Nanocomposite-Based Electrochemical Sensors for Neurotransmitters Detection in Neurodegenerative Diseases
Abstract
:1. Introduction
2. Diagnostic Biomarkers of NDs
3. Sensitive Nanocomposite Materials
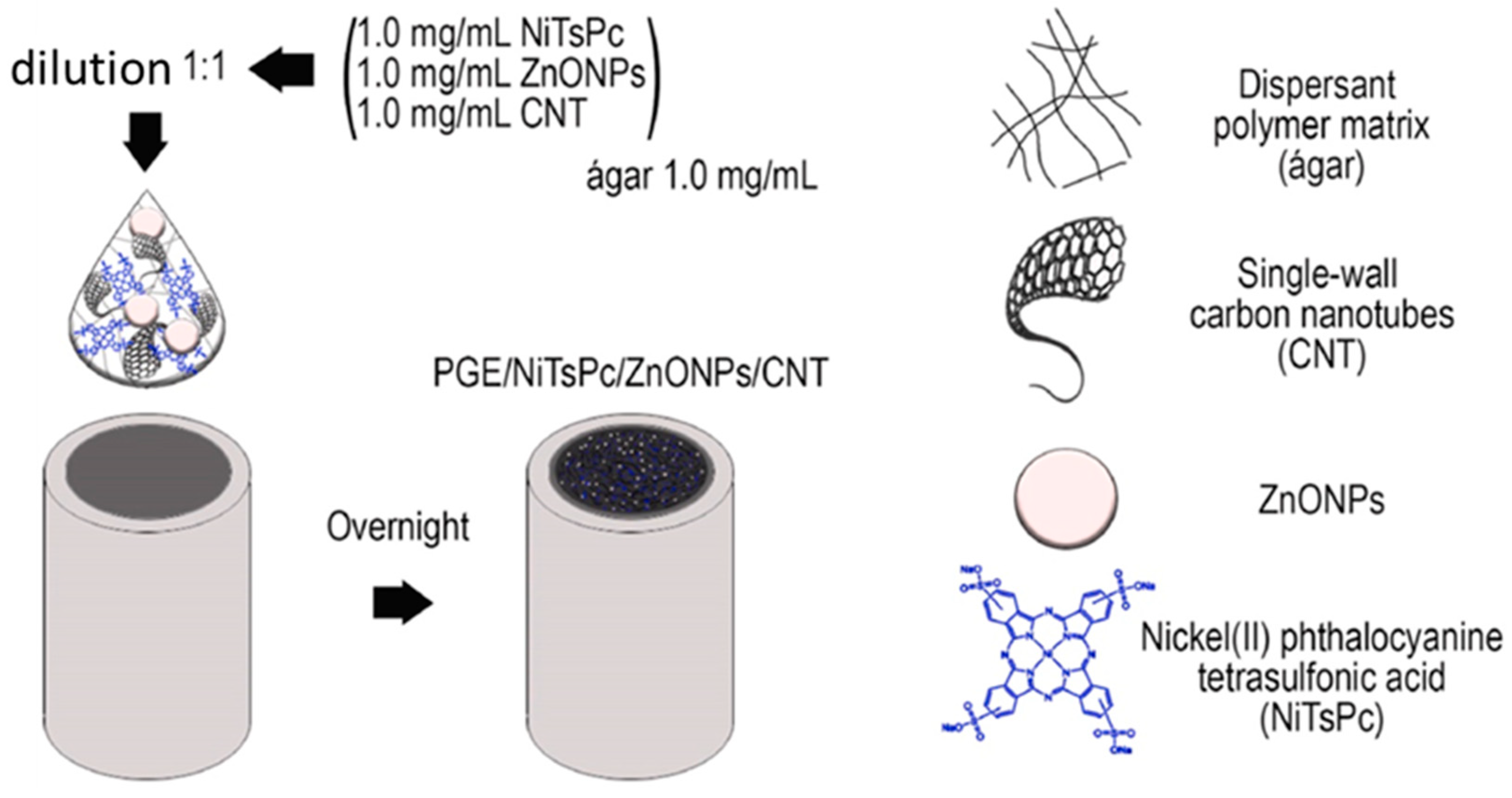
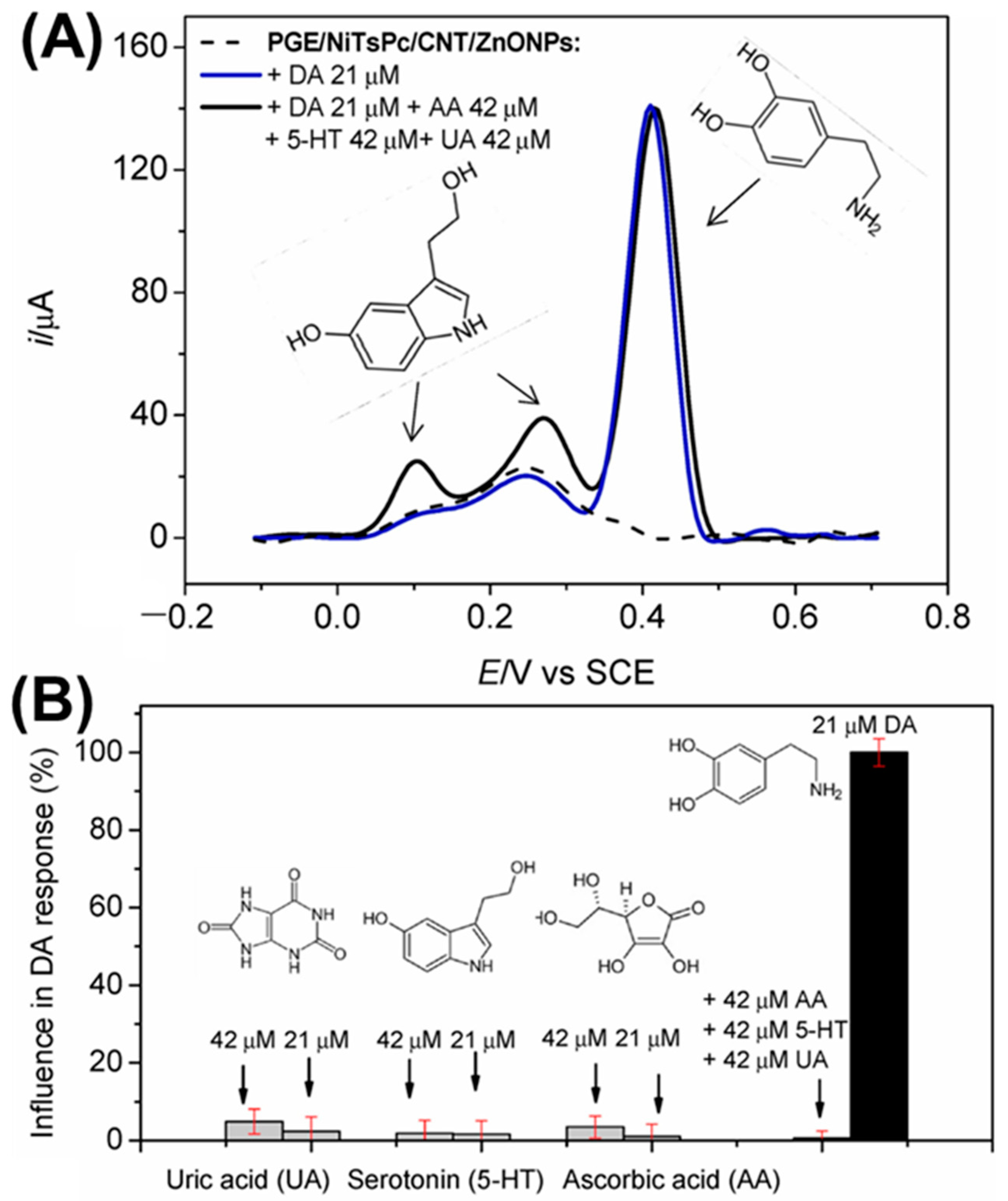
3.1. Carbon-Based NCs
3.2. Polymer-Based NCs
3.3. Molecularly Imprinted Polymers (MIPs)
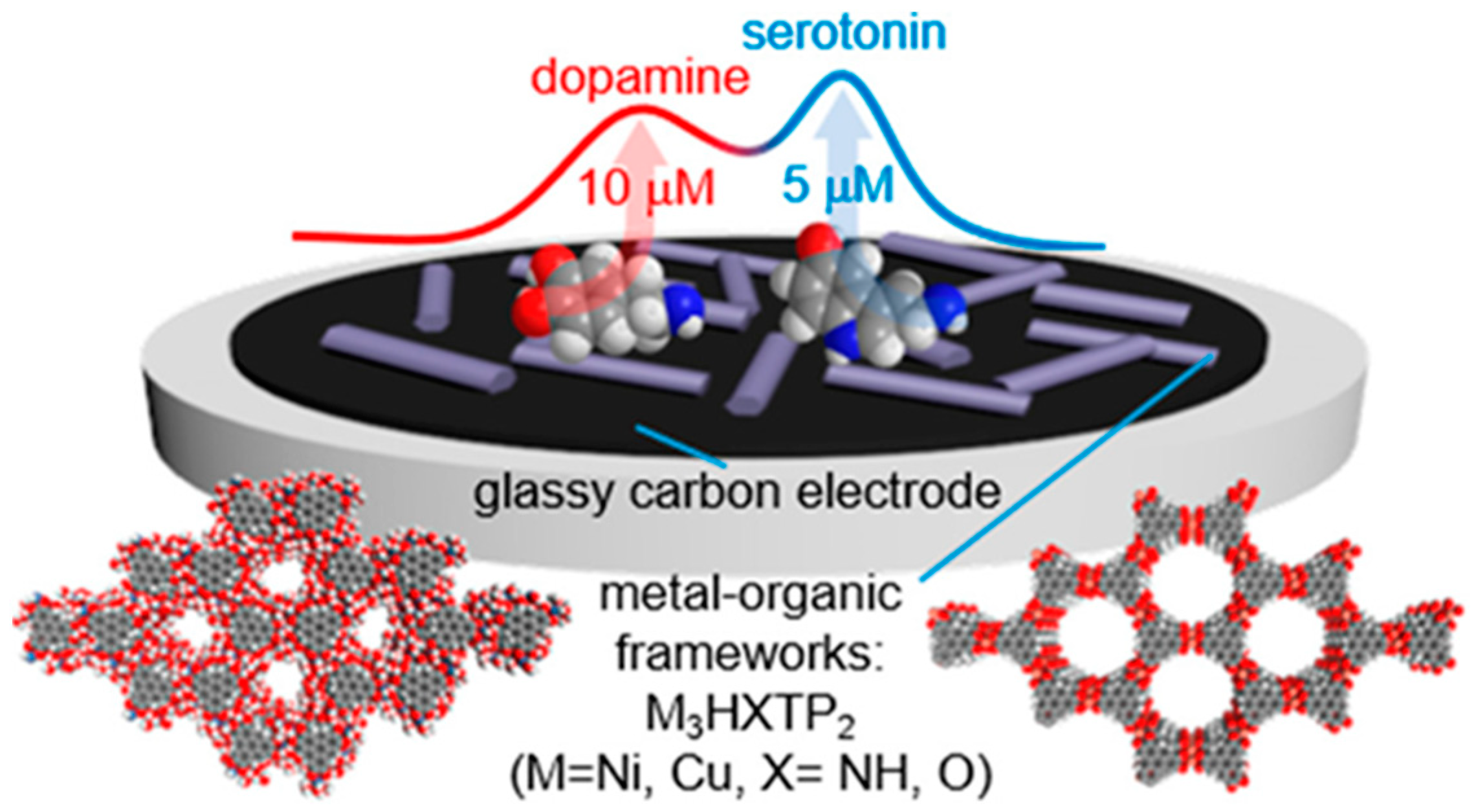
3.4. Metal-Organic Frameworks (MOFs)
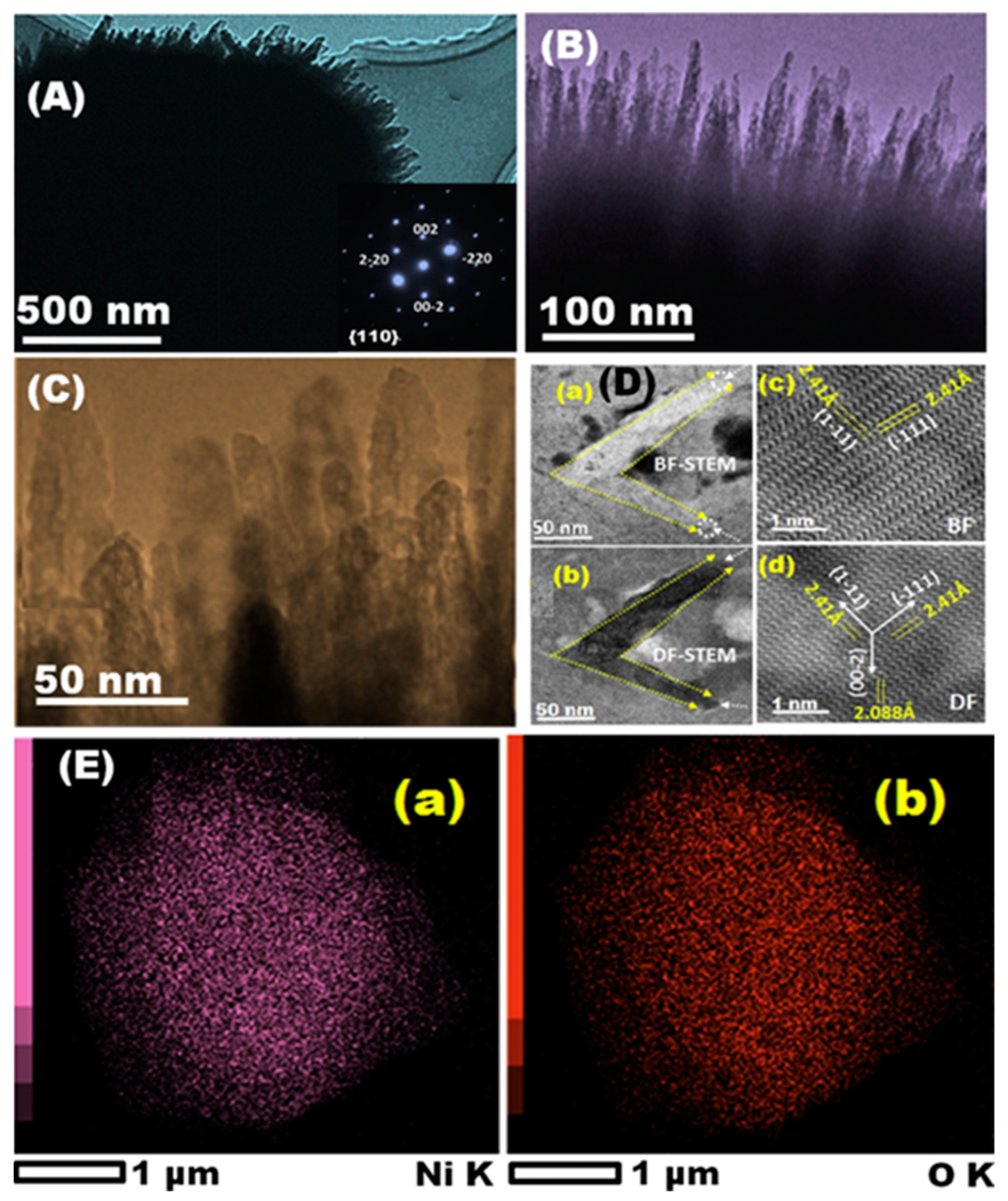
3.5. Metals and Metal Oxide NCs
3.6. Transition Metal Oxide, Carbide, and Sulfide NCs
3.7. Ionic Liquids
3.8. Other NCs
4. Versatile Electrochemical Technique in NTs Analysis
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Y.-L.; Wang, N.; Sun, F.-R.; Cao, X.-P.; Zhang, W.; Yu, J.-T. Tau in neurodegenerative disease. Ann. Transl. Med. 2018, 6, 175. [Google Scholar] [CrossRef] [PubMed]
- Dichgans, J.; Schulz, J.B. Aging in parts? Systemic aging of the nervous system. Nervenarzt 1999, 70, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, U.; Kayed, R. Amyloid β, Tau, and α-Synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases. Prog. Neurobiol. 2022, 214, 102270. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R. What causes neurodegenerative disease? Folia Neuropathol. 2020, 58, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, S.M.; Rochitta, G.; McMahon, C.P.; Craig, J.D.; Killiron, S.J.; O’Brien, K.B.; Serra, P.A.; Lowry, J.P.; O’Neill, R.D. Modifications of Poly(o-phenylenediamine) permselective layer on Pt-Ir for biosensor application in neurochemical monitoring. Sensors 2007, 7, 420–437. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Martin, W.R.W. MR spectroscopy in neurodegenerative disease. Mol. Imaging Biol. 2007, 9, 196–203. [Google Scholar] [CrossRef]
- Bicker, J.; Fortuna, A.; Alves, G.; Falcao, A. Liquid chromatographic methods for the quantification of catecholamines and their metabolites in several biological samples-a review. Anal. Chim. Acta 2013, 768, 12–34. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, Y.; Chen, B.; Ma, F.; Hao, L.; Li, G.; Ouyang, C.; Li, L. Visualization and identification of neurotransmitters in crustacean brain via multifaceted mass spectrometric approaches. ACS Chem. Neurosci. 2019, 10, 1222–1229. [Google Scholar] [CrossRef]
- Kennedy, R.T.; Watson, C.J.; Haskins, W.E.; Powell, D.H.; Strecker, R.E. In vivo neurochemical monitoring by microdialysis and capillary separations. Curr. Opin. Chem. Biol. 2002, 6, 659–665. [Google Scholar] [CrossRef]
- Moukhles, H.; Bosler, O.; Bolam, J.P.; Vallee, A.; Umbriaco, D.; Geffard, M.; Doucet, G. Quantitative and morphometric data indicate precise cellular interactions between serotonin terminals and postsynaptic targets in rat substantia nigra. Neuroscience 1997, 76, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Yang, Y.; Wang, L.; Chen, S.; Du, Y.; Song, Y. Electrochemical sensors based on metal-porous carbon nanozymes for dopamine, uric Acid and furazolidone. Chemosensors 2022, 10, 458. [Google Scholar] [CrossRef]
- Shen, M.; Colombo, M.L. Electrochemical nanoprobes for the chemical detection of neurotransmitters. Anal. Methods 2015, 7, 7095–7105. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, C.-Y.; Xu, C.-L. A highly sensitive non-enzymatic glucose sensor based on bimetallic Cu-Ag superstructures. Biosens. Bioelectron. 2015, 63, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Batra, B.; Kumari, S.; Pundir, C.S. Construction of glutamate biosensor based on covalent immobilization of glutamate oxidase on polypyrrole nanoparticles/polyaniline modified gold electrode. Enzyme Microb. Technol. 2014, 57, 69–77. [Google Scholar] [CrossRef]
- Available online: https://pubmed.ncbi.nlm.nih.gov/?term=nanocomposite+based+electrochemical+sensors&filter =datesearch.y_5 (accessed on 18 January 2023).
- Liu, X.; Ma, T.; Pinna, N.; Zhang, J. Two-dimensional nanostructured materials for gas sensing. Adv. Funct. Mater. 2017, 27, 1702168. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Olivera, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef]
- Thanh, T.D.; Balamurugan, J.; Lee, S.H.; Kim, N.H.; Lee, J.H. Novel porous gold-palladium nanoalloy network-supported graphene as an advanced catalyst for non-enzymatic hydrogen peroxide sensing. Biosens. Bioelectron. 2016, 85, 669–678. [Google Scholar] [CrossRef]
- Yang, P.; Tong, X.; Wang, G.; Gao, Z.; Guo, X.; Qin, Y. NiO/SiC nanocomposite prepared by atomic layer deposition used as a novel electrocatalyst for nonenzymatic glucose sensing. ACS Appl. Mater. Interfaces 2015, 7, 4772–4777. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Sullivan, P.; Holmes, C.; Mash, D.C.; Kopin, I.J.; Sharabi, Y. Determinants of denervation-independent depletion of putamen dopamine in Parkinson’s disease and multiple system atrophy. Park. Relat. Disord. 2017, 35, 88–91. [Google Scholar] [CrossRef]
- Kleppner, S.R.; Tobin, A.J. GABA signalling: Therapeutic targets for epilepsy, Parkinson’s disease and Huntington’s disease. Expert Opin. Ther. Targets 2001, 5, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Fei, J.; Hu, S. Simultaneous determination of dopamine and serotonin on a glassy carbon electrode coated with a film of carbon nanotubes. Anal. Biochem. 2003, 318, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Eulenburg, V.; Gomeza, J. Neurotransmitter transporters expressed in glial cells as regulators of synapse function. Brain Res. Rev. 2010, 63, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Hinzman, J.M.; Thomas, T.C.; Burmeister, J.J.; Quintero, J.E.; Huettl, P.; Pomerleau, F.; Gerhardt, G.A.; Lifshitz, J. Diffuse brain injury elevates tonic glutamate levels and potassium-evoked glutamate release in discrete brain regions at two days post-injury: An enzyme-based microelectrode array study. J. Neurotrauma 2010, 27, 889–899. [Google Scholar] [CrossRef]
- Parsons, M.P.; Raymond, L.A. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 2014, 82, 279–293. [Google Scholar] [CrossRef]
- Baluta, S.; Malecha, K.; Swist, A.; Cabaj, J. Fluorescence sensing platforms for epinephrine detection based on low temperature cofired ceramics. Sensors 2020, 20, 1429. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Beitollahi, H.; Mohseni, M.A.S.; Benvidi, A.; Naeimi, H.; Nejati-Barzoki, M.; Taghavinia, N. Simultaneous determination of epinephrine and acetaminophen concentrations using a novel carbon paste electrode prepared with 2,2′-[1,2 butanediylbis(nitriloethylidyne)]-bis-hydroquinone and TiO(2) nanoparticles. Colloids Surf. B Biointerfaces 2010, 76, 82–87. [Google Scholar] [CrossRef]
- Available online: https://www.myupchar.com/en/test/catecholamines-blood (accessed on 18 January 2023).
- Kumata, H.; Nishimura, R.; Nakanishi, C.; Inoue, C.; Tezuka, Y.; Endo, H.; Miyagi, S.; Tominaga, T.; Unno, M.; Kamei, T. Surgical strategy for an adult patient with a catecholamine-producing ganglioneuroblastoma and a cerebral aneurysm: A case report. Surg. Case Rep. 2018, 4, 119. [Google Scholar] [CrossRef]
- Hsine, Z.; Mlika, R.; Jaffrezic-Renault, N.; Korri-Youssoufi, H. Review—Recent progress in graphene based modified electrodes for electrochemical detection of dopamine. Chemosensors 2022, 10, 249. [Google Scholar] [CrossRef]
- Wang, S.; Guo, P.; Ma, G.; Wei, J.; Wang, Z.; Cui, L.; Sun, L.; Wang, A. Three-dimensional hierarchical mesoporous carbon for regenerative electrochemical dopamine sensor. Electrochim. Acta 2020, 360, 137016. [Google Scholar] [CrossRef]
- Dong, L.; Fan, L.; Yigang, D. Hierarchical porous carbon derived from pyrolysis of sodium citrate for sensitive determination of dopamine and uric acid. J. Electrochem. Soc. 2019, 166, B1585–B1593. [Google Scholar] [CrossRef]
- Liu, S.; Gao, S.; Fei, T.; Zhang, T. Highly sensitive and selective dopamine detection utilizing nitrogen-doped mesoporous carbon prepared by a molten glucose-assisted hard-template approach. Chempluschem 2019, 84, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.-Y.; Meng, Y.-N.; Xiao, P.-W.; Zhao, Z.-Q.; Wei, Z.-X.; Han, B.-H. Nitrogen-doped graphene aerogels as efficient supercapacitor electrodes and gas adsorbents. ACS Appl. Mater. Interfaces 2015, 7, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Wang, B.; Zheng, J.; Weng, B.; Li, C. Sensitive dopamine sensor based on three dimensional and macroporous carbon aerogel microelectrode. Int. J. Electrochem. Sci. 2018, 13, 4379–4389. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; An, S.; Choe, S.R.; Kwak, C.H.; Huh, Y.S.; Lee, J.; Han, Y.-K. Fabrication of 3D honeycomb-like porous polyurethane-functionalized reduced graphene oxide for detection of dopamine. Biosens. Bioelectron. 2016, 86, 122–128. [Google Scholar] [CrossRef]
- Keisham, B.; Seksenyan, A.; Denyer, S.; Kheirkhah, P.; Arnone, G.D.; Avalos, P.; Bhimani, A.D.; Svendsen, C.; Berry, V.; Mehta, A.I. Quantum capacitance based amplified graphene phononics for studying neurodegenerative diseases. ACS Appl. Mater. Interfaces 2019, 11, 169–175. [Google Scholar] [CrossRef]
- Suriyaprakash, J.; Bala, K.; Shan, L.; Wu, L.; Gupta, N. Molecular engineered carbon-based sensor for ultrafast and specific detection of neurotransmitters. ACS Appl. Mater. Interfaces 2021, 13, 60878–60893. [Google Scholar] [CrossRef]
- Liu, X.-P.; Tong, J.; Yuan, Z.; Yang, Y.; Mao, C.-J.; Niu, H.-L.; Jin, B.-K.; Zhang, S.-Y. Highly sensitive electrochemical dopamine sensor from poly(diallyldimethylammonium chloride)-functionalized graphene nanoribbon/gold nanoparticle nanocomposite. J. Nanosci. Nanotechnol. 2016, 16, 1645–1649. [Google Scholar] [CrossRef]
- Mohanan, V.M.A.; Kunnummal, A.K.; Biju, V.M.N. Selective electrochemical detection of dopamine based on molecularly imprinted poly(5-amino 8-hydroxy quinoline) immobilized reduced graphene oxide. J. Mater. Sci. 2018, 53, 10627–10639. [Google Scholar] [CrossRef]
- Gao, F.; Cai, X.; Wang, X.; Gao, C.; Liu, S.; Gao, F.; Wang, Q. Highly sensitive and selective detection of dopamine in the presence of ascorbic acid at graphene oxide modified electrode. Sens. Actuators B Chem. 2013, 186, 380–387. [Google Scholar] [CrossRef]
- Chu, K.; Wang, F.; Zhao, X.-L.; Wang, X.-W.; Tian, Y. Electrochemical dopamine sensor based on P-doped graphene: Highly active metal-free catalyst and metal catalyst support. Mater. Sci. Eng. C 2017, 81, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Yang, B.; Zhang, Z.; Kong, J.; Huang, G.; Mei, Y. Self-rolled TiO2 microscroll/graphene composite for electrochemical dopamine sensing. Prog. Nat. Sci. 2020, 30, 337–342. [Google Scholar] [CrossRef]
- Tezerjani, M.D.; Benvidi, A.; Dehghani Firouzabadi, A.; Mazloum-Ardakani, M.; Akbari, A. Epinephrine electrochemical sensor based on a carbon paste electrode modified with hydroquinone derivative and graphene oxide nano-sheets: Simultaneous determination of epinephrine, acetaminophen and dopamine. Measurement 2017, 101, 183–189. [Google Scholar] [CrossRef]
- Yadav, S.K.; Agrawal, B.; Oyama, M.; Goyal, R.N. Graphene modified palladium sensor for electrochemical analysis of norepinephrine in pharmaceuticals and biological fluids. Electrochim. Acta 2014, 125, 622–629. [Google Scholar]
- Ma, Y.; Han, J.; Tong, Z.; Qin, J.; Wang, M.; Suhr, J.; Nam, J.; Xiao, L.; Jia, S.; Chen, X. Porous carbon boosted non-enzymatic glutamate detection with ultra-high sensitivity in broad range using cu ions. Nanomaterials 2022, 12, 1987. [Google Scholar] [CrossRef]
- Alamry, K.A.; Hussein, M.A.; Choi, J.-W.; El-Said, W.A. Non-enzymatic electrochemical sensor to detect γ-aminobutyric acid with ligand-based on graphene oxide modified gold electrode. J. Electroanal. Chem. 2020, 879, 114789. [Google Scholar] [CrossRef]
- Nirala, N.R.; Abraham, S.; Kumar, V.; Bansal, A.; Srivastava, A.; Saxena, P.S. Colorimetric detection of cholesterol based on highly efficient peroxidase mimetic activity of graphene quantum dots. Sens. Actuators B Chem. 2015, 218, 42–50. [Google Scholar] [CrossRef]
- Jang, H.-S.; Kim, D.; Lee, C.; Yan, B.; Qin, X.; Piao, Y. Nafion coated Au nanoparticle-graphene quantum dot nanocomposite modified working electrode for voltammetric determination of dopamine. Inorg. Chem. Commun. 2019, 105, 174–181. [Google Scholar] [CrossRef]
- Pang, P.; Yan, F.; Li, H.; Li, H.; Zhang, Y.; Wang, H.; Wu, Z.; Yang, W. Graphene quantum dots and Nafion composite as an ultrasensitive electrochemical sensor for the detection of dopamine. Anal. Methods 2016, 8, 4912–4918. [Google Scholar] [CrossRef]
- Luhana, C.; Moyo, I.; Tshenkeng, K.; Mashazi, P. In-sera selectivity detection of catecholamine neurotransmitters using covalent composite of cobalt phthalocyanine and aminated graphene quantum dots. Microchem. J. 2022, 180, 107605. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Tong, L.; Tong, Q.-X. Graphene quantum dots/multiwalled carbon nanotubes composite-based electrochemical sensor for detecting dopamine release from living cells. ACS Sustain. Chem. Eng. 2020, 8, 1644–1650. [Google Scholar] [CrossRef]
- Arumugasamy, S.K.; Govindaraju, S.; Yun, K. Electrochemical sensor for detecting dopamine using graphene quantum dots incorporated with multiwall carbon nanotubes. Appl. Surf. Sci. 2020, 508, 145294. [Google Scholar] [CrossRef]
- Galal, A.; Atta, N.F.; El-Ads, E.H.; El-Gohary, A.R.M. Fabrication of β-cyclodextrin/glycine/carbon nanotubes electrochemical neurotransmitters sensor—Application in ultra-sensitive determination of DOPAC in human serum. Electroanalysis 2018, 30, 1678–1688. [Google Scholar] [CrossRef]
- Lin, R.; Lim, T.M.; Tran, T. Carbon nanotube band electrodes for electrochemical sensors. Electrochem. Commun. 2018, 86, 135–139. [Google Scholar] [CrossRef]
- Merkoçi, A.; Pumera, M.; Llopis, X.; Pérez, B.; del Valle, M.; Alegret, S. New materials for electrochemical sensing VI: Carbon nanotubes. TrAC Trends Anal. Chem. 2005, 24, 826–838. [Google Scholar] [CrossRef]
- Chapin, A.A.; Rajasekaran, P.R.; Quan, D.N.; Hu, L.; Herberholz, J.; Bentley, W.E.; Ghodssi, R. Electrochemical measurement of serotonin by Au-CNT electrodes fabricated on microporous cell culture membranes. Microsyst. Nanoeng. 2020, 6, 90. [Google Scholar] [CrossRef]
- Rajarathinam, T.; Thirumalai, D.; Kwon, M.; Lee, S.; Jayaraman, S.; Paik, H.-J.; Lee, J.; Chang, S.-C. Screen-printed carbon electrode modified with de-bundled single-walled carbon nanotubes for voltammetric determination of norepinephrine in ex vivo rat tissue. Bioelectrochemistry 2022, 146, 108155. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.; Shi, W.; Jiang, M.; Tian, L.; Su, M.; Wu, J.; Liu, Q.; Yu, C.; Gu, H. Pt nanoparticle decorated carbon nanotubes nanocomposite based sensing platform for the monitoring of cell-secreted dopamine. Sens. Actuators B Chem. 2021, 330, 129311. [Google Scholar] [CrossRef]
- Vlckova, M.; Schwarz, M.A. Determination of cationic neurotransmitters and metabolites in brain homogenates by microchip electrophoresis and carbon nanotube-modified amperometry. J. Chromatogr. A 2007, 1142, 214–221. [Google Scholar] [CrossRef]
- Mercante, L.A.; Pavinatto, A.; Iwaki, L.E.O.; Scagion, V.P.; Zucolotto, V.; Oliveira, O.N., Jr.; Mattoso, L.H.C.; Correa, D.S. Electrospun polyamide 6/poly(allylamine hydrochloride) nanofibers functionalized with carbon nanotubes for electrochemical detection of dopamine. ACS Appl. Mater. Interfaces 2015, 7, 4784–4790. [Google Scholar] [CrossRef]
- Kumar, S.; Awasthi, A.; Sharma, M.D.; Singh, K.; Singh, D. Functionalized multiwall carbon nanotube-molybdenum disulphide nanocomposite based electrochemical ultrasensitive detection of neurotransmitter epinephrine. Mater. Chem. Phys. 2022, 290, 126656. [Google Scholar] [CrossRef]
- Koteshwara Reddy, K.; Satyanarayana, M.; Yugender Goud, K.; Vengatajalabathy Gobi, K.; Kim, H. Carbon nanotube ensembled hybrid nanocomposite electrode for direct electrochemical detection of epinephrine in pharmaceutical tablets and urine. Mater. Sci. Eng. C 2017, 79, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Atta, N.F.; Ahmed, Y.M.; Galal, A. Electrochemical determination of neurotransmitters at crown ether modified carbon nanotube composite: Application for sub-nano-sensing of serotonin in human serum. Electroanalysis 2018, 31, 1204–1214. [Google Scholar] [CrossRef]
- Fagan-Murphy, A.; Patel, B.A. Compressed multiwall carbon nanotube composite electrodes provide enhanced electroanalytical performance for determination of serotonin. Electrochim. Acta 2014, 138, 392–399. [Google Scholar] [CrossRef]
- Gorduk, O. Differential pulse voltammetric determination of serotonin using an acid-activated multiwalled carbon nanotube—Over-oxidized poly(3,4-ethylenedioxythiophene) modified pencil graphite electrode. Anal. Lett. 2019, 53, 1034–1052. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, M.; Cao, J.; Niu, R.; Li, P.; Zhou, G.; Yu, Y.; van den Berg, A.; Shui, L. Electrochemical sensor integrated microfluidic device for sensitive and simultaneous quantification of dopamine and 5-hydroxytryptamine. Sens. Actuators B Chem. 2018, 273, 873–883. [Google Scholar] [CrossRef]
- Bonetto, M.C.; Muñoz, F.F.; Diz, V.E.; Sacco, N.J.; Cortón, E. Fused and unzipped carbon nanotubes, electrochemically treated, for selective determination of dopamine and serotonin. Electrochim. Acta 2018, 283, 338–348. [Google Scholar] [CrossRef]
- Gupta, P.; Tsai, K.; Ruhunage, C.K.; Gupta, V.K.; Rahm, C.E.; Jiang, D.; Alvarez, N.T. True picomolar neurotransmitter sensor based on open-ended carbon nanotubes. Anal. Chem. 2020, 92, 8536–8545. [Google Scholar] [CrossRef]
- Mendoza, A.; Asrat, T.; Liu, F.; Wonnenberg, P.; Zestos, A.G. Carbon nanotube yarn microelectrodes promote high temporal measurements of serotonin using fast scan cyclic voltammetry. Sensors 2020, 20, 1173. [Google Scholar] [CrossRef]
- Clark, J.; Chen, Y.; Hinder, S.; Silva, S.R.P. Highly Sensitive Dopamine Detection Using a Bespoke Functionalised Carbon Nanotube Microelectrode Array. Electroanalysis 2017, 29, 2365–2376. [Google Scholar] [CrossRef]
- Gerard, M.; Chaubey, A.; Malhotra, B.D. Application of conducting polymers to biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, M.-M.; Bai, F.-Q.; Guo, Z.-Z.; Liu, H.; Zhong, G.-X.; Peng, H.-P.; Chen, W.; Lin, X.-H.; Lei, Y.; et al. An electrochemical biosensor for sensitive detection of nicotine-induced dopamine secreted by PC12 cells. J. Electroanal. Chem. 2019, 832, 217–224. [Google Scholar] [CrossRef]
- Selvolini, G.; Lazzarini, C.; Marrazza, G. Electrochemical nanocomposite single-use sensor for dopamine detection. Sensors 2019, 19, 3097. [Google Scholar] [CrossRef] [PubMed]
- Koluaçık, E.; Karabiberoğlu, Ş.U.; Dursun, Z. Electrochemical determination of serotonin using pre-treated multi-walled carbon nanotube-polyaniline composite electrode. Electroanalysis 2018, 30, 2977–2987. [Google Scholar] [CrossRef]
- Peairs, M.J.; Ross, A.E.; Venton, B.J. Comparison of Nafion- and overoxidized polypyrrole-carbon nanotube electrodes for neurotransmitter detection. Anal. Methods 2011, 3, 2379–2386. [Google Scholar] [CrossRef]
- Kathiresan, V.; Thirumalai, D.; Rajarathinam, T.; Yeom, M.; Lee, J.; Kim, S.; Yoon, J.-H.; Chang, S.-C. A simple one-step electrochemical deposition of bioinspired nanocomposite for the non-enzymatic detection of dopamine. J. Anal. Sci. Technol. 2021, 12, 5. [Google Scholar] [CrossRef]
- Ghanbari, K.; Bonyadi, S. An electrochemical sensor based on Pt nanoparticles decorated over-oxidized polypyrrole/reduced graphene oxide nanocomposite for simultaneous determination of two neurotransmitters dopamine and 5-Hydroxy tryptamine in the presence of ascorbic acid. Int. J. Polym. Anal. Charact. 2020, 25, 105–125. [Google Scholar] [CrossRef]
- Ran, G.; Chen, X.; Xia, Y. Electrochemical detection of serotonin based on a poly(bromocresol green) film and Fe3O4 nanoparticles in a chitosan matrix. RSC Adv. 2017, 7, 1847–1851. [Google Scholar] [CrossRef]
- Ran, G.; Chen, C.; Gu, C. Serotonin sensor based on a glassy carbon electrode modified with multiwalled carbon nanotubes, chitosan, and poly(p-aminobenzenesulfonate). Microchim. Acta 2015, 182, 1323–1328. [Google Scholar] [CrossRef]
- Huang, J.; Xu, W.; Gong, Y.; Weng, S.; Lin, X. Selective and reliable electrochemical sensor based on Polythionine/AuNPs composites for epinephrine detection in serum. Int. J. Electrochem. Sci. 2016, 11, 8193–8203. [Google Scholar] [CrossRef]
- Yan, X.; Lu, N.; Gu, Y.; Li, C.; Zhang, T.; Liu, H.; Zhang, Z.; Zhai, S. Catalytic activity of biomimetic model of cytochrome P450 in oxidation of dopamine. Talanta 2018, 179, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Thirumalai, D.; Lee, S.; Kwon, M.; Paik, H.J.; Lee, J.; Chang, S.-C. Disposable voltammetric sensor modified with block copolymer-dispersed graphene for simultaneous determination of dopamine and ascorbic acid in ex vivo mouse brain tissue. Biosensors 2021, 11, 368. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.V.; Lopes, C.B.; da Silva, W.C.; de Paiva, Y.G.; dos Santos Silva, F.D.; Lima, P.R.; Kubota, L.T.; Goulart, M.O.F. Electropolymerization of ferulic acid on multi-walled carbon nanotubes modified glassy carbon electrode as a versatile platform for NADH, dopamine and epinephrine separate detection. Microchem. J. 2017, 133, 460–467. [Google Scholar] [CrossRef]
- Sadanandhan, N.K.; Cheriyathuchenaaramvalli, M.; Devaki, S.J.; Ravindranatha Menon, A.R. PEDOT-reduced graphene oxide-silver hybrid nanocomposite modified transducer for the detection of serotonin. J. Electroanal. Chem. 2017, 794, 244–253. [Google Scholar] [CrossRef]
- da Silva, Q.G.; Vieira Barbosa, N.; de Pieri Troiani, E.; Censi Faria, R. Electrochemical determination of norepinephrine on cathodically pretreated Poly(1,5-diaminonaphthalene) modified electrode. Electroanalysis 2011, 23, 1359–1364. [Google Scholar] [CrossRef]
- Vashist, S.K.; Zheng, D.; Al-Rubeaan, K.; Luong, J.H.T.; Sheu, F.-S. Advances in carbon nanotube based electrochemical sensors for bioanalytical applications. Biotechnol. Adv. 2011, 29, 169–188. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Vittal, R.; Nien, P.-C.; Ho, K.-C. Enhancing dopamine detection using a glassy carbon electrode modified with MWCNTs, quercetin, and Nafion®. Biosens. Bioelectron. 2009, 24, 3504–3509. [Google Scholar] [CrossRef]
- Cui, F.; Zhou, Z.; Zhou, H.S. Molecularly imprinted polymers and surface imprinted polymers based electrochemical biosensor for infectious diseases. Sensors 2020, 20, 996. [Google Scholar] [CrossRef]
- Morelli, I.; Chiono, V.; Vozzi, G.; Ciardelli, G.; Silvestri, D.; Giusti, P. Molecularly imprinted submicronspheres for applications in a novel model biosensor-film. Sens. Actuators B Chem. 2010, 150, 394–401. [Google Scholar] [CrossRef]
- Song, W.; Chen, Y.; Xu, J.; Yang, X.-R.; Tian, D.-B. Dopamine sensor based on molecularly imprinted electrosynthesized polymers. J. Solid State Electrochem. 2010, 14, 1909–1914. [Google Scholar] [CrossRef]
- Tertis, M.; Florea, A.; Adumitrachioaie, A.; Cernat, A.; Bogdan, D.; Barbu-Tudoran, L.; Jaffrezic Renault, N.; Sandulescu, R.; Cristea, C. Detection of dopamine by a biomimetic electrochemical sensor based on polythioaniline-bridged gold nanoparticles. ChemPlusChem 2017, 82, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Amatatongchai, M.; Sitanurak, J.; Sroysee, W.; Sodanat, S.; Chairam, S.; Jarujamrus, P.; Nacapricha, D.; Lieberzeit, P.A. Highly sensitive and selective electrochemical paper-based device using a graphite screen-printed electrode modified with molecularly imprinted polymers coated Fe3O4@Au@SiO2 for serotonin determination. Anal. Chim. Acta 2019, 1077, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Ahnfeldt, T.; Stock, N. New functionalized flexible Al-MIL-53-X (X = -Cl, -Br, -CH3, -NO2, -(OH)2) solids: Syntheses, characterization, sorption, and breathing behavior. Inorg. Chem. 2011, 50, 9518–9526. [Google Scholar] [CrossRef]
- Zhou, K.; Shen, D.; Li, X.; Chen, Y.; Hou, L.; Zhang, Y.; Sha, J. Molybdenum oxide-based metal-organic framework/polypyrrole nanocomposites for enhancing electrochemical detection of dopamine. Talanta 2020, 209, 120507. [Google Scholar] [CrossRef]
- Moghzi, F.; Soleimannejad, J.; Sanudo, E.C.; Janczak, J. Dopamine sensing based on ultrathin fluorescent metal-organic nanosheets. ACS Appl. Mater. Interfaces 2020, 12, 44499–44507. [Google Scholar] [CrossRef]
- Chen, S.S.; Han, P.-C.; Kuok, W.-K.; Lu, J.-Y.; Gu, Y.; Ahamad, T.; Alshehri, S.M.; Ayalew, H.; Yu, H.-H.; Wu, K.C.-W. Synthesis of MOF525/PEDOT composites as microelectrodes for electrochemical sensing of dopamine. Polymers 2020, 12, 1976. [Google Scholar] [CrossRef]
- Yu, G.; Xia, J.; Zhang, F.; Wang, Z. Hierarchical and hybrid RGO/ZIF-8 nanocomposite as electrochemical sensor for ultrasensitive determination of dopamine. J. Electroanal. Chem. 2017, 801, 496–502. [Google Scholar] [CrossRef]
- Ko, M.; Mendecki, L.; Eagleton, A.M.; Durbin, C.G.; Stolz, R.M.; Meng, Z.; Mirica, K.A. Employing conductive metal-organic frameworks for voltammetric detection of neurochemicals. J. Am. Chem. Soc. 2020, 142, 11717–11733. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, T.; Niu, Y.; Ye, B.-C. Electrochemical detection of glutamate by metal–organic frameworks-derived Ni@NC electrocatalysts. Microchem. J. 2022, 175, 107229. [Google Scholar] [CrossRef]
- Li, J.; Qu, J.; Yang, R.; Qu, L.; Harrington, P.B. A snsitive and selective electrochemical sensor based on Graphene quantum dot/gold nanoparticle nanocomposite modified electrode for the determination of quercetin in biological samples. Electroanalysis 2016, 28, 1322–1330. [Google Scholar] [CrossRef]
- Sipuka, D.S.; Arotiba, O.A.; Sebokolodi, T.I.; Tsekeli, T.R.; Nkosi, D. Gold-dendrimer nanocomposite based electrochemical sensor for dopamine. Electroanalysis 2022, 34. [Google Scholar] [CrossRef]
- Li, X.; Lu, X.; Kan, X. 3D electrochemical sensor based on poly(hydroquinone)/gold nanoparticles/nickel foam for dopamine sensitive detection. J. Electroanal. Chem. 2017, 799, 451–458. [Google Scholar] [CrossRef]
- Tertiș, M.; Cernat, A.; Lacatiș, D.; Florea, A.; Bogdan, D.; Suciu, M.; Săndulescu, R.; Cristea, C. Highly selective electrochemical detection of serotonin on polypyrrole and gold nanoparticles-based 3D architecture. Electrochem. Commun. 2017, 75, 43–47. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, Y.; Tang, C.; Zhu, X.; Lu, X.; Liu, L.; Chen, Z.; Li, L. Voltammetric determination of 5-hydroxytryptamine based on the use of platinum nanoparticles coated with molecularly imprinted silica. Microchim. Acta 2018, 185, 219. [Google Scholar] [CrossRef]
- Goyal, R.N.; Aziz, M.A.; Oyama, M.; Chatterjee, S.; Rana, A.R.S. Nanogold based electrochemical sensor for determination of norepinephrine in biological fluids. Sens. Actuators B Chem. 2011, 153, 232–238. [Google Scholar] [CrossRef]
- Pitiphattharabun, S.; Meesombad, K.; Panomsuwan, G.; Jongprateep, O. MWCNT/Ti-doped ZnO nanocomposite as electrochemical sensor for detecting glutamate and ascorbic acid. Int. J. Appl. Ceram. Technol. 2021, 19, 467–479. [Google Scholar] [CrossRef]
- Yang, Z.; Hu, G.; Chen, X.; Zhao, J.; Zhao, G. The nano-Au self-assembled glassy carbon electrode for selective determination of epinephrine in the presence of ascorbic acid. Colloids Surf. B Biointerfaces 2007, 54, 230–235. [Google Scholar] [CrossRef]
- Wu, B.; Yeasmin, S.; Liu, Y.; Cheng, L.-J. Sensitive and selective electrochemical sensor for serotonin detection based on ferrocene-gold nanoparticles decorated multiwall carbon nanotubes. Sens. Actuators B Chem. 2022, 354, 131216. [Google Scholar] [CrossRef]
- da Silva, V.N.C.; Farias, E.A.O.; Araujo, A.R.; Xavier Magalhaes, F.E.; Neves Fernandes, J.R.; Teles Souza, J.M.; Eiras, C.; da Silva, D.A.; do Vale Bastos, V.H.; Teixeira, S.S. Rapid and selective detection of dopamine in human serum using an electrochemical sensor based on zinc oxide nanoparticles, nickel phthalocyanines, and carbon nanotubes. Biosens. Bioelectron. 2022, 210, 114211. [Google Scholar] [CrossRef]
- Truţă, F.; Tertis, M.; Cristea, C.; Graur, F. Simultaneous detection of dopamine and serotonin in real complex matrices. Curr. Anal. Chem. 2021, 17, 374–384. [Google Scholar] [CrossRef]
- Figueiredo-Filho, L.C.S.; Silva, T.A.; Vicentini, F.C.; Fatibello-Filho, O. Simultaneous voltammetric determination of dopamine and epinephrine in human body fluid samples using a glassy carbon electrode modified with nickel oxide nanoparticles and carbon nanotubes within a dihexadecylphosphate film. Analyst 2014, 139, 2842–2849. [Google Scholar] [CrossRef] [PubMed]
- Emran, M.Y.; Shenashen, M.A.; Mekawy, M.; Azzam, A.M.; Akhtar, N.; Gomaa, H.; Selim, M.M.; Faheem, A.; El-Safty, S.A. Ultrasensitive in-vitro monitoring of monoamine neurotransmitters from dopaminergic cells. Sens. Actuators B Chem. 2018, 259, 114–124. [Google Scholar] [CrossRef]
- Sun, D.; Li, H.; Li, M.; Li, C.; Dai, H.; Sun, D.; Yang, B. Electrodeposition synthesis of a NiO/CNT/PEDOT composite for simultaneous detection of dopamine, serotonin, and tryptophan. Sens. Actuators B Chem. 2018, 259, 433–442. [Google Scholar] [CrossRef]
- Ghanbari, K.; Moloudi, M. Flower-like ZnO decorated polyaniline/reduced graphene oxide nanocomposites for simultaneous determination of dopamine and uric acid. Anal. Biochem. 2016, 512, 91–102. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Tao, L.; Min, Q.; Xiang, J.; Wang, Q.; Xie, J.; Yue, Y.; Wu, S.; Li, X.; et al. A disposable electrochemical sensor for simultaneous determination of norepinephrine and serotonin in rat cerebrospinal fluid based on MWNTs-ZnO/chitosan composites modified screen-printed electrode. Biosens. Bioelectron. 2015, 65, 31–38. [Google Scholar] [CrossRef]
- Mphuthi, N.G.; Adekunle, A.S.; Ebenso, E.E. Electrocatalytic oxidation of epinephrine and norepinephrine at metal oxide doped phthalocyanine/MWCNT composite sensor. Sci. Rep. 2016, 6, 26938. [Google Scholar] [CrossRef]
- Jamal, M.; Hasan, M.; Mathewson, A.; Razeeb, K.M. Disposable sensor based on enzyme-free Ni nanowire array electrode to detect glutamate. Biosens. Bioelectron. 2013, 40, 213–218. [Google Scholar] [CrossRef]
- Samdani, K.J.; Samdani, J.S.; Kim, N.H.; Lee, J.H. FeMoO4 based, enzyme-free electrochemical biosensor for ultrasensitive detection of norepinephrine. Biosens. Bioelectron. 2016, 81, 445–453. [Google Scholar] [CrossRef]
- Paramparambath, S.; Shafath, S.; Maurya, M.R.; Cabibihan, J.-J.; Al-Ali, A.; Malik, R.A.; Sadasivuni, K.K. Nonenzymatic electrochemical sensor based on CuO-MgO composite for dopamine detection. IEEE Sens. J. 2021, 21, 25597–25605. [Google Scholar] [CrossRef]
- Zhong, Z.; Wang, J.; Jiang, S.; Li, M.; Lin, J.; Pan, J.; Tao, X.; Xie, A.; Luo, S. Novel electrochemical sensor based on Fe3O4-ZrO2-graphene oxide for determination of dopamine. Ionics 2022, 28, 4853–4865. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Joseph Anthuvan, A.; Chen, S.-M.; Chinnuswamy, V.; Kadirvelu, K. Trace level electrochemical determination of the neurotransmitter dopamine in biological samples based on iron oxide nanoparticle decorated graphene sheets. Inorg. Chem. Front. 2018, 5, 705–718. [Google Scholar] [CrossRef]
- Vinay, M.M.; Arthoba Nayaka, Y. Iron oxide (Fe2O3) nanoparticles modified carbon paste electrode as an advanced material for electrochemical investigation of paracetamol and dopamine. J. Sci. Adv. Mater. Devices 2019, 4, 442–450. [Google Scholar] [CrossRef]
- Nehru, L.; Chinnathambi, S.; Fazio, E.; Neri, F.; Leonardi, S.G.; Bonavita, A.; Neri, G. Electrochemical sensing of serotonin by a modified MnO(2)-graphene electrode. Biosensors 2020, 10, 33. [Google Scholar] [CrossRef]
- Tomé, L.I.N.; Brett, C.M.A. Polymer/iron oxide nanoparticle modified glassy carbon electrodes for the enhanced detection of epinephrine. Electroanalysis 2019, 31, 704–710. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Maleki, M.; Khoshroo, A. High-performance electrochemical sensor based on electrodeposited iron oxide nanoparticle: Catecholamine as analytical probe. J. Iran. Chem. Soc. 2017, 14, 1659–1664. [Google Scholar] [CrossRef]
- Shahzad, F.; Iqbal, A.; Zaidi, S.A.; Hwang, S.-W.; Koo, C.M. Nafion-stabilized two-dimensional transition metal carbide (Ti3C2Tx MXene) as a high-performance electrochemical sensor for neurotransmitter. J. Ind. Eng. Chem. 2019, 79, 338–344. [Google Scholar] [CrossRef]
- Ni, M.; Chen, J.; Wang, C.; Wang, Y.; Huang, L.; Xiong, W.; Zhao, P.; Xie, Y.; Fei, J. A high-sensitive dopamine electrochemical sensor based on multilayer Ti3C2 MXene, graphitized multi-walled carbon nanotubes and ZnO nanospheres. Microchem. J. 2022, 178, 107410. [Google Scholar] [CrossRef]
- Boobphahom, S.; Siripongpreda, T.; Zhang, D.; Qin, J.; Rattanawaleedirojn, P.; Rodthongkum, N. TiO(2)/MXene-PVA/GO hydrogel-based electrochemical sensor for neurological disorder screening via urinary norepinephrine detection. Microchim. Acta 2021, 188, 387. [Google Scholar] [CrossRef]
- Samdani, K.J.; Joh, D.W.; Rath, M.K.; Lee, K.T. Electrochemical mediatorless detection of norepinephrine based on MoO3 nanowires. Electrochim. Acta 2017, 252, 268–274. [Google Scholar] [CrossRef]
- Huang, K.-J.; Liu, Y.-J.; Liu, Y.-M.; Wang, L.-L. Molybdenum disulfide nanoflower-chitosan-Au nanoparticles composites based electrochemical sensing platform for bisphenol A determination. J. Hazard. Mater. 2014, 276, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraj, K.; Dinakaran, T.; Lee, Y.; Kim, S.; Kim, H.S.; Lee, J.; Chang, S.-C. One-step construction of a molybdenum disulfide/multi-walled carbon nanotubes/polypyrrole nanocomposite biosensor for the ex-vivo detection of dopamine in mouse brain tissue. Biochem. Biophys. Res. Commun. 2017, 494, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Pramoda, K.; Moses, K.; Maitra, U.; Rao, C.N.R. Superior performance of a MoS2-RGO composite and a borocarbonitride in the electrochemical detection of dopamine, uric acid, and adenine. Electroanalysis 2015, 27, 1892–1898. [Google Scholar] [CrossRef]
- Balu, S.; Palanisamy, S.; Velusamy, V.; Yang, T.C.K.; El-Shafey, E.I. Tin disulfide nanorod-graphene-beta-cyclodextrin nanocomposites for sensing dopamine in rat brains and human blood serum. Mater. Sci. Eng. C 2020, 108, 110367. [Google Scholar] [CrossRef]
- Sun, Y.; Fei, J.; Hou, J.; Zhang, Q.; Liu, Y.; Hu, B. Simultaneous determination of dopamine and serotonin using a carbon nanotubes-ionic liquid gel modified glassy carbon electrode. Microchim. Acta 2009, 165, 373–379. [Google Scholar] [CrossRef]
- Liu, P.; Dong, M.; Lu, J.; Guo, H.; Lu, X.; Liu, X. Simultaneous determination of 5-hydroxytryptamine and dopamine using ionic liquid functionalized graphene. Ionics 2015, 21, 1111–1119. [Google Scholar] [CrossRef]
- Li, Y.; Ji, Y.; Ren, B.; Jia, L.; Ma, G.; Liu, X. Carboxyl-functionalized mesoporous molecular sieve/colloidal gold modified nano-carbon ionic liquid paste electrode for electrochemical determination of serotonin. Mater. Res. Bull. 2019, 109, 240–245. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Khoshroo, A. High sensitive sensor based on functionalized carbon nanotube/ionic liquid nanocomposite for simultaneous determination of norepinephrine and serotonin. J. Electroanal. Chem. 2014, 717–718, 17–23. [Google Scholar] [CrossRef]
- Umapathi, S.; Masud, J.; Coleman, H.; Nath, M. Electrochemical sensor based on CuSe for determination of dopamine. Microchim. Acta 2020, 187, 440. [Google Scholar] [CrossRef]
- Pramanik, S.; Kumar, Y.; Karmakar, P.; Das, D.K. Nanosized Gadoliniumorthoferrite-based electrochemical sensor for the determination of dopamine. Nano LIFE 2021, 11, 3. [Google Scholar] [CrossRef]
- Yan, X.; Gu, Y.; Li, C.; Zheng, B.; Li, Y.; Zhang, T.; Zhang, Z.; Yang, M. Morphology-controlled synthesis of Bi2S3 nanorods-reduced graphene oxide composites with high-performance for electrochemical detection of dopamine. Sens. Actuators B Chem. 2018, 257, 936–943. [Google Scholar] [CrossRef]
- Ramachandran, A.; Panda, S.; Karunakaran Yesodha, S. Physiological level and selective electrochemical sensing of dopamine by a solution processable graphene and its enhanced sensing property in general. Sens. Actuators B Chem. 2018, 256, 488–497. [Google Scholar] [CrossRef]
- Thanh, T.D.; Balamurugan, J.; Hien, H.V.; Kim, N.H.; Lee, J.H. A novel sensitive sensor for serotonin based on high-quality of AuAg nanoalloy encapsulated graphene electrocatalyst. Biosens. Bioelectron. 2017, 96, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Manbohi, A.; Ahmadi, S.H. Sensitive and selective detection of dopamine using electrochemical microfluidic paper-based analytical nanosensor. Sens. Bio-Sens. Res. 2019, 23, 100270. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, H.; Yu, Z.; Quhe, R.; Zhou, S.; Wang, Y.; Liu, C.C.; Zhong, H.; Han, N.; Lu, J.; et al. Rise of silicene: A competitive 2D material. Prog. Mater. Sci. 2016, 83, 24–151. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Pazhamalai, P.; Kim, S.-J. Two-dimensional siloxene nanosheets: Novel high-performance supercapacitor electrode materials. Energy Environ. Sci. 2018, 11, 1595–1602. [Google Scholar] [CrossRef]
- Parra, V.; Arrieta, Á.A.; Fernández-Escudero, J.A.; García, H.; Apetrei, C.; Rodríguez-Méndez, M.L.; de Saja, J.A. E-tongue based on a hybrid array of voltammetric sensors based on phthalocyanines, perylene derivatives and conducting polymers: Discrimination capability towards red wines elaborated with different varieties of grapes. Sens. Actuators B Chem. 2006, 115, 54–61. [Google Scholar] [CrossRef]
- Diab, N.; Morales, D.M.; Andronescu, C.; Masoud, M.; Schuhmann, W. A sensitive and selective graphene/cobalt tetrasulfonated phthalocyanine sensor for detection of dopamine. Sens. Actuators B Chem. 2019, 285, 17–23. [Google Scholar] [CrossRef]
- Uwaya, G.E.; Fayemi, O.E. Electrochemical detection of serotonin in banana at green mediated PPy/Fe3O4NPs nanocomposites modified electrodes. Sens. Bio-Sens. Res. 2020, 28, 100338. [Google Scholar] [CrossRef]
- Ramos, M.M.V.; Carvalho, J.H.S.; de Oliveira, P.R.; Janegitz, B.C. Determination of serotonin by using a thin film containing graphite, nanodiamonds and gold nanoparticles anchored in casein. Measurement 2020, 149, 106979. [Google Scholar] [CrossRef]
- Babaei, A.; Taheri, A.R. Nafion/Ni(OH)2 nanoparticles-carbon nanotube composite modified glassy carbon electrode as a sensor for simultaneous determination of dopamine and serotonin in the presence of ascorbic acid. Sens. Actuators B Chem. 2013, 176, 543–551. [Google Scholar] [CrossRef]
- Sivasubramanian, R.; Biji, P. Preparation of copper (I) oxide nanohexagon decorated reduced graphene oxide nanocomposite and its application in electrochemical sensing of dopamine. Mater. Sci. Eng. B 2016, 210, 10–18. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Behpour, M.; Mortazavi, M.; Khoobi, A. Fabrication of a graphene oxide nano-sheet modified electrode for determination of dopamine in the presence of tyrosine: A multivariate optimization strategy. J. Mol. Liq. 2016, 215, 31–38. [Google Scholar] [CrossRef]
- Hou, J.; Xu, C.; Zhao, D.; Zhou, J. Facile fabrication of hierarchical nanoporous AuAg alloy and its highly sensitive detection towards dopamine and uric acid. Sens. Actuators B Chem. 2016, 225, 241–248. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, G.; Tian, K.; Xu, C. A highly sensitive and stable electrochemical sensor for simultaneous detection towards ascorbic acid, dopamine, and uric acid based on the hierarchical nanoporous PtTi alloy. Biosens. Bioelectron. 2016, 82, 119–126. [Google Scholar] [CrossRef]
- Liu, B.; Ouyang, X.; Ding, Y.; Luo, L.; Xu, D.; Ning, Y. Electrochemical preparation of nickel and copper oxides-decorated graphene composite for simultaneous determination of dopamine, acetaminophen and tryptophan. Talanta 2016, 146, 114–121. [Google Scholar] [CrossRef]
- Han, H.S.; You, J.-M.; Jeong, H.; Jeon, S. Synthesis of graphene oxide grafted poly(lactic acid) with palladium nanoparticles and its application to serotonin sensing. Appl. Surf. Sci. 2013, 284, 438–445. [Google Scholar] [CrossRef]
- Xue, C.; Wang, X.; Zhu, W.; Han, Q.; Zhu, C.; Hong, J.; Zhou, X.; Jiang, H. Electrochemical serotonin sensing interface based on double-layered membrane of reduced graphene oxide/polyaniline nanocomposites and molecularly imprinted polymers embedded with gold nanoparticles. Sens. Actuators B Chem. 2014, 196, 57–63. [Google Scholar] [CrossRef]
- Dinesh, B.; Veeramani, V.; Chen, S.-M.; Saraswathi, R. In situ electrochemical synthesis of reduced graphene oxide-cobalt oxide nanocomposite modified electrode for selective sensing of depression biomarker in the presence of ascorbic acid and dopamine. J. Electroanal. Chem. 2017, 786, 169–176. [Google Scholar] [CrossRef]
- Babaei, A.; Taheri, A.R.; Aminikhah, M. Nanomolar simultaneous determination of levodopa and serotonin at a novel carbon ionic liquid electrode modified with Co(OH)2 nanoparticles and multi-walled carbon nanotubes. Electrochim. Acta 2013, 90, 317–325. [Google Scholar] [CrossRef]
- Jamal, M.; Chakrabarty, S.; Shao, H.; McNulty, D.; Yousuf, M.A.; Furukawa, H.; Khosla, A.; Razeeb, K.M. A non- enzymatic glutamate sensor based on nickel oxide nanoparticle. Microsyst. Technol. 2018, 24, 4217–4223. [Google Scholar] [CrossRef]
- Hussain, M.M.; Rahman, M.M.; Asiri, A.M.; Awual, M.R. Non-enzymatic simultaneous detection of l-glutamic acid and uric acid using mesoporous Co3O4 nanosheets. RSC Adv. 2016, 6, 80511–80521. [Google Scholar] [CrossRef]
- Ahmad, H.M.N.; Si, B.; Dutta, G.; Csoros, J.R.; Seitz, W.R.; Song, E. Non-enzymatic electrochemical detection of glutamate using templated polymer-based target receptors. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; Volume 2019, pp. 613–616. [Google Scholar]
- Zeynaloo, E.; Yang, Y.-P.; Dikici, E.; Landgraf, R.; Bachas, L.-G.; Daunert, S. Design of a mediator-free, non-enzymatic electrochemical biosensor for glutamate detection. Nanomedicine 2021, 31, 102305. [Google Scholar] [CrossRef] [PubMed]
- Moraes, F.C.; Golinelli, D.L.C.; Mascaro, L.H.; Machado, S.A.S. Determination of epinephrine in urine using multi-walled carbon nanotube modified with cobalt phthalocyanine in a paraffin composite electrode. Sens. Actuators B Chem. 2010, 148, 492–497. [Google Scholar] [CrossRef]
- Ma, X.; Chen, M.; Li, X.; Purushothaman, A.; Li, F. Electrochemical detection of norepinephrine in the presence of epinephrine, uric acid and ascorbic acid using a graphene-modified electrode. Int. J. Electrochem. Sci 2012, 7, 991–1000. [Google Scholar]
- Bian, C.; Zeng, Q.; Xiong, H.; Zhang, X.; Wang, S. Electrochemistry of norepinephrine on carbon-coated nickel magnetic nanoparticles modified electrode and analytical applications. Bioelectrochemistry 2010, 79, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Vilian, A.T.E.; Chen, S.-M.; Hung, Y.-T.; Ali, M.A.; Al-Hemaid, F.M.A. Electrochemical oxidation and determination of norepinephrine in the presence of acetaminophen using MnO2 nanoparticle decorated reduced graphene oxide sheets. Anal. Methods 2014, 6, 6504–6513. [Google Scholar] [CrossRef]
- Anandaraj, S.; Chen, T.-W.; Chen, S.-M.; Elaiyappillai, E.; Loganathan, S.; Ibrahimsa, S.L.; Johnson, P.M.; Selvin, P.S.; Weng, W.-H.; Leung, W.-H. Electrochemical detection of norepinephrine using sponge-like Co3O4 modified screen printed carbon electrode. Int. J. Electrochem. Sci. 2017, 12, 10524–10533. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, K.; Zhao, L.; Chai, S.; Zhang, J.; Zhang, X.; Zou, Q. A novel sensor made of Antimony doped Tin oxide-silica composite sol on a glassy carbon electrode modified by single-walled carbon nanotubes for detection of norepinephrine. Mater. Sci. Eng. C 2017, 80, 180–186. [Google Scholar] [CrossRef]
- Hui, N.; Sun, X.; Niu, S.; Luo, X. PEGylated polyaniline nanofibers: Antifouling and conducting biomaterial for electrochemical DNA sensing. ACS. Appl. Mater. Interfaces 2017, 9, 2914–2923. [Google Scholar] [CrossRef]
- Jackson, B.P.; Dietz, S.M.; Wightman, R.M. Fast-scan cyclic voltammetry of 5-hydroxytryptamine. Anal. Chem. 1995, 67, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Meunier, C.J.; McCarty, G.S.; Sombers, L.A. Drift subtraction for Fast-scan cyclic voltammetry using double-waveform partial-least-squares regression. Anal. Chem. 2019, 91, 7319–7327. [Google Scholar] [CrossRef] [PubMed]
- Venton, B.J.; Cao, Q. Fundamentals of fast-scan cyclic voltammetry for dopamine detection. Analyst 2020, 145, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Jo, T.; Yoshimi, K.; Takahashi, T.; Oyama, G.; Hattori, N. Dual use of rectangular and triangular waveforms in voltammetry using a carbon fiber microelectrode to differentiate norepinephrine from dopamine. J. Electroanal. Chem. 2017, 802, 1–7. [Google Scholar] [CrossRef]
- Park, C.; Oh, Y.; Shin, H.; Kim, J.; Kang, Y.; Sim, J.; Cho, H.U.; Lee, H.K.; Jung, S.J.; Blaha, C.D.; et al. Fast cyclic square-wave voltammetry to enhance neurotransmitter selectivity and sensitivity. Anal. Chem. 2018, 90, 13348–13355. [Google Scholar] [CrossRef]
- Lucio Boschen, S.; Trevathan, J.; Hara, S.A.; Asp, A.; Lujan, J.L. Defining a path toward the use of Fast-scan cyclic voltammetry in human studies. Front. Neurosci. 2021, 15, 728092. [Google Scholar] [CrossRef]

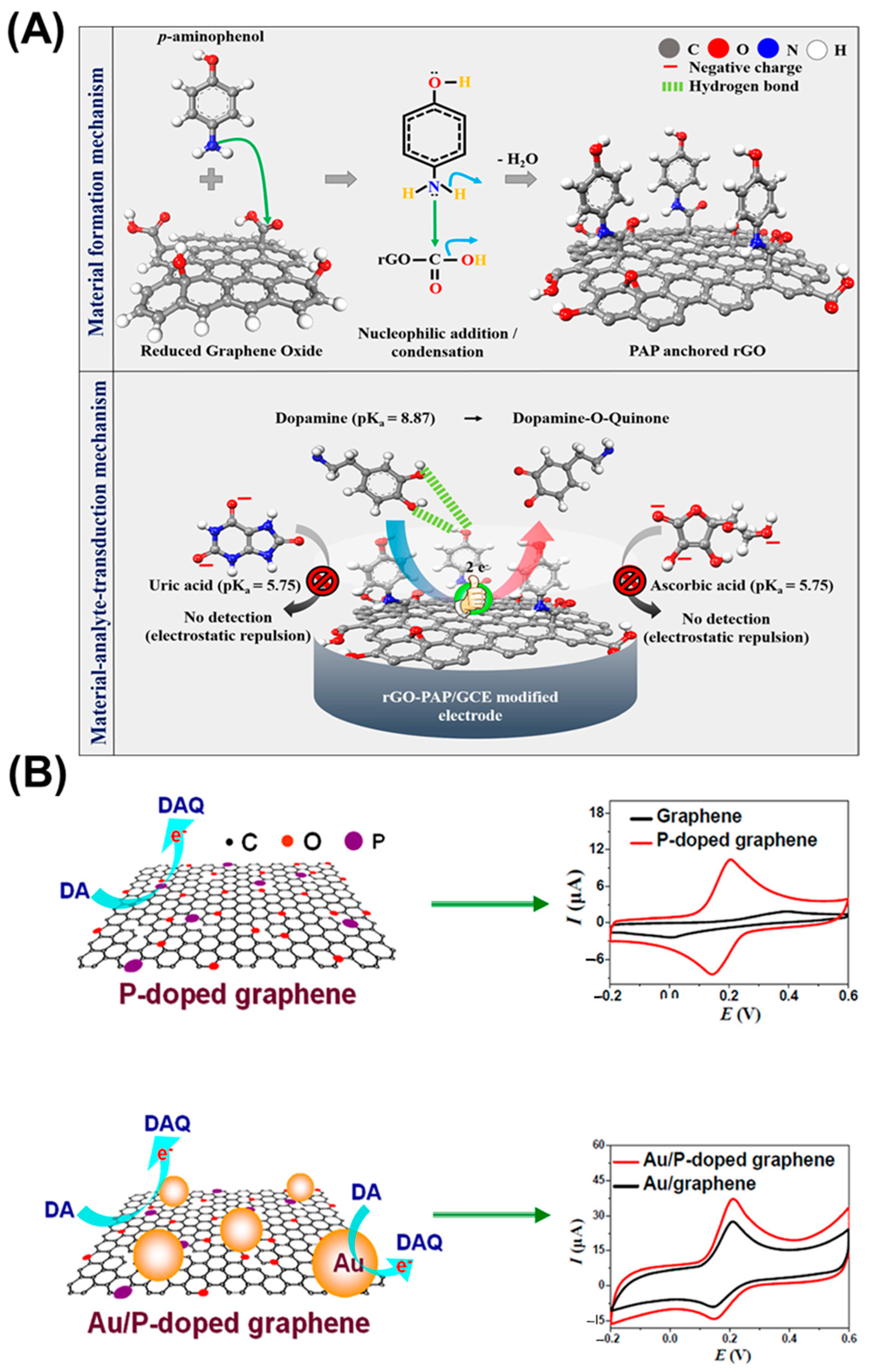
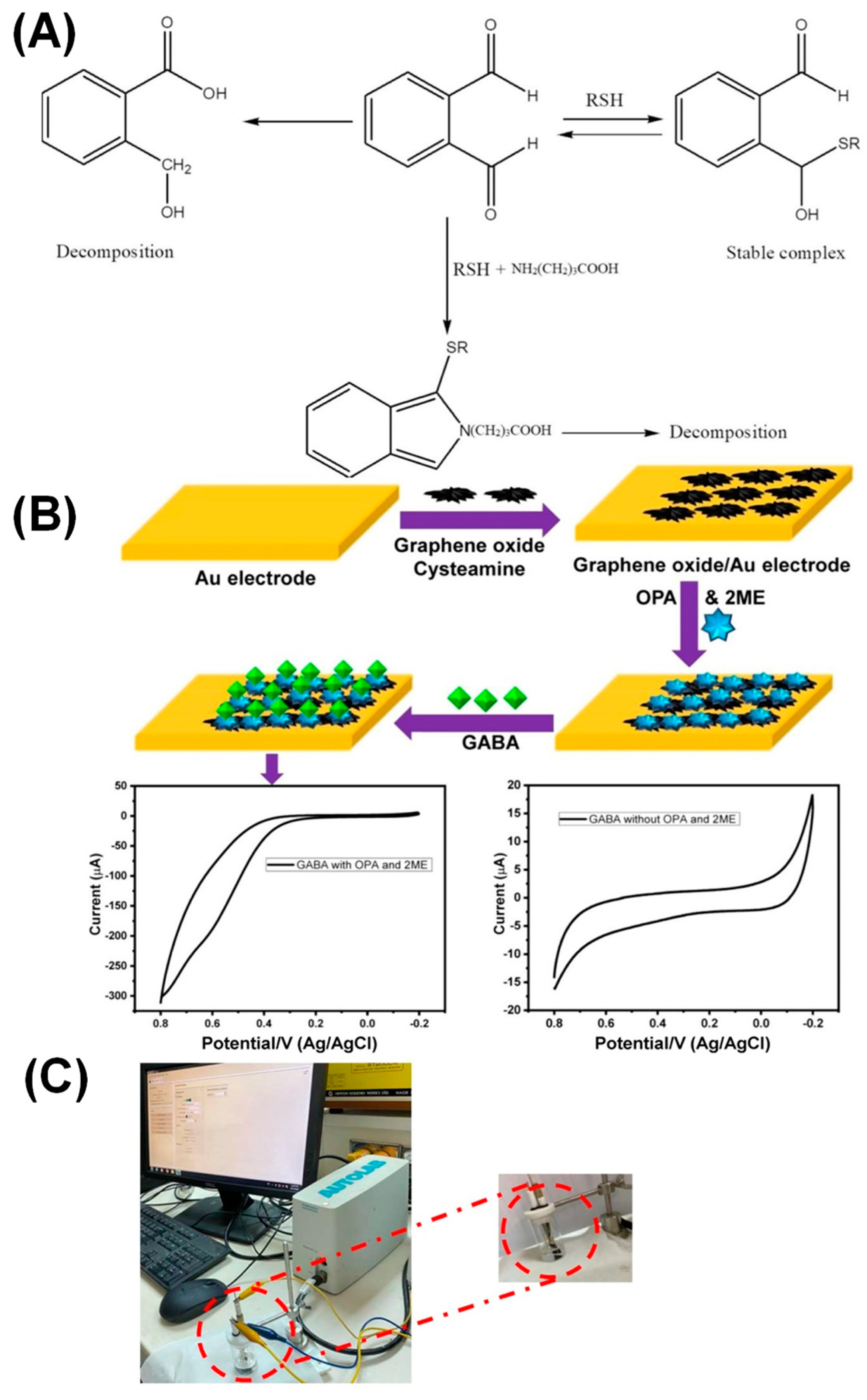
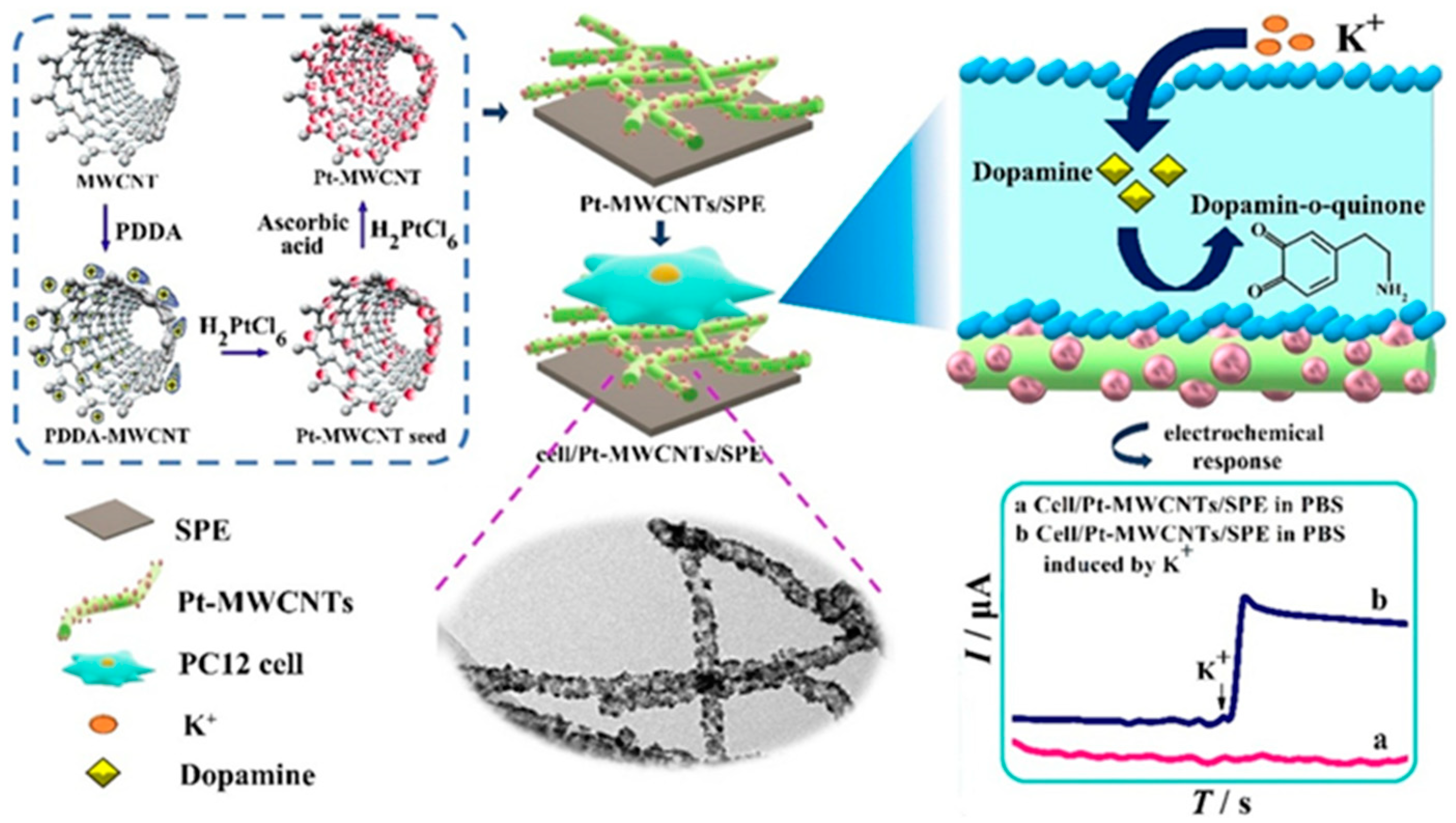
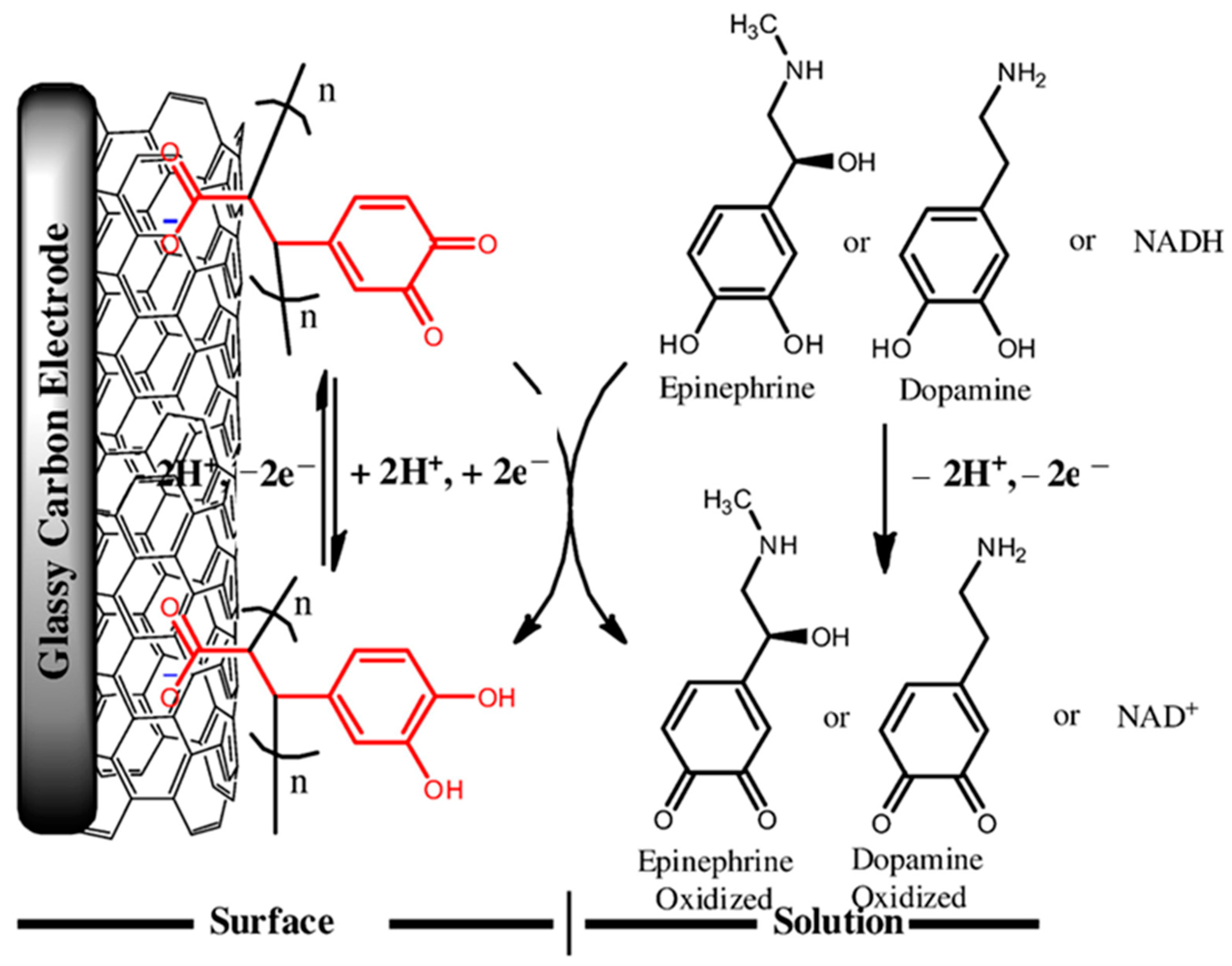


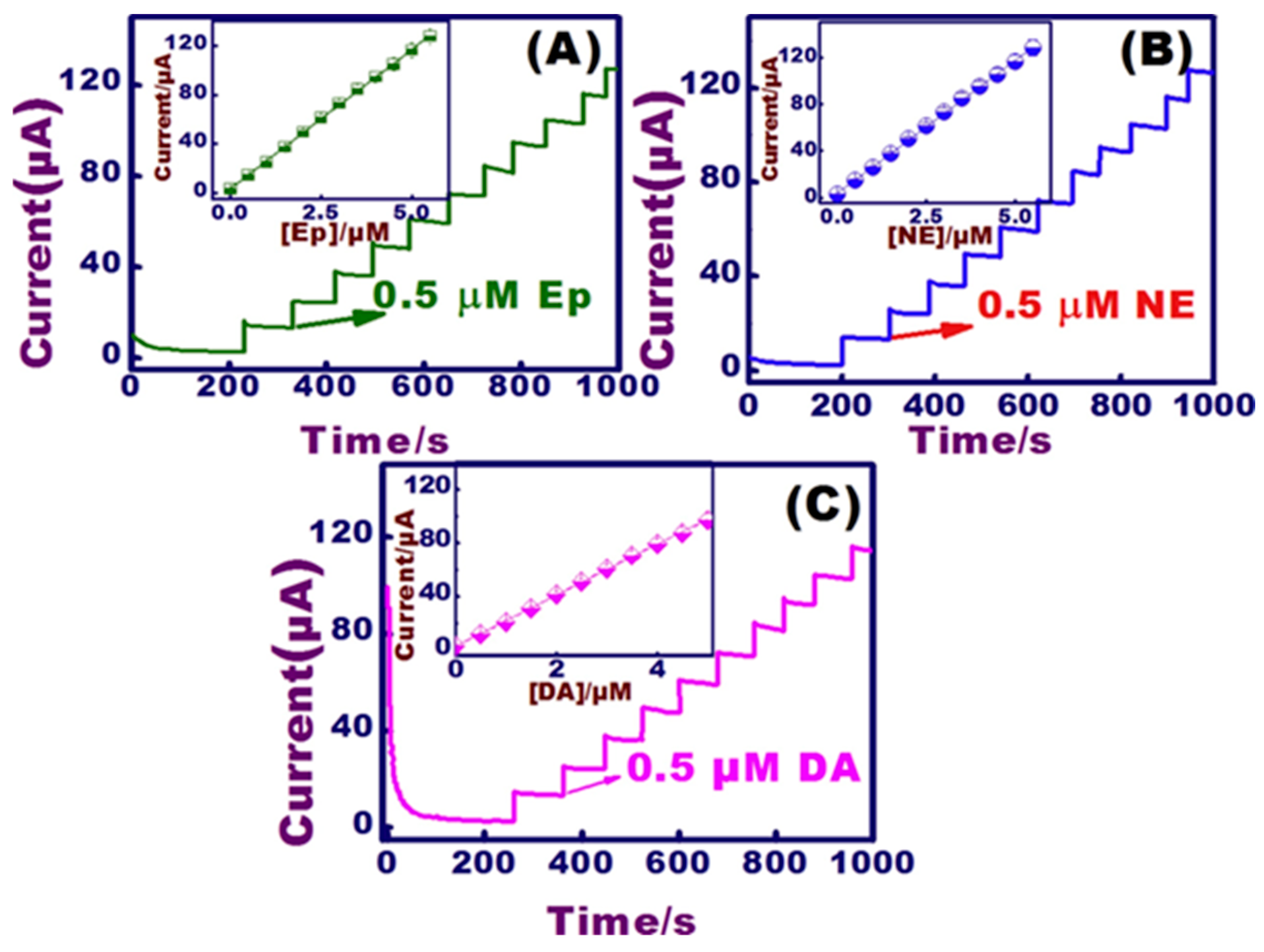
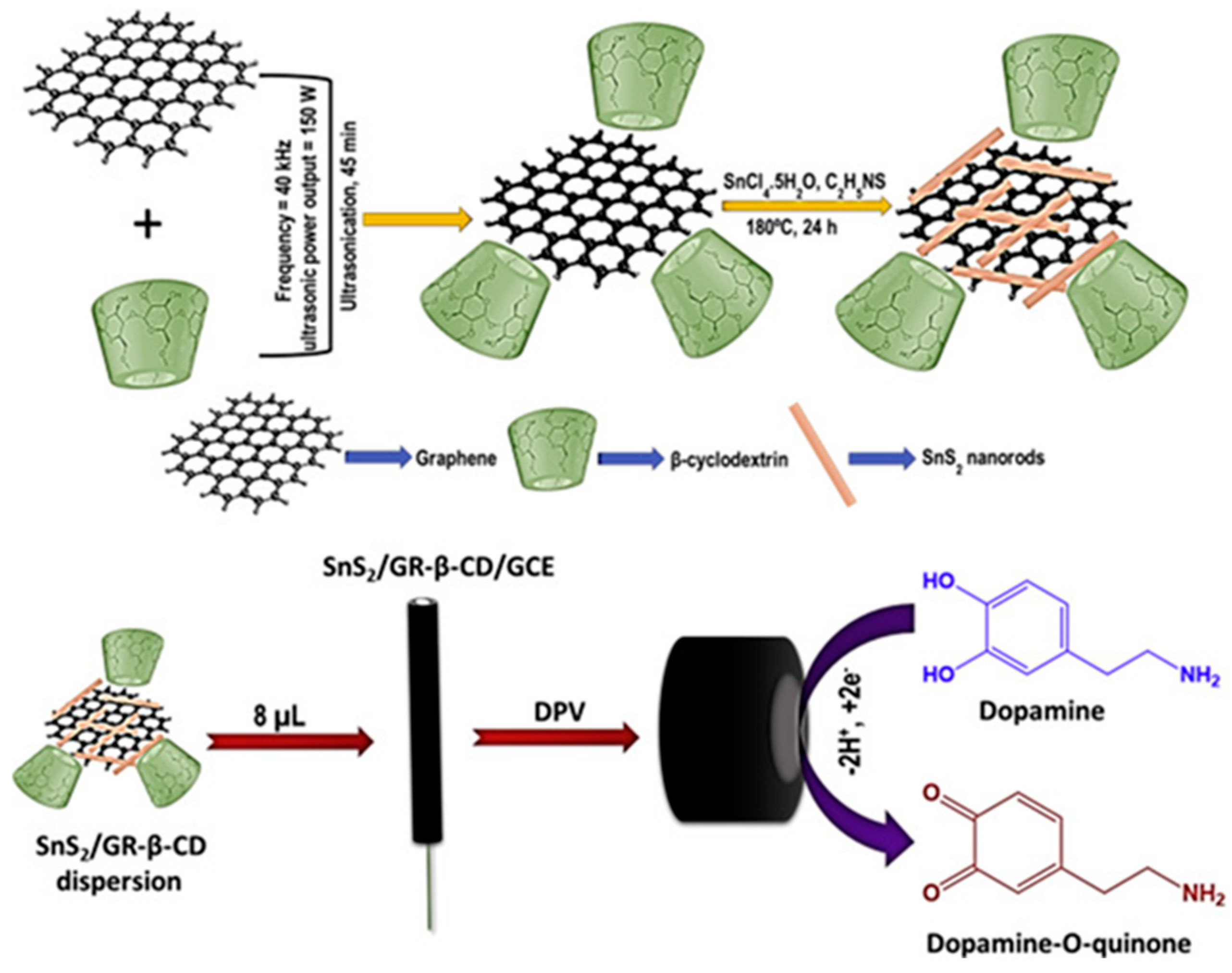
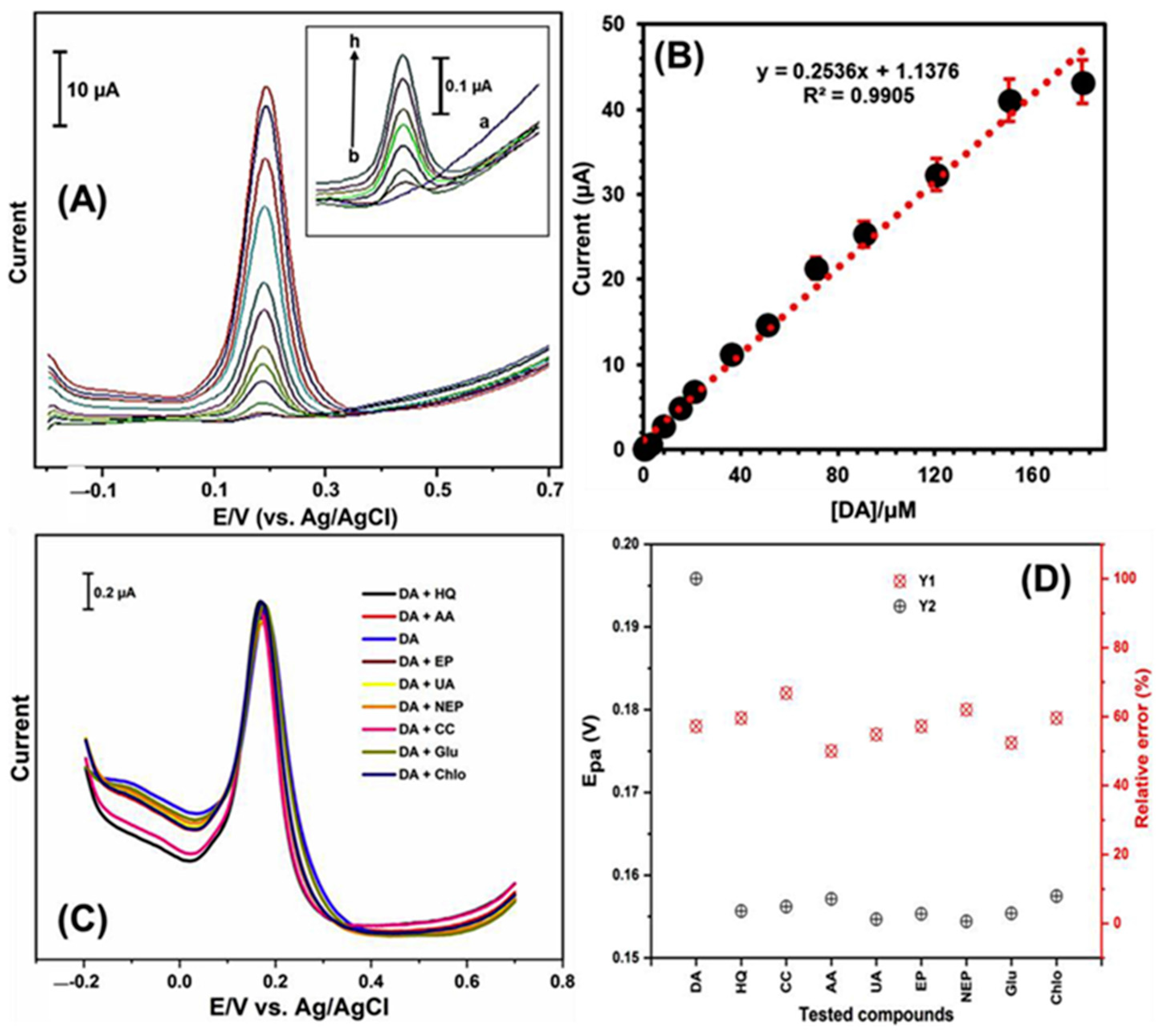

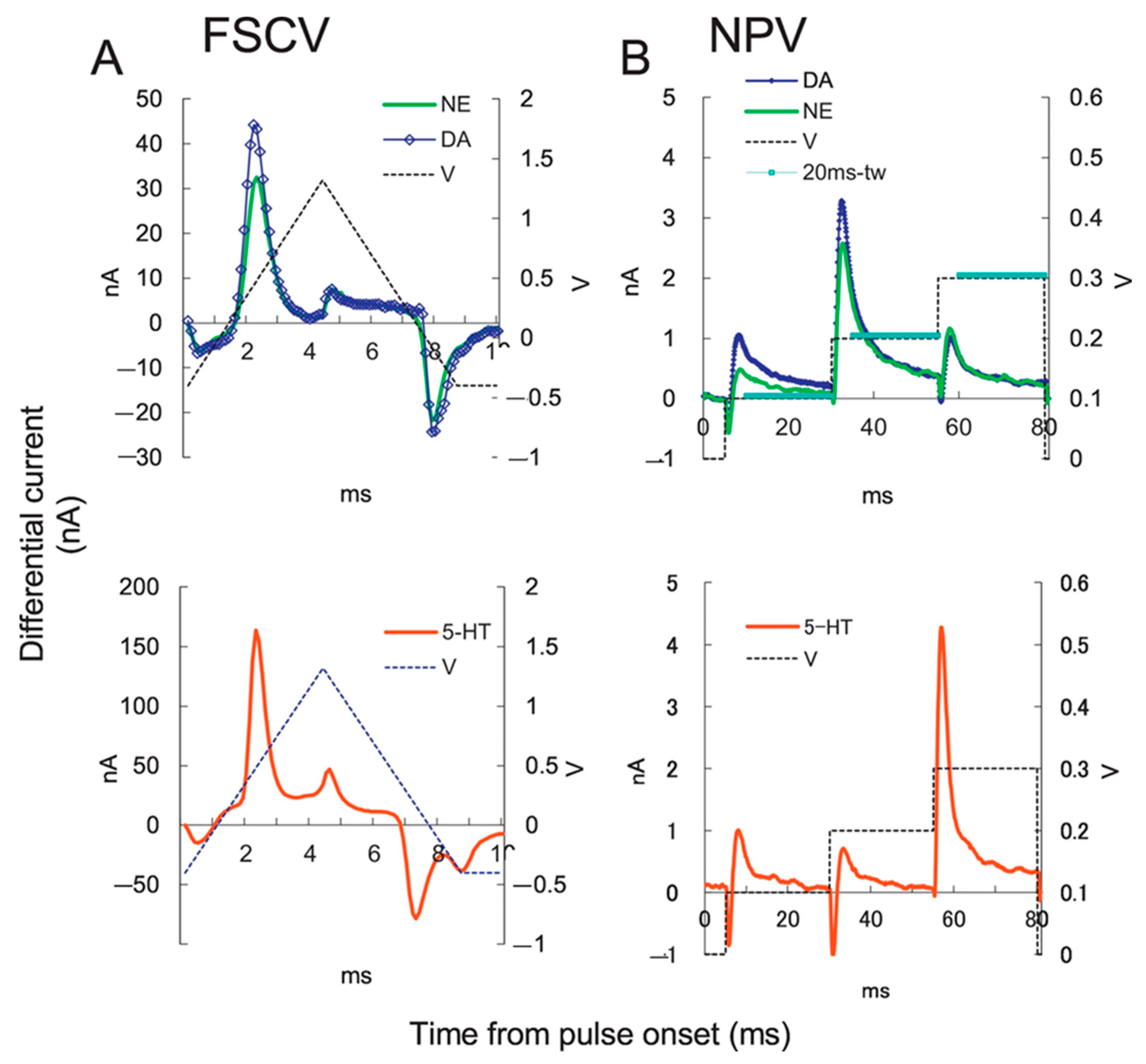
| Target | NC Modification | Linear Range (µM) | LOD (µM) | Sensitivity (µA µM−1 cm−2) | Electrochemical Technique | Tested Real Samples | Reference |
|---|---|---|---|---|---|---|---|
| DA | rGO/3D porous PU | 0.0001–0.00115 | 0.000001 | - | DPV | Human serum and urine | [37] |
| rGO/Bi2S3/GCE | 0.01–40.0 | 0.0123 | 2.046 | CV, DPV | Human urine | [143] | |
| rGO-poly(FeTFPP)/GCE | 0.05–300 | 0.023 | - | CV, EIS, DPV | Human urine and lake water | [83] | |
| rGO-ZIF-8/GCE | 0.10–100 | 0.03 | 0.153 | CV, DPV | Human serum | [100] | |
| rGO-Cu2O/GCE | 10.0–900 | 0.05 | 0.520 | CV, DPV | Human blood and urine | [154] | |
| Poly-FA/MWCNT/GCE | 5.00–120 | 2.21 | 0.037 | CV, CA | Pharmaceutical samples | [85] | |
| PGr/GCE | 0.005–1.0 | 0.001 | 0.004 | CV, DPV | Human blood | [144] | |
| CPE/GO | 0.08–2.30 | 0.0086 | 0.489 | EIS, LSV, DPV, Chronocoulometry | Human blood | [155] | |
| HNP-AuAg/GCE | 5.00–335 | 0.20 | 0.399 | CV, DPV, CA | - | [156] | |
| HNP-Pt-Ti/GCE | 0.004–500 | 3.20 | 0.186 | CV, DPV, CA | Human serum | [157] | |
| NiO-CuO/Graphene/GCE | 0.50–20.0 | 0.170 | 9.406 | CV, EIS, SWV | Human serum and pharmaceutical samples | [158] | |
| 3D Macroporous carbon aerogel | 0.20–90.0 | - | 0.030 | CV, DPV, CA | Human serum | [36] | |
| Wrinkled rGO/SPCE | 0.10–300 | 0.134 | - | CV, LSV | Mouse brain tissues | [84] | |
| rGO/PAP/GCE | 0.01–100 | - | 0.0075 | CV, EIS, DPV, CA | Human serum | [39] | |
| Au/P-doped Graphene | 0.10–180 | - | 0.002 | CV, DPV, CA | Human serum and urine | [43] | |
| GQDs- MWCNTs/GCE | 0.25–250 | 0.095 | 0.520 | CV, EIS, DPV, CA | Human serum | [54] | |
| poly-celestine blue/GCE | 0.01–0.7 1.0–10.0 | 0.0012 | 17.01 | CV, EIS, DPV | PC12 cell lines | [74] | |
| MWCNT-PANI/GCE | 0.0000001–0.000012 | 0.000000033 | - | CV, EIS, DPV | Artificial urine | [76] | |
| rGO/MWCNTs/PPy | 0.025–0.1 | 0.0023 | 8.96 | CV, EIS, CA | - | [78] | |
| Pt NPs/PPy/rGO | 0.1–250.0 | 0.042 | - | CV, EIS, DPV | Human blood | [79] | |
| MIP electrode | 0.0000005–0.00004 | 0.13 | - | DPV | Human blood and pharmaceutical samples | [92] | |
| Thioaniline- AuNPs/MIP | 0.000000001–0.000005 | - | 0.0000038 | CV, EIS, DPV | Human serum | [93] | |
| CuTRZMoO4/PPy/GCE | 1.0–100.0 | 0.08 | - | CV, EIS | Human serum | [97] | |
| Porphyrin MOF/PEDOT/ | 4.0–100.0 | - | 0.011 | CV, DPV | - | [99] | |
| 5-HT | GO-g-PLA-Pd | 0.10–100 | 0.08 | - | CV, EIS, CA | - | [159] |
| rGO/PANI/AuNPs-MIPs | 0.20–10.0 | 0.0117 | - | CV, DPV, Chronocoulometry | Human blood | [160] | |
| rGO-Co3O4 | 0.10–51.0 | 48.7 | 22 | CV, EIS, DPV | Human blood | [161] | |
| Nafion/Co(OH)2–MWCNTs | 0.05–75.0 | 0.023 | - | CV, DPV, CA | Human serum | [162] | |
| CNT/IL | 5.00–900 | 2.00 | - | CV, EIS, DPV, LSV | Human blood and water | [150] | |
| poly(bromocresol green)/GCE | 0.50–100 | 0.08 | - | CV, DPV | Human serum | [80] | |
| poly(p-aminobenzene sulfonic acid)/MWCNT/CS | 0.10–100 | 0.08 | - | CV, EIS, DPV | Human serum | [81] | |
| Fe3O4/Au/SiO2/MIP | 0.01–1000 | 0.002 | - | LSV | Human urine and pharmaceutical samples | [94] | |
| GLU | NiO/CS/GCE | 1000–8000 | 272 | 0.011 | CV, CA | - | [163] |
| Co3O4 nanosheets/GCE | 0.0001–1,000,000 | 0.00001 | 0.00095 | CV | Human serum and urine | [164] | |
| Pt/NiNAE | 500–8000 | 83 | 0.096 | CV, CA | - | [120] | |
| NiMOF/GCE | 0.005–500.0 | 0.00167 | - | CV | Human urine | [102] | |
| PNIPAM/4-VP/MAA | - | 1.7 | 0.0086 | CV, EIS | - | [165] | |
| GluBP/AuNP/SPCE | 0.10–0.8 | 0.15 | 0.0011 | CV, EIS | - | [166] | |
| EP | Paraffin/MWCNT/CoPc | 1.33–5.50 | 0.0156 | 5.920 | CV, DPV | Human urine | [167] |
| p-FA/MWCNT/GCE | 73.0–1406 | 22.2 | 0.004 | CV, CA | Pharmaceutical samples | [85] | |
| NiONPs-MWCNT-DHP/GCE | 0.3–9.5 | 0.082 | 0.390 | CV, DPV, SWV | Human urine, cerebrospinal and lung fluids | [114] | |
| EDDPT/GO/CPE | 1.5–600 | 0.65 | 0.076 | CV, CA, DPV | Human serum and pharmaceutical samples | [45] | |
| MWCNT-CS | 0.05–10 | 0.03 | - | CV, EIS, DPV | Artificial urine and pharmaceutical samples | [64] | |
| Graphene film | 0.6–120 | 0.4 | - | CV | Pharmaceutical samples | [168] | |
| C-Ni/GCE | 0.2–80 | 6.0 | - | CV, DPV | - | [169] | |
| NE | rGO–MnO2/GCE | 0.2–800 | 0.02 | - | CV, SWV | Human urine | [170] |
| Co3O4/SPCE | 0.1–1525 | 0.075 | - | CV, EIS, CA | Human serum and urine | [171] | |
| ATO/MIP/SWCNT/GCE | 0.099–15 | 0.033 | - | CV | Human blood | [172] | |
| Graphene/Pd | 0.099–15 | 0.07 | 0.0174 | CV, EIS, SWV | Human urine and pharmaceutical samples | [46] | |
| DSWCNTs/SPCE | 0.1–2.0 | 0.062 | - | CV, DPV | Rat brain | [59] | |
| poly(1,5-diaminonaphthalene) | 9.90–90.9 | 1.82 | - | CV, DPV | Pharmaceutical samples | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajarathinam, T.; Kang, M.; Hong, S.; Chang, S.-C. Nanocomposite-Based Electrochemical Sensors for Neurotransmitters Detection in Neurodegenerative Diseases. Chemosensors 2023, 11, 103. https://doi.org/10.3390/chemosensors11020103
Rajarathinam T, Kang M, Hong S, Chang S-C. Nanocomposite-Based Electrochemical Sensors for Neurotransmitters Detection in Neurodegenerative Diseases. Chemosensors. 2023; 11(2):103. https://doi.org/10.3390/chemosensors11020103
Chicago/Turabian StyleRajarathinam, Thenmozhi, Mijeong Kang, Sungmoo Hong, and Seung-Cheol Chang. 2023. "Nanocomposite-Based Electrochemical Sensors for Neurotransmitters Detection in Neurodegenerative Diseases" Chemosensors 11, no. 2: 103. https://doi.org/10.3390/chemosensors11020103





