A Sensitive Immunochromatographic Test Strip Based on Hydrophobic Quantum Dots Incorporated into Mg/Fe Nanoflowers for HCG Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Characterization
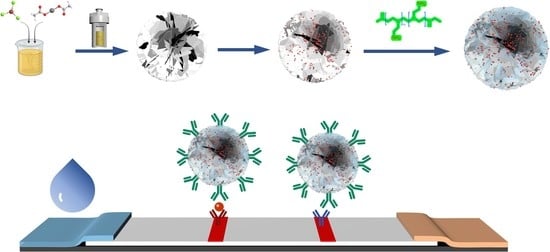
2.2. Preparation Procedures of the Red-Emitting Hydrophobic CdSe/ZnS QDs
2.3. Preparation Procedures of MF@CdSe/ZnS@PEI NFs [36]
2.4. Preparation Procedures of Ab2-MF@CdSe/ZnS@PEI
2.5. Fabrication Procedures of the MF@CdSe/ZnS@PEI-ICTS
2.6. Detection of HCG Standard Samples with the MF@CdSe/ZnS@PEI-ICTS
3. Results and Discussion
3.1. Preparation and Characterization of MF@CdSe/ZnS@PEI NFs
3.2. Performance of MF@CdSe/ZnS@PEI-ICTS for HCG Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, L.; Qu, X. Cancer biomarker detection: Recent achievements and challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, T.; de la Guardia, M.; Baradaran, B. Lateral flow assays towards point-of-care cancer detection: A review of current progress and future trends. TrAC Trends Anal. Chem. 2020, 125, 115842. [Google Scholar] [CrossRef]
- Plotz, C.M.; Singer, J.M. The latex fixation test: II. Results in rheumatoid arthritis. Am. J. Med. 1956, 21, 893–896. [Google Scholar] [CrossRef]
- Brucker, M.C.; Macmullen, N.J. What’s New in Pregnancy Tests. J. Obstet. Gynecol. Neonatal Nurs. 1985, 14, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-C.; Chou, C.-C.; Yang, Y.-Q.; Wei-Kai, T.; Wang, Y.-T.; Chan, Y.-H. Multiplexed Detection of Tumor Markers with Multicolor Polymer Dot-Based Immunochromatography Test Strip. Anal. Chem. 2018, 90, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, B.; Wen, S.; Liao, J.; Lin, G.; Zhou, J.; Jin, D. Quantitative Lateral Flow Strip Sensor Using Highly Doped Upconversion Nanoparticles. Anal. Chem. 2018, 90, 12356–12360. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, K.; Cao, B.; Shen, L.; Ke, X.; Cui, D.; Zhong, C.; Li, W. Multifunctional Nano-Sunflowers with Color-Magnetic-Raman Properties for Multimodal Lateral Flow Immunoassay. Anal. Chem. 2021, 93, 3626–3634. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lin, Y.-Y.; Wang, J.; Wu, H.; Wai, C.M.; Lin, Y. Disposable Electrochemical Immunosensor Diagnosis Device Based on Nanoparticle Probe and Immunochromatographic Strip. Anal. Chem. 2007, 79, 7644–7653. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Ao, L.; Luo, Y.; Liao, T.; Huang, L.; Zhuo, D.; Jiang, C.; Wang, J.; Hu, J. Quantum dots assembly enhanced and dual-antigen sandwich structured lateral flow immunoassay of SARS-CoV-2 antibody with simultaneously high sensitivity and specificity. Biosens. Bioelectron. 2022, 198, 113810. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liao, T.; Wang, J.; Ao, L.; Su, W.; Hu, J. Brilliant Pitaya-Type Silica Colloids with Central–Radial and High-Density Quantum Dots Incorporation for Ultrasensitive Fluorescence Immunoassays. Adv. Funct. Mater. 2018, 28, 1705380. [Google Scholar] [CrossRef]
- Wang, T.; Zhuang, J.; Lynch, J.; Chen, O.; Wang, Z.; Wang, X.; LaMontagne, D.; Wu, H.; Wang, Z.; Cao, Y.C. Self-Assembled Colloidal Superparticles from Nanorods. Science 2012, 338, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Nguyen, T.D.; Yang, M.; Lee, B.; Santos, A.; Podsiadlo, P.; Tang, Z.; Glotzer, S.C.; Kotov, N.A. Self-assembly of self-limiting monodisperse supraparticles from polydisperse nanoparticles. Nat. Nanotechnol. 2011, 6, 580–587. [Google Scholar] [CrossRef]
- Yi, C.; Zhang, S.; Webb, K.T.; Nie, Z. Anisotropic Self-Assembly of Hairy Inorganic Nanoparticles. Acc. Chem. Res. 2017, 50, 12–21. [Google Scholar] [CrossRef]
- Foubert, A.; Beloglazova, N.V.; De Saeger, S. Comparative study of colloidal gold and quantum dots as labels for multiplex screening tests for multi-mycotoxin detection. Anal. Chim. Acta 2017, 955, 48–57. [Google Scholar] [CrossRef]
- Deng, H.; Liu, Q.; Wang, X.; Huang, R.; Liu, H.; Lin, Q.; Zhou, X.; Xing, D. Quantum dots-labeled strip biosensor for rapid and sensitive detection of microRNA based on target-recycled nonenzymatic amplification strategy. Biosens. Bioelectron. 2017, 87, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Cai, J.; Zhang, B.; Zhao, Q.; Piao, J.; Peng, W.; Gao, W.; Zhou, D.; Zhao, M.; Chang, J. A review of fluorescent signal-based lateral flow immunochromatographic strips. J. Mater. Chem. B 2017, 5, 5079–5091. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, D.; He, R.; Guo, Q.; Wang, K.; Zhang, X.; Huang, P.; Cui, D. A Novel Quantum Dots–Based Point of Care Test for Syphilis. Nanoscale Res. Lett. 2010, 5, 875. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, Z.-L.; Wen, C.-Y.; Tang, M.; Wu, L.-L.; Liu, C.; Zhu, L.; Pang, D.-W. Sensitive and Quantitative Detection of C-Reaction Protein Based on Immunofluorescent Nanospheres Coupled with Lateral Flow Test Strip. Anal. Chem. 2016, 88, 6577–6584. [Google Scholar] [CrossRef]
- Gao, F.; Lei, C.; Liu, Y.; Song, H.; Kong, Y.; Wan, J.; Yu, C. Rational Design of Dendritic Mesoporous Silica Nanoparticles’ Surface Chemistry for Quantum Dot Enrichment and an Ultrasensitive Lateral Flow Immunoassay. ACS Appl. Mater. Interfaces 2021, 13, 21507–21515. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, W.; Yang, Q.; Gong, X.; Guo, W.; Dong, C.; Liu, J.; Xuan, L.; Chang, J. Rapid and Quantitative Detection of Prostate Specific Antigen with a Quantum Dot Nanobeads-Based Immunochromatography Test Strip. ACS Appl. Mater. Interfaces 2014, 6, 6406–6414. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Huang, X.; Shao, Y.; Zheng, L.; Guo, L.; Xiong, Y. Size-Dependent Immunochromatographic Assay with Quantum Dot Nanobeads for Sensitive and Quantitative Detection of Ochratoxin A in Corn. Anal. Chem. 2017, 89, 7062–7068. [Google Scholar] [CrossRef] [PubMed]
- Joumaa, N.; Lansalot, M.; Théretz, A.; Elaissari, A.; Sukhanova, A.; Artemyev, M.; Nabiev, I.; Cohen, J.H.M. Synthesis of Quantum Dot-Tagged Submicrometer Polystyrene Particles by Miniemulsion Polymerization. Langmuir 2006, 22, 1810–1816. [Google Scholar] [CrossRef]
- Wang, C.; Yang, X.; Gu, B.; Liu, H.; Zhou, Z.; Shi, L.; Cheng, X.; Wang, S. Sensitive and Simultaneous Detection of SARS-CoV-2-Specific IgM/IgG Using Lateral Flow Immunoassay Based on Dual-Mode Quantum Dot Nanobeads. Anal. Chem. 2020, 92, 15542–15549. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Wang, D.; Huo, Z.; Chen, W.; Liu, L.; Liang, X.; Chen, C.; Wang, X.; Peng, Q.; Li, Y. A Versatile Bottom-up Assembly Approach to Colloidal Spheres from Nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 6650–6653. [Google Scholar] [CrossRef]
- Zhuang, J.; Wu, H.; Yang, Y.; Cao, Y.C. Controlling Colloidal Superparticle Growth Through Solvophobic Interactions. Angew. Chem. Int. Ed. 2008, 47, 2208–2212. [Google Scholar] [CrossRef]
- Gao, X.; Nie, S. Doping Mesoporous Materials with Multicolor Quantum Dots. J. Phys. Chem. B 2003, 107, 11575–11578. [Google Scholar] [CrossRef]
- Yang, F.; Skripka, A.; Tabatabaei, M.S.; Hong, S.H.; Ren, F.; Huang, Y.; Oh, J.K.; Martel, S.; Liu, X.; Vetrone, F.; et al. Magnetic Photoluminescent Nanoplatform Built from Large-Pore Mesoporous Silica. Chem. Mater. 2019, 31, 3201–3210. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Q.; Wang, J.; Foda, M.; Liu, J.; Cai, K.; Han, H. A brilliant sandwich type fluorescent nanostructure incorporating a compact quantum dot layer and versatile silica substrates. Chem. Commun. 2014, 50, 2896–2899. [Google Scholar] [CrossRef]
- Lu, Z.; Gao, C.; Zhang, Q.; Chi, M.; Howe, J.Y.; Yin, Y. Direct Assembly of Hydrophobic Nanoparticles to Multifunctional Structures. Nano Lett. 2011, 11, 3404–3412. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, L.; Huang, L.; Wang, J.; Liu, J.; Hu, C.; Han, H. Quantum dots decorated gold nanorod as fluorescent-plasmonic dual-modal contrasts agent for cancer imaging. Biosens. Bioelectron. 2015, 74, 16–23. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Shi, W.; Lu, C. Confinement Effect in Layered Double Hydroxide Nanoreactor: Improved Optical Sensing Selectivity. Anal. Chem. 2016, 88, 8188–8193. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, C.; Zhang, M. Gold Nanoclusters@Ru(bpy)32+-Layered Double Hydroxide Ultrathin Film as a Cathodic Electrochemiluminescence Resonance Energy Transfer Probe. Anal. Chem. 2015, 87, 8026–8032. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, K.; Tian, R.; Lin, Y.; Lu, C. Highly dispersed layered double oxide hollow spheres with sufficient active sites for adsorption of methyl blue. Nanoscale 2018, 10, 23191–23197. [Google Scholar] [CrossRef]
- Mubarak, M.; Islam, M.S.; Yoon, D.-Y.; Lee, J.H.; Park, H.J.; Bae, J.-S.; Lee, H.-J. Flower-like Mg/Fe-layered double oxide nanospheres with ultrahigh adsorption efficiency for anionic organic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126446. [Google Scholar] [CrossRef]
- Mubarak, M.; Jeon, H.; Islam, M.S.; Yoon, C.; Bae, J.-S.; Hwang, S.-J.; Choi, W.S.; Lee, H.-J. One-pot synthesis of layered double hydroxide hollow nanospheres with ultrafast removal efficiency for heavy metal ions and organic contaminants. Chemosphere 2018, 201, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Gong, X.; Song, T.; Yang, J.; Zhu, S.; Li, Y.; Cui, Y.; Li, Y.; Zhang, B.; Chang, J. Quantum dot-based immunochromatography test strip for rapid, quantitative and sensitive detection of alpha fetoprotein. Biosens. Bioelectron. 2011, 30, 145–150. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, R.; Guo, X.; Liang, J.; Deng, Q.; Li, M.; An, T.; Liu, T.; Wu, Y. Simultaneous quantitation of cytokeratin-19 fragment and carcinoembryonic antigen in human serum via quantum dot-doped nanoparticles. Biosens. Bioelectron. 2017, 91, 60–65. [Google Scholar] [CrossRef]
- Cho, J.; Jung, Y.K.; Lee, J.-K. Kinetic studies on the formation of various II–VI semiconductor nanocrystals and synthesis of gradient alloy quantum dots emitting in the entire visible range. J. Mater. Chem. 2012, 22, 10827–10833. [Google Scholar] [CrossRef]
- Xu, L.-D.; Zhang, Q.; Ding, S.-N.; Xu, J.-J.; Chen, H.-Y. Ultrasensitive Detection of Severe Fever with Thrombocytopenia Syndrome Virus Based on Immunofluorescent Carbon Dots/SiO2 Nanosphere-Based Lateral Flow Assay. ACS Omega 2019, 4, 21431–21438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.D.; Zhu, J.; Ding, S.N. Immunoassay of SARS-CoV-2 nucleocapsid proteins using novel red emission-enhanced carbon dot-based silica spheres. Analyst 2021, 146, 5055–5060. [Google Scholar] [CrossRef]
- Kim, M.; Kim, M.S.; Kweon, S.H.; Jeong, S.; Kang, M.H.; Kim, M.I.; Lee, J.; Doh, J. Simple and Sensitive Point-of-Care Bioassay System Based on Hierarchically Structured Enzyme-Mimetic Nanoparticles. Adv. Healthc. Mater. 2015, 4, 1311–1316. [Google Scholar] [CrossRef]
- Kim, M.S.; Kweon, S.H.; Cho, S.; An, S.S.A.; Kim, M.I.; Doh, J.; Lee, J. Pt-Decorated Magnetic Nanozymes for Facile and Sensitive Point-of-Care Bioassay. ACS Appl. Mater. Interfaces 2017, 9, 35133–35140. [Google Scholar] [CrossRef]
- Huang, P.; Liu, J.; Wei, F.; Zhu, Y.; Wang, X.; Cao, C.; Song, W. Size-selective adsorption of anionic dyes induced by the layer space in layered double hydroxide hollow microspheres. Mater. Chem. Front. 2017, 1, 1550–1555. [Google Scholar] [CrossRef]
- Géraud, E.; Rafqah, S.; Sarakha, M.; Forano, C.; Prevot, V.; Leroux, F. Three Dimensionally Ordered Macroporous Layered Double Hydroxides: Preparation by Templated Impregnation/Coprecipitation and Pattern Stability upon Calcination. Chem. Mater. 2008, 20, 1116–1125. [Google Scholar] [CrossRef]
- Wang, B.; Wu, H.; Yu, L.; Xu, R.; Lim, T.-T.; Lou, X.W. Template-free Formation of Uniform Urchin-like α-FeOOH Hollow Spheres with Superior Capability for Water Treatment. Adv. Mater. 2012, 24, 1111–1116. [Google Scholar] [CrossRef]
- Kim, K.M.; Jeon, J.H.; Kim, Y.Y.; Lee, H.K.; Park, O.O.; Wang, D.H. Effects of ligand exchanged CdSe quantum dot interlayer for inverted organic solar cells. Org. Electron. 2015, 25, 44–49. [Google Scholar] [CrossRef]
- Granados-Oliveros, G.; Pineros, B.S.G.; Calderon, F.G.O. CdSe/ZnS quantum dots capped with oleic acid and L-glutathione: Structural properties and application in detection of Hg2+. J. Mol. Struct. 2022, 1254, 132293. [Google Scholar] [CrossRef]
- Cingarapu, S.; Yang, Z.; Sorensen, C.M.; Klabunde, K.J. Synthesis of CdSe/ZnS and CdTe/ZnS Quantum Dots: Refined Digestive Ripening. J. Nanomater. 2012, 2012, 312087. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Q.; Luo, S.; He, L.; Fan, R.; Zhang, S.; Yang, C.; Chen, Y. Dual near-infrared fluorescence-based lateral flow immunosensor for the detection of zearalenone and deoxynivalenol in maize. Food Chem. 2021, 336, 127718. [Google Scholar] [CrossRef]
- Zhang, A.; Guo, W.; Ke, H.; Zhang, X.; Zhang, H.; Huang, C.; Yang, D.; Jia, N.; Cui, D. Sandwich-format ECL immunosensor based on Au star@BSA-Luminol nanocomposites for determination of human chorionic gonadotropin. Biosens. Bioelectron. 2018, 101, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Zhang, D.; He, Y.; Yang, Y.; Chen, P.; Yang, H.; Zhou, Z.; Liu, Y.; Wang, Y. New photothermal immunoassay of human chorionic gonadotropin using Prussian blue nanoparticle-based photothermal conversion. Anal. Bioanal. Chem. 2019, 411, 6837–6845. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, H.-B.; Zhong, Z.-T.; Li, C.-Q.; Chen, W.; Liu, B.; Zhao, Y.-D. A smartphone-based rapid quantitative detection platform for lateral flow strip of human chorionic gonadotropin with optimized image algorithm. Microchem. J. 2020, 157, 105038. [Google Scholar] [CrossRef]
- Wang, X.; Xue, C.-H.; Yang, D.; Jia, S.-T.; Ding, Y.-R.; Lei, L.; Gao, K.-Y.; Jia, T.-T. Modification of a nitrocellulose membrane with nanofibers for sensitivity enhancement in lateral flow test strips. RSC Adv. 2021, 11, 26493–26501. [Google Scholar] [CrossRef]
- Danthanarayana, A.N.; Finley, E.; Vu, B.; Kourentzi, K.; Willson, R.C.; Brgoch, J. A multicolor multiplex lateral flow assay for high-sensitivity analyte detection using persistent luminescent nanophosphors. Anal. Methods 2020, 12, 272–280. [Google Scholar] [CrossRef]
- Liang, H.; Ning, G.; Wang, L.; Li, C.; Zheng, J.; Zeng, J.; Zhao, H.; Li, C.-P. Covalent Framework Particles Modified with MnO2 Nanosheets and Au Nanoparticles as Electrochemical Immunosensors for Human Chorionic Gonadotropin. ACS Appl. Nano Mater. 2021, 4, 4593–4601. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhang, Q.; Bao, N.; Ding, S.-N. A Sensitive Immunochromatographic Test Strip Based on Hydrophobic Quantum Dots Incorporated into Mg/Fe Nanoflowers for HCG Detection. Chemosensors 2023, 11, 114. https://doi.org/10.3390/chemosensors11020114
Liu H, Zhang Q, Bao N, Ding S-N. A Sensitive Immunochromatographic Test Strip Based on Hydrophobic Quantum Dots Incorporated into Mg/Fe Nanoflowers for HCG Detection. Chemosensors. 2023; 11(2):114. https://doi.org/10.3390/chemosensors11020114
Chicago/Turabian StyleLiu, Hao, Qing Zhang, Ning Bao, and Shou-Nian Ding. 2023. "A Sensitive Immunochromatographic Test Strip Based on Hydrophobic Quantum Dots Incorporated into Mg/Fe Nanoflowers for HCG Detection" Chemosensors 11, no. 2: 114. https://doi.org/10.3390/chemosensors11020114