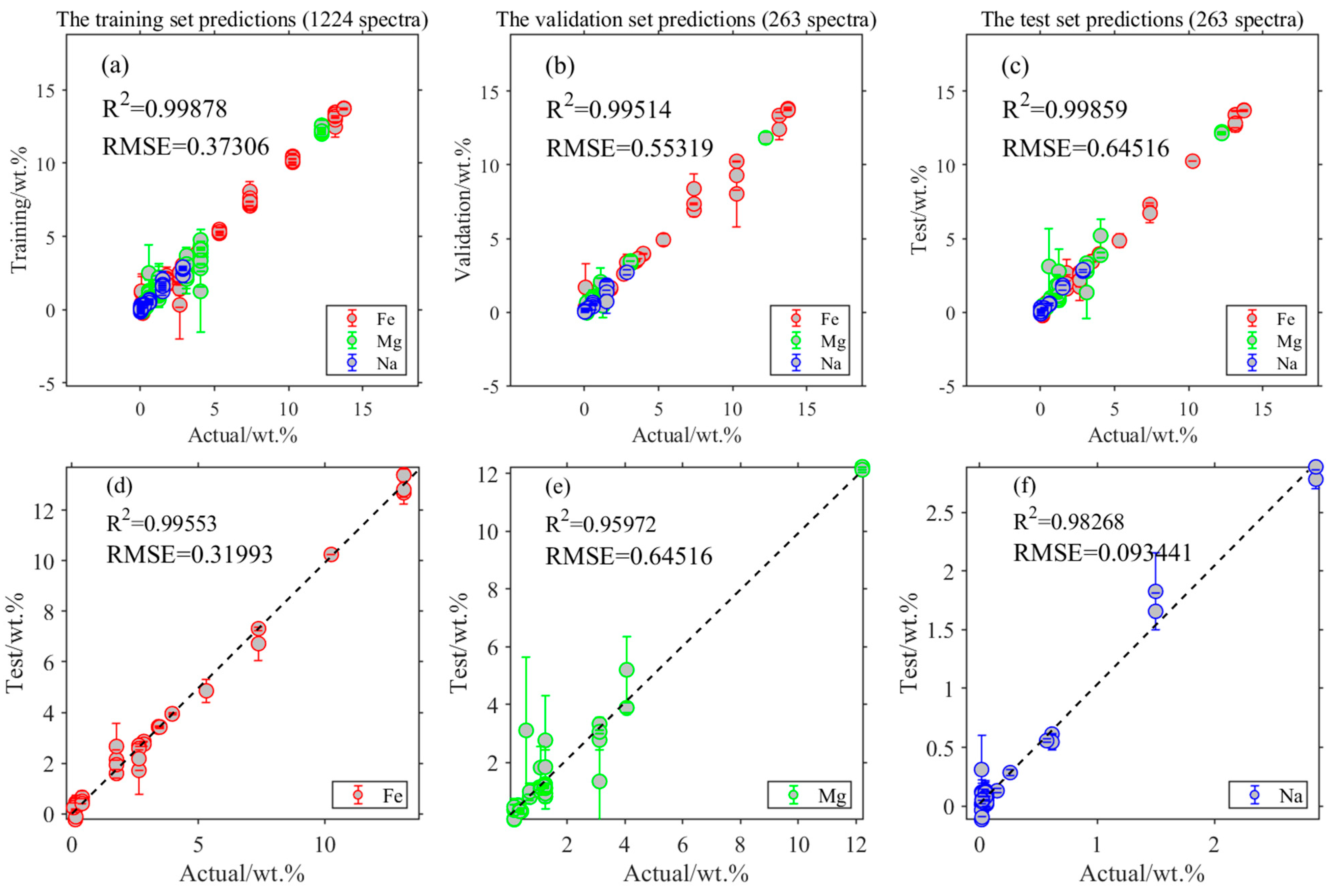
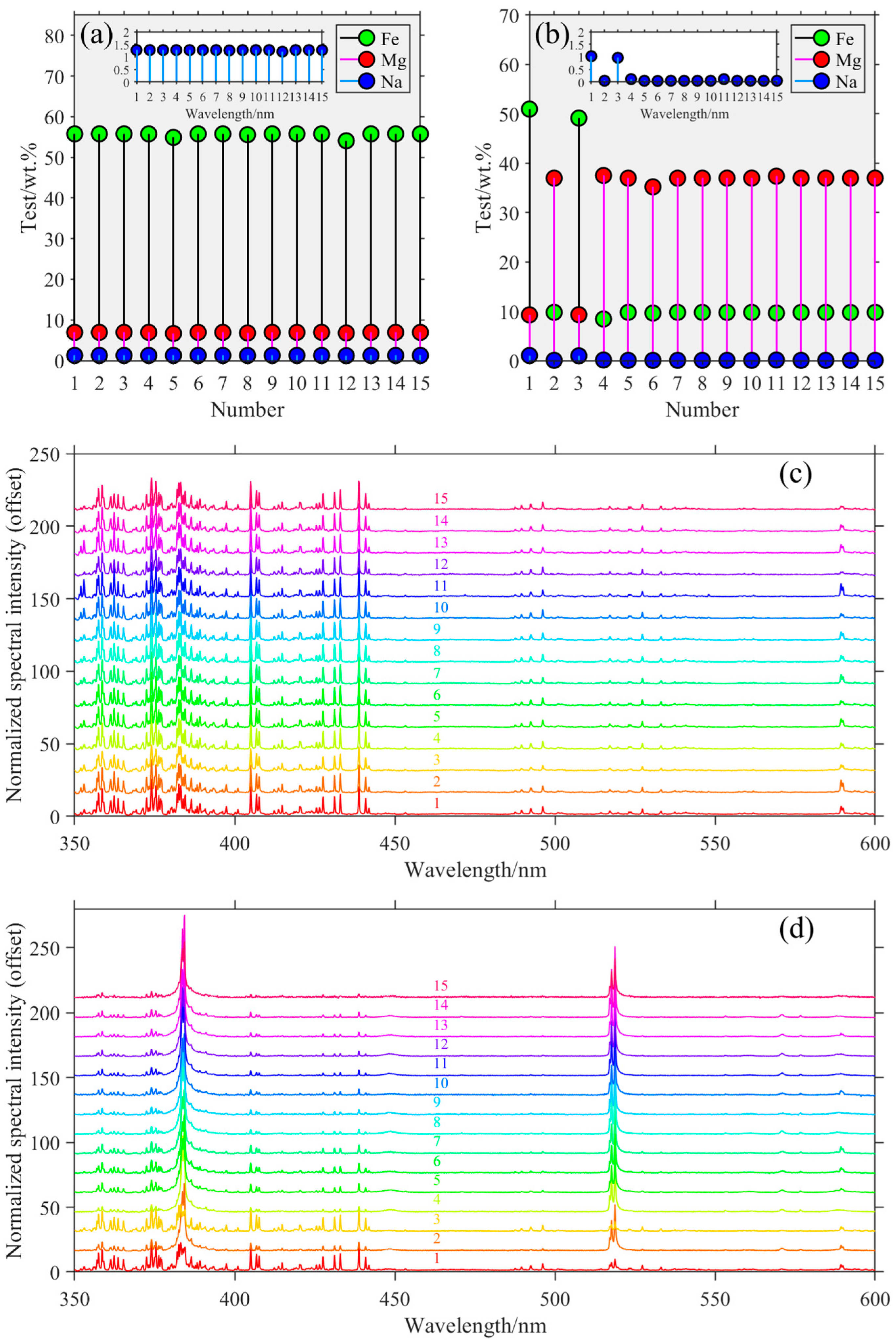
3.3. Element Quantitative Analysis
4. Discussion
4.1. Physical and Chemical Matrix Effects of Quantitative Models
4.2. Relevance of Analyzed Elements Fe, Mg, and Na for Meteorite Analysis
4.3. The General Utility of the Approach and Its Comparison with State-of-the-Art Meteorite Analysis Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miziolek, A.W.; Palleschi, V.; Schechter, I. Laser-Induced Breakdown Spectroscopy (LIBS)—Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Asquini, C.P. Laser Induced Breakdown Spectroscopy (LIBS); Woodhead Publishing: Sawston, UK, 2013. [Google Scholar]
- Chen, S.; Weijie, X.; Yongqi, T.; Yuqing, Z.; Zengqi, Y.; Long, Z.; Sahar, S.; Mengting, W.; Fengye, C.; Jin, Y. From machine learning to transfer learning in laser-induced breakdown spectroscopy analysis of rocks for Mars exploration. Sci. Rep. 2021, 11, 21379. [Google Scholar]
- Dyar, M.D.; Tucker, J.M.; Humphries, S.; Clegg, S.M.; Wiens, R.C.; Lane, M.D. Strategies for Mars remote Laser-Induced Breakdown Spectroscopy analysis of sulfur in geological samples. Spectrochim. Acta Part B At. Spectrosc. 2011, 66, 39–56. [Google Scholar]
- Farley, K.A.; Williford, K.H.; Stack, K.M.; Bhartia, R.; Chen, A.; de la Torre, M.; Hand, K.; Goreva, Y.; Herd, C.D.K.; Hueso, R.; et al. Mars 2020 Mission Overview. Space Sci. Rev. 2020, 216, 142. [Google Scholar] [CrossRef]
- Manrique, J.A.; Lopez-Reyes, G.; Cousin, A.; Rull, F.; Maurice, S.; Wiens, R.C.; Madsen, M.B.; Madariaga, J.M.; Gasnault, O.; Aramendia, J.; et al. SuperCam Calibration Targets: Design and Development. Space Sci. Rev. 2020, 216, 138. [Google Scholar] [CrossRef]
- Xu, W.; Liu, X.; Yan, Z.; Li, L.; Zhang, Z.; Kuang, Y.; Jiang, H.; Yu, H.; Yang, F.; Liu, C.; et al. The MarSCoDe Instrument Suite on the Mars Rover of China’s Tianwen-1 Mission. Space Sci. Rev. 2021, 217, 64. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, T.; Huang, S. Influence of the pressure and temperature on LIBS for gas concentration measurements. Spectrochim. Acta Part B At. Spectrosc. 2019, 155, 24–33. [Google Scholar]
- Tran, M.; Smith, B.W.; Hahn, D.W.; Winefordner, J.D. Detection of Gaseous and Particulate Fluorides by Laser-Induced Breakdown Spectroscopy. Appl. Spectrosc. 2001, 55, 1455–1461. [Google Scholar] [CrossRef]
- Ripoll, L.; Navarro-González, J.; Legnaioli, S.; Palleschi, V.; Hidalgo, M. Evaluation of Thin Film Microextraction for trace elemental analysis of liquid samples using LIBS detection. Talanta 2021, 223, 121736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.C.; Hu, Z.Q.; Su, Y.B.; Hai, B.; Zhu, X.L.; Zhu, J.F.; Ma, X. Simple method for liquid analysis by laser-induced breakdown spectroscopy (LIBS). Opt. Express 2018, 26, 18794–18802. [Google Scholar] [CrossRef]
- Rifai, K.; Laflamme, M.; Constantin, M.; Vidal, F.; Sabsabi, M.; Blouin, A.; Bouchard, P.; Fytas, K.; Castello, M.; Kamwa, B.N. Analysis of gold in rock samples using laser-induced breakdown spectroscopy: Matrix and heterogeneity effects. Spectrochim. Acta Part B At. Spectrosc. 2017, 134, 33–41. [Google Scholar] [CrossRef]
- Killinger, D.K.; Allen, S.D.; Waterbury, R.D.; Stefano, C.; Dottery, E.L. Enhancement of Nd:YAG LIBS emission of a remote target using a simultaneous CO_2 laser pulse. Opt. Express 2007, 15, 12905–12915. [Google Scholar] [CrossRef]
- Qiang, H.E.; Xiong, W.; Hong-peng, W.; Ru-jun, Y. Study on the Distribution of Ca Elements in Ammonite Stones Based on micro LIBS. Spectrosc. Spectr. Anal. 2019, 39, 2917–2921. [Google Scholar]
- Chen, X.; Li, X.; Yang, S.; Yu, X.; Liu, A. Discrimination of lymphoma using laser-induced breakdown spectroscopy conducted on whole blood samples. Biomed. Opt. Express 2018, 9, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Rehse, S.J.; Diedrich, J.; Palchaudhuri, S. Identification and discrimination of Pseudomonas aeruginosa bacteria grown in blood and bile by laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 1169–1176. [Google Scholar] [CrossRef]
- Meng, D.; Zhao, N.; Ma, M.; Fang, L.; Gu, Y.; Jia, Y.; Liu, J.; Liu, W. Application of a mobile laser-induced breakdown spectroscopy system to detect heavy metal elements in soil. Appl. Opt. 2017, 56, 5204–5210. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.K.; Pati, J.K.; Rai, A.K. Chapter 16—LIBS Study of Geological Samples Coupled with Chemometric Methods. In Laser-Induced Breakdown Spectroscopy, 2nd ed.; Singh, J.P., Thakur, S.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 369–384. [Google Scholar] [CrossRef]
- Pérez-Diez, S.; Fernández-Menéndez, L.J.; Veneranda, M.; Morillas, H.; Prieto-Taboada, N.; de Vallejuelo, S.F.-O.; Bordel, N.; Martellone, A.; De Nigris, B.; Osanna, M.; et al. Chemometrics and elemental mapping by portable LIBS to identify the impact of volcanogenic and non-volcanogenic degradation sources on the mural paintings of Pompeii. Anal. Chim. Acta 2021, 1168, 338565. [Google Scholar] [CrossRef]
- Sun, L.; Yu, H.; Cong, Z.; Xin, Y. On-line semi-quantitative analysis of molten steel composition using laser-induced breakdown spectroscopy. Chin. J. Sci. Instrum. 2011, 32, 2602–2608. [Google Scholar]
- Cai, J.; Dong, M.; Zhang, Y.; Chen, Y.; Liang, Y.; Lu, J. Temporally and spatially resolved study of laser-induced plasma generated on coals with different volatile matter contents. Spectrochim. Acta Part B At. Spectrosc. 2021, 180, 106195. [Google Scholar] [CrossRef]
- Rammelkamp, K.; Schröder, S.; Kubitza, S.; Vogt, D.S.; Frohmann, S.; Hansen, P.B.; Böttger, U.; Hanke, F.; Hübers, H.-W. Low-level LIBS and Raman data fusion in the context of in situ Mars exploration. J. Raman Spectrosc. 2019, 51, 1682–1701. [Google Scholar]
- Wang, Q.; Xiao, J.; Li, Y.; Lu, Y.; Guo, J.; Tian, Y.; Ren, L. Mid-level data fusion of Raman spectroscopy and laser-induced breakdown spectroscopy: Improving ores identification accuracy. Anal. Chim. Acta 2023, 1240, 340772. [Google Scholar]
- Moros, J.; ElFaham, M.M.; Laserna, J.J. Dual-Spectroscopy Platform for the Surveillance of Mars Mineralogy Using a Decisions Fusion Architecture on Simultaneous LIBS-Raman Data. Anal. Chem. 2018, 90, 2079–2087. [Google Scholar] [CrossRef]
- Raneri, S.; Botto, A.; Campanella, B.; Momčilović, M.; Palleschi, V.; Poggialini, F.; Sciuto, C.; Gattiglia, G.; Volpintesta, F.; Selvaraj, T.; et al. Increasing resolution in chemical mapping of geomaterials: From X-ray fluorescence to laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2022, 194, 106482. [Google Scholar] [CrossRef]
- Sun, H.; Song, C.; Lin, X.; Gao, X. Identification of meat species by combined laser-induced breakdown and Raman spectroscopies. Spectrochim. Acta Part B At. Spectrosc. 2022, 194, 106456. [Google Scholar] [CrossRef]
- Chen, J.; Pisonero, J.; Chen, S.; Wang, X.; Fan, Q.; Duan, Y. Convolutional neural network as a novel classification approach for LIBS applications in lithological recognition. Spectrochim. Acta Part B At. Spectrosc. 2020, 166, 105801. [Google Scholar] [CrossRef]
- Chengxu, L.; Bo, W.; Jiang, X.; Zhang, J.; Kang, N.; Yanwei, Y. Detection of K in the soil using time-resolved laser induced breakdown spectroscopy based on convolutional neural networks. Plasma Sci. Technol. 2019, 21, 034014. [Google Scholar]
- Stead, C.V.; Tomlinson, E.L.; Kamber, B.S.; Babechuk, M.G.; McKenna, C.A. Rare Earth Element Determination in Olivine by Laser Ablation-Quadrupole-ICP-MS: An Analytical Strategy and Applications. Geostand. Geoanalytical Res. 2017, 41, 197–212. [Google Scholar] [CrossRef]
- Bsdok, B.; Altenberger, U.; Concha-Perdomo, A.E.; Wilke FD, H.; Gil-Rodríguez, J.G. The Santa Rosa de Viterbo meteorite, Colombia. New work on it’s petrological, geochemical and economical characterization. J. S. Am. Earth Sci. 2020, 104, 102779. [Google Scholar] [CrossRef]
- Deon, F.; Förster, H.J.; van Ruitenbeek, F.; Appelt, O. Performance of analytical techniques (SWIR imaging, XRD, EPMA) for the identification of minerals frequently formed during natural and technological geothermal processes. Int. J. Terr. Heat Flow Appl. Geotherm. 2022, 5, 34–44. [Google Scholar] [CrossRef]
- Ning, X.; Selesnick, I.W.; Duval, L. Chromatogram baseline estimation and denoising using sparsity (BEADS). Chemom. Intell. Lab. Syst. 2014, 139, 156–167. [Google Scholar] [CrossRef]
- Wang, H.; Fang, P.; Yan, X.; Zhou, Y.; Cheng, Y.; Yao, L.; Jia, J.; He, J.; Wan, X. Study on the Raman spectral characteristics of dynamic and static blood and its application in species identification. J. Photochem. Photobiol. B Biol. 2022, 232, 112478. [Google Scholar] [CrossRef]
- Wang, H.; Wan, X. Effect of chlorophyll fluorescence quenching on quantitative analysis of adulteration in extra virgin olive oil. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119183. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xin, Y.; Wan, X. Spectral detection technology of vegetable oil: Spectral analysis of porphyrins and terpenoids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 261, 119965. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xin, Y.; Ma, H.; Fang, P.; Li, C.; Wan, X.; He, Z.; Jia, J.; Ling, Z. Rapid detection of Chinese-specific peony seed oil by using confocal Raman spectroscopy and chemometrics. Food Chem. 2021, 362, 130041. [Google Scholar] [CrossRef]
- van der Maaten, L.J.P.; Postma, E.O.; van den Herik, H.J. Dimensionality Reduction: A Comparative Review. Tilburg University Technical Report, TiCC-TR 2009-005, 2009. J. Mach. Learn. Res. 2009, 10, 13. [Google Scholar]
- van der Maaten, L.J.P.; Hinton, G.E. Visualizing High-Dimensional Data Using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Hecht-Nielsen, R. Theory of the backpropagation neural network. Neural Netw. 1988, 1, 445. [Google Scholar] [CrossRef]
- Harris, J.R.; Murray, R.; Hirose, T. IHS transform for the integration of radar imagery with other remotely sensed data. Photogramm. Eng. Remote Sens. 1990, 56, 1631–1641. [Google Scholar]
- Rahmani, S.; Strait, M.; Merkurjev, D.; Moeller, M.; Wittman, T. An adaptive IHS pan-sharpening method. IEEE Geosci. Remote Sens. Lett. 2010, 7, 746–750. [Google Scholar] [CrossRef]
- Gosetti, F.; Mazzucco, E.; Gennaro, M.C.; Marengo, E. Simultaneous determination of sixteen underivatized biogenic amines in human urine by HPLC-MS/MS. Anal. Bioanal. Chem. 2012, 405, 907–916. [Google Scholar] [CrossRef]
- Alan, E.R. Mineralogy of meteorite groups. Meteorit. Planet. Sci. 1997, 32, 153–328. [Google Scholar]
- Chen, M.; Xie, X. Ahmed EL GORESY. A shock-produced (Mg, Fe)SiO3 glass in the Suizhou meteorite. Meteorit. Planet. Sci. 2010, 39, 1797–1808. [Google Scholar] [CrossRef]
- Chopelas, A. Single crystal Raman spectra of forsterite, fayalite, and monticellite. Am. Meteoriteogist 1991, 76, 1101–1109. [Google Scholar] [CrossRef]
- Mouri, T.; Enami, M. Raman spectroscopic study of olivine-group minerals. J. Miner. Pet. Sci. 2008, 103, 100–104. [Google Scholar] [CrossRef]
- Wang, A.; Kuebler, K.; Jolliff, B.; Haskin, L.A. Mineralogy of a Martian meteorite as determined by Raman spectroscopy. J. Raman Spectrosc. 2004, 35, 504–514. [Google Scholar] [CrossRef]
- Gatan Inc. Improving Sample Analysis Using Cathodoluminescence (CL) and Energy Dispersive Spectroscopy (EDS). AZoM. Available online: https://www.azom.com/article.aspx?ArticleID=20761 (accessed on 5 May 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Xin, Y.; Fang, P.; Wang, Y.; Duan, M.; Wu, W.; Yang, R.; Liu, S.; Zhang, L.; Wan, X. Quantitative Analysis of Meteorite Elements Based on the Multidimensional Scaling–Back Propagation Neural Network Algorithm Combined with Raman Mapping-Assisted Micro-Laser Induced Breakdown Spectroscopy. Chemosensors 2023, 11, 567. https://doi.org/10.3390/chemosensors11110567
Wang H, Xin Y, Fang P, Wang Y, Duan M, Wu W, Yang R, Liu S, Zhang L, Wan X. Quantitative Analysis of Meteorite Elements Based on the Multidimensional Scaling–Back Propagation Neural Network Algorithm Combined with Raman Mapping-Assisted Micro-Laser Induced Breakdown Spectroscopy. Chemosensors. 2023; 11(11):567. https://doi.org/10.3390/chemosensors11110567
Chicago/Turabian StyleWang, Hongpeng, Yingjian Xin, Peipei Fang, Yian Wang, Mingkang Duan, Wenming Wu, Ruidong Yang, Sicong Liu, Liang Zhang, and Xiong Wan. 2023. "Quantitative Analysis of Meteorite Elements Based on the Multidimensional Scaling–Back Propagation Neural Network Algorithm Combined with Raman Mapping-Assisted Micro-Laser Induced Breakdown Spectroscopy" Chemosensors 11, no. 11: 567. https://doi.org/10.3390/chemosensors11110567




