Validation of a HS–GC–FID Method for the Quantification of Sevoflurane in the Blood, Urine, Brain and Lungs for Forensic Purposes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Stock Solutions, Standards and Quality Controls
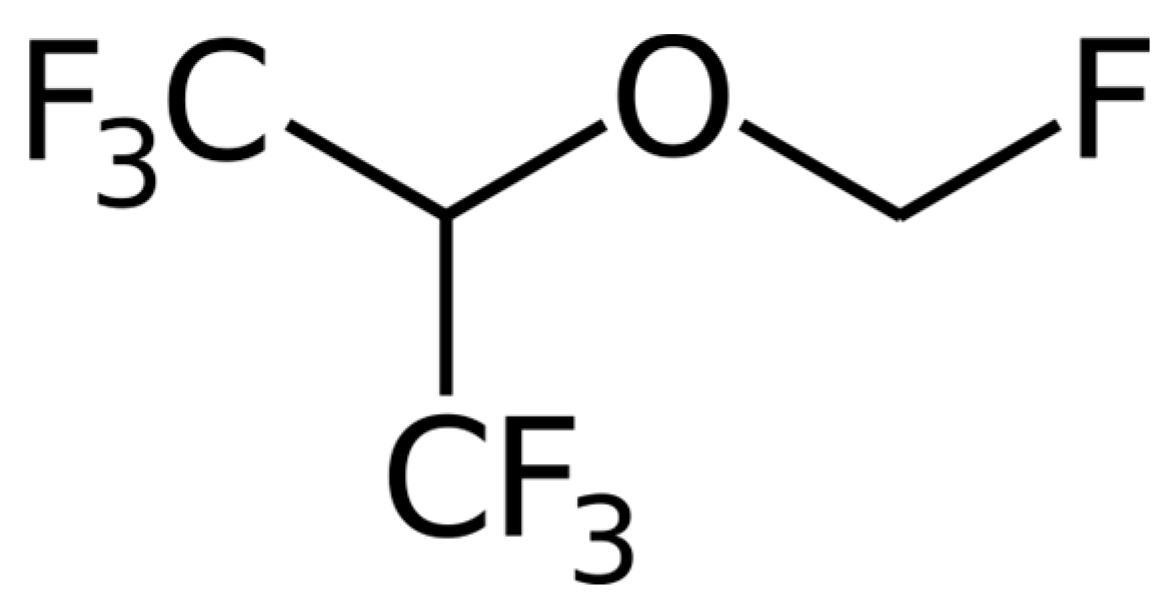
2.3. Instrumentation and Method Optimization
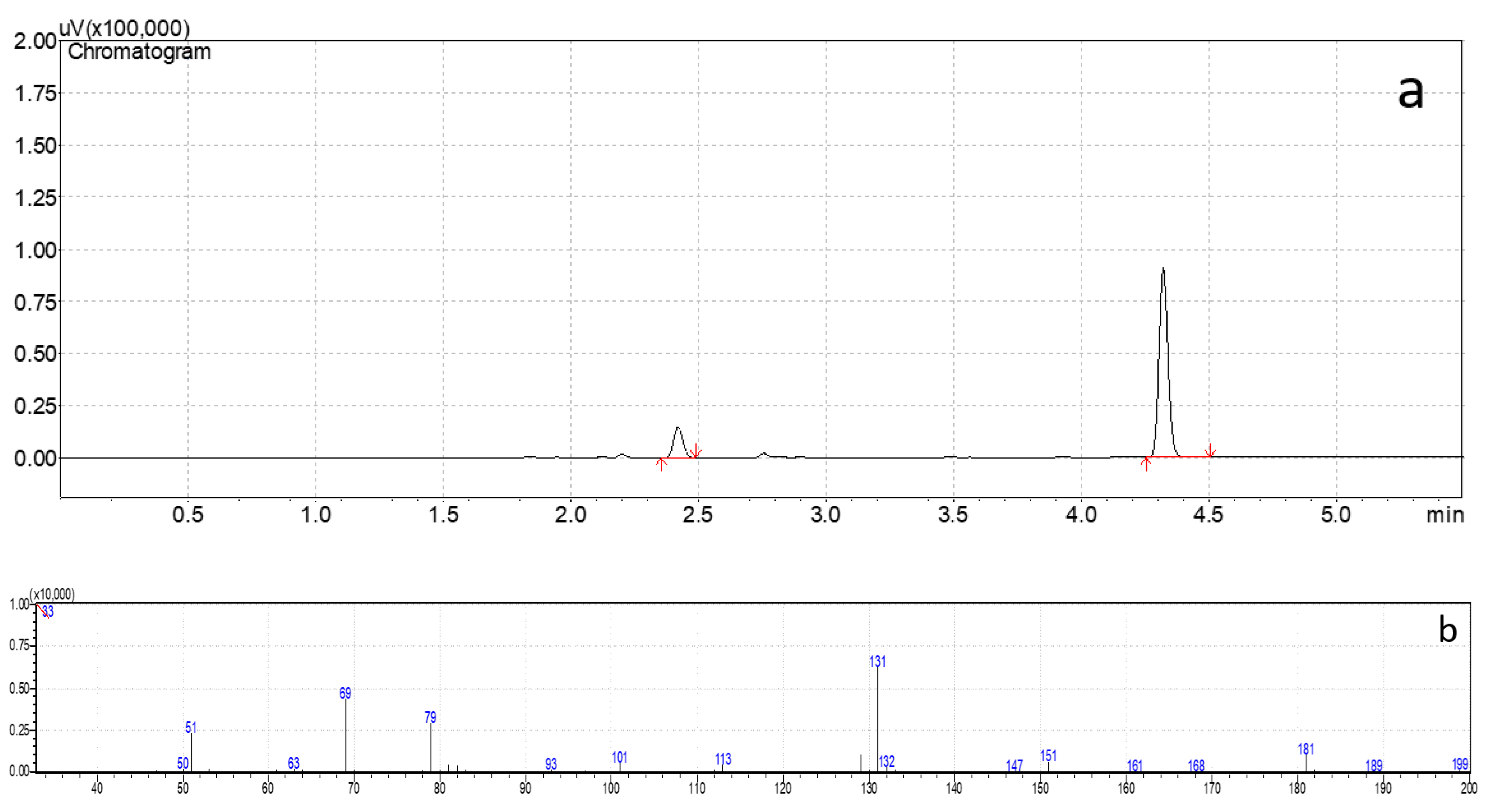
2.4. Headspace Procedure
2.5. Validation Plan
2.5.1. Interferences Studies
2.5.2. Linearity and Carryover
2.5.3. Limit of Detection and Limit of Quantification
2.5.4. Precision and Accuracy
2.5.5. Stability Studies
2.5.6. Method Application
3. Results
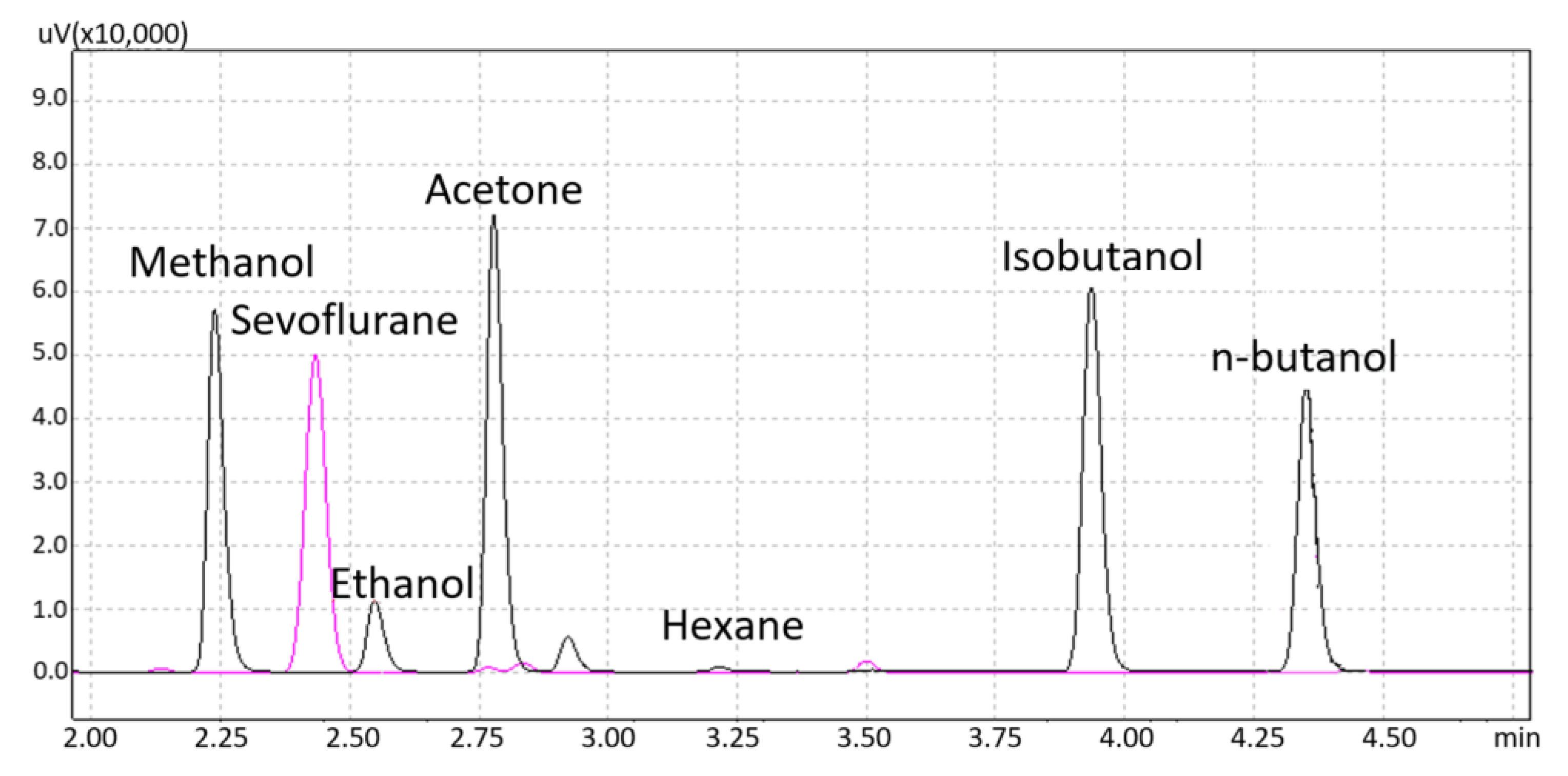
3.1. Interferences Studies
3.2. Linearity and Carryover
3.3. Limit of Detection and Limit of Quantification
3.4. Precision and Accuracy
3.5. Stability Studies
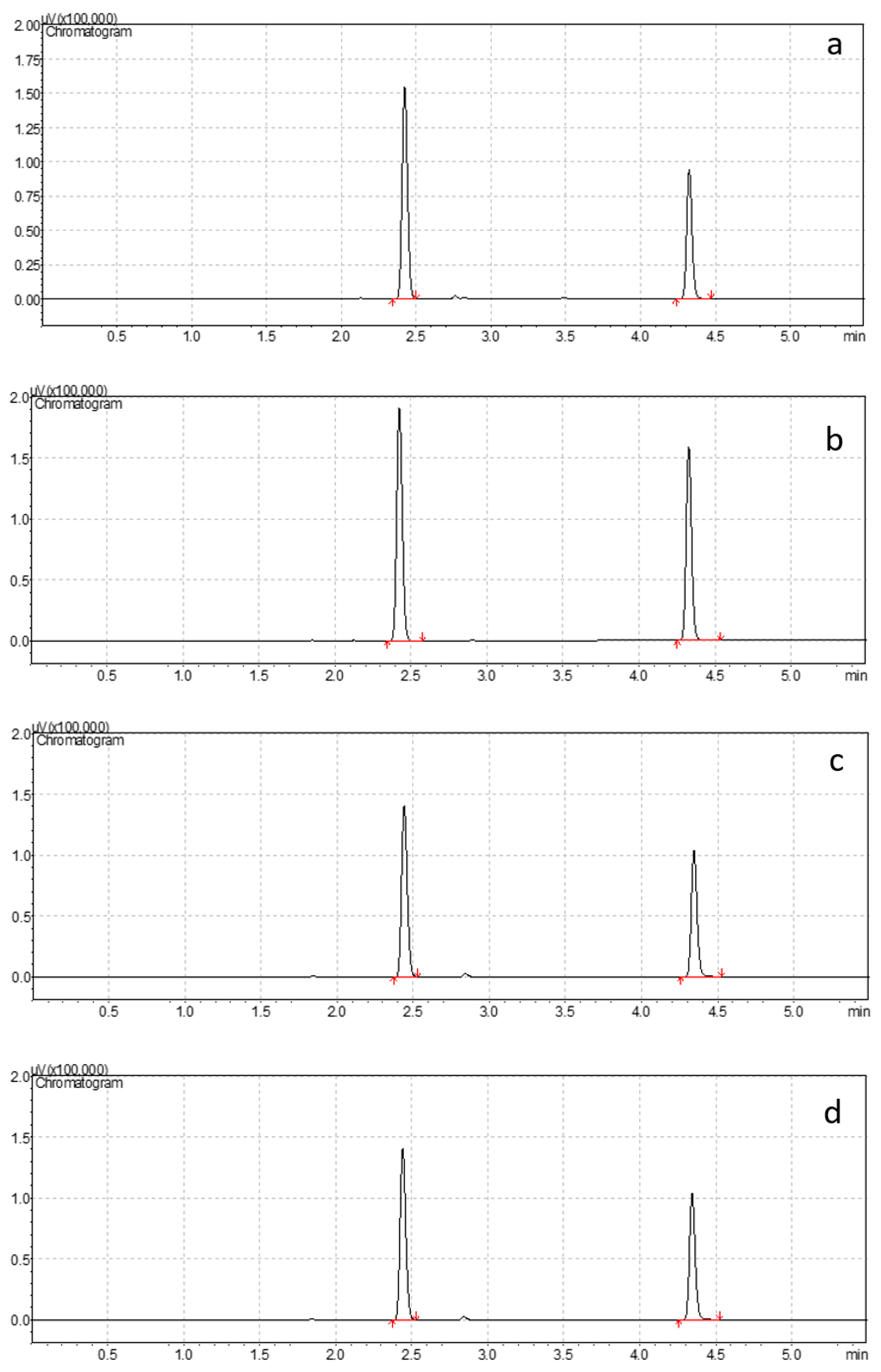
3.6. Method Application
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Keeffe, N.J.; Healy, T.E. The role of new anesthetic agents. Pharm. Ther. 1999, 84, 233–248. [Google Scholar] [CrossRef]
- Behne, M.; Wilke, H.J.; Harder, S. Clinical pharmacokinetics of sevoflurane. Clin. Pharmacokinet. 1999, 36, 13–26. [Google Scholar] [CrossRef]
- Delgado-Herrera, L.; Ostroff, R.D.; Rogers, S.A. Sevoflurance: Approaching the ideal inhalational anesthetic. a pharmacologic, pharmacoeconomic, and clinical review. CNS Drug Rev. 2001, 7, 48–120. [Google Scholar] [CrossRef]
- Wille, S.M.; Lambert, W.E. Volatile substance abuse--post-mortem diagnosis. Forensic Sci. Int. 2004, 142, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Pelletti, G.; Rossi, F.; Garagnani, M.; Barone, R.; Roffi, R.; Pelotti, S. Medico-legal implications of toluene abuse and toxicity. Review of cases along with blood concentrations. Leg Med. 2018, 34, 48–57. [Google Scholar] [CrossRef]
- Burrows, D.L.; Nicolaides, A.; Stephens, G.C.; Ferslew, K.E. The distribution of sevoflurane in a sevoflurane induced death. J. Forensic Sci. 2004, 49, 394–397. [Google Scholar]
- Epstein, R.H.; Gratch, D.M.; Grunwald, Z. Development of a scheduled drug diversion surveillance system based on an analysis of atypical drug transactions. Anesth. Analg. 2007, 105, 1053–1060. [Google Scholar] [CrossRef]
- Hayashi, T.; Buschmann, C.; Riesselmann, B.; Roscher, S.; Tsokos, M. Circumstantial and toxicological features of deaths from self-administered intravenous anesthetic/narcotic agents. Forensic Sci. Med. Pathol. 2013, 9, 138–144. [Google Scholar] [CrossRef]
- Johnstone, R.E.; Katz, R.L.; Stanley, T.H. Homicides using muscle relaxants, opioids, and anesthetic drugs: Anesthesiologist assistance in their investigation and prosecution. Anesthesiology 2011, 114, 713–716. [Google Scholar] [CrossRef]
- Derrington, M.C.; Smith, G. A review of studies of anaesthetic risk, morbidity and mortality. Br. J. Anaesth. 1987, 59, 815–833. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C.M.; Young, T.; Laster, M.J.; Eger, E.I., 2nd; Garg, U. Sevoflurane concentrations in blood, brain, and lung after sevoflurane-induced death. J. Forensic Sci. 2007, 52, 1408–1410. [Google Scholar] [CrossRef]
- Levine, B.; Cox, D.; Jufer-Phipps, R.A.; Li, L.; Jacobs, A.; Fowler, D. A fatality from sevoflurane abuse. J. Anal. Toxicol. 2007, 31, 534–536. [Google Scholar] [CrossRef]
- Cantrell, F.L. A fatal case of sevoflurane abuse. Clin. Toxicol. 2008, 46, 918–919. [Google Scholar] [CrossRef]
- Kovatsi, L.; Giannakis, D.; Arzoglou, V.; Samanidou, V. Development and validation of a direct headspace GC-FID method for the determination of sevoflurane, desflurane and other volatile compounds of forensic interest in biological fluids: Application on clinical and post-mortem samples. J. Sep. Sci. 2011, 34, 1004–1010. [Google Scholar] [CrossRef]
- Pihlainen, K.; Ojanperä, I. Analytical toxicology of fluorinated inhalation anaesthetics. Forensic Sci. Int. 1998, 97, 117–133. [Google Scholar] [CrossRef]
- Pelletti, G.; Rossi, F.; Garagnani, M.; Barone, R.; Roffi, R.; Fais, P.; Pelotti, S. Optimization of cloned enzyme donor immunoassay cut-offs for drugs of abuse in post-mortem whole blood. Forensic Sci. Int. 2020, 312, 110291. [Google Scholar] [CrossRef]
- Giorgetti, A.; Barone, R.; Pelletti, G.; Garagnani, M.; Pascali, J.; Haschimi, B.; Auwärter, V. Development and validation of a rapid LC-MS/MS method for the detection of 182 novel psychoactive substances in whole blood. Drug Test. Anal. 2022, 14, 202–223. [Google Scholar] [CrossRef]
- Pelletti, G.; Verstraete, A.G.; Reyns, T.; Barone, R.; Rossi, F.; Garagnani, M.; Pelotti, S. Prevalence of therapeutic drugs in blood of drivers involved in traffic crashes in the area of Bologna, Italy. Forensic Sci. Int. 2019, 302, 109914. [Google Scholar] [CrossRef]
- Scientific Working Group for Forensic Toxicology. Scientific Working Group for Forensic Toxicology (SWGTOX) standard practices for method validation in forensic toxicology. J. Anal. Toxicol. 2013, 37, 452–474. [Google Scholar] [CrossRef]
- Larry, B. (Ed.) Principles of Forensic Toxicology; American Association for Clinical Chemistry: Washington, DC, USA, 1999; ISBN 1-890883-07-7. [Google Scholar]
- Pelletti, G.; Garagnani, M.; Barone, R.; Boscolo-Berto, R.; Rossi, F.; Morotti, A.; Roffi, R.; Fais, P.; Pelotti, S. Validation and preliminary application of a GC-MS method for the determination of putrescine and cadaverine in the human brain: A promising technique for PMI estimation. Forensic Sci. Int. 2019, 297, 221–227. [Google Scholar] [CrossRef]
- Romolo, F.S.; di Luca, N.M.; Ciallella, C.; Bottoni, E.; Fiore, P.A.; Cappelletti, S.; Giuliani, N.; Augsburger, M.; Varlet, V. Volatile lipophilic substances management in case of fatal sniffing. J. Forensic Leg. Med. 2017, 52, 35–39. [Google Scholar] [CrossRef] [Green Version]
- Soejima, M.; Tanaka, N.; Oshima, T.; Kinoshita, H.; Koda, Y. Detection of helium in a fire victim: A case report. Forensic Sci. Int. 2021, 318, 110613. [Google Scholar] [CrossRef]
- Pahor, K.; Olson, G.; Forbes, S.L. Post-mortem detection of gasoline residues in lung tissue and heart blood of fire victims. Int. J. Leg. Med. 2013, 127, 923–930. [Google Scholar] [CrossRef]
- Saito, K.; Takayasu, T.; Nishigami, J.; Kondo, T.; Ohtsuji, M.; Lin, Z.; Ohshima, T. Determination of the volatile anesthetics halothane, enflurane, isoflurane, and sevoflurane in biological specimens by pulse-heating GC-MS. J. Anal. Toxicol. 1995, 19, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.C.; Hwang, K.L.; Shen, C.H.; Wang, H.F.; Ho, W.M. Simultaneous determination of fluorinated inhalation anesthetics in blood by gas chromatography-mass spectrometry combined with a headspace autosampler. J. Chromatogr. B Biomed. Sci. Appl. 2001, 759, 307–318. [Google Scholar] [CrossRef]
- Kojima, T.; Ishii, A.; Watanabe-Suzuki, K.; Kurihara, R.; Seno, H.; Kumazawa, T.; Suzuki, O.; Katsumata, Y. Sensitive determination of four general anaesthetics in human whole blood by capillary gas chromatography with cryogenic oven trapping. J. Chromatogr. B Biomed. Sci. Appl. 2001, 762, 103–108. [Google Scholar] [CrossRef]
- Bourdeaux, D.; Sautou-Miranda, V.; Montagner, A.; Perbet, S.; Constantin, J.M.; Bazin, J.E.; Chopineau, J. Simple assay of plasma sevoflurane and its metabolite hexafluoroisopropanol by headspace GC-MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 45–50. [Google Scholar] [CrossRef]
- Accorsi, A.; Morrone, B.; Domenichini, I.; Valenti, S.; Raffi, G.B.; Violante, F.S. Urinary sevoflurane and hexafluoro-isopropanol as biomarkers of low-level occupational exposure to sevoflurane. Int. Arch. Occup. Environ. Health. 2005, 78, 369–378. [Google Scholar] [CrossRef]
- Astier, A. Chromatographic determination of volatile solvents and their metabolites in urine for monitoring occupational exposure. J. Chromatogr. 1993, 643, 389–398. [Google Scholar] [CrossRef]
- Ghimenti, S.; Tabucchi, S.; Bellagambi, F.G.; Lomonaco, T.; Onor, M.; Trivella, M.G.; Fuoco, R.; Di Francesco, F. Determination of sevoflurane and isopropyl alcohol in exhaled breath by thermal desorption gas chromatography-mass spectrometry for exposure assessment of hospital staff. J. Pharm. Biomed. Anal. 2015, 106, 218–223. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, F.J.; Wang, F.Y.; Wang, Y.Y.; Guo, J.; Kanhar, G.M.; Chen, J.; Liu, J.; Zhou, C.; Yan, M.; et al. Simultaneous on-line monitoring of propofol and sevoflurane in balanced anesthesia by direct resistive heating gas chromatography. J. Chromatogr. A 2017, 1506, 93–100. [Google Scholar] [CrossRef]
- Accorsi, A.; Barbieri, A.; Raffi, G.B.; Violante, F.S. Biomonitoring of exposure to nitrous oxide, sevoflurane, isoflurane and halothane by automated GC/MS headspace urinalysis. Int. Arch. Occup. Environ. Health. 2001, 74, 541–548. [Google Scholar] [CrossRef]
- Jafari, A.; Bargeshadi, R.; Jafari, F.; Mohebbi, I.; Hajaghazadeh, M. Environmental and biological measurements of isoflurane and sevoflurane in operating room personnel. Int. Arch. Occup. Environ. Health. 2018, 91, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Woydt, L.; Bernhard, M.; Kirsten, H.; Burkhardt, R.; Hammer, N.; Gries, A.; Dreßler, J.; Ondruschka, B. Intra-individual alterations of serum markers routinely used in forensic pathology depending on increasing post-mortem interval. Sci. Rep. 2018, 8, 12811. [Google Scholar] [CrossRef]
- Pelletti, G.; Cecchetto, G.; Viero, A.; Fais, P.; Weber, M.; Miotto, D.; Montisci, M.; Viel, G.; Giraudo, C. Accuracy, precision and inter-rater reliability of micro-CT analysis of false starts on bones. A preliminary validation study. Leg Med. 2017, 29, 38–43. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime (UNDC). Laboratory and Scientific Section United Nations Office on Drugs and Crime. Guidelines for the Forensic Analysis of Drugs Facilitating Sexual Assault and Other Criminal Acts. United Nations. December 2011. Available online: https://www.unodc.org/documents/scientific/forensic_analys_of_drugs_facilitating_sexual_assault_and_other_criminal_acts.pdf (accessed on 24 November 2022).
- Grocholska, P.; Popiel, D.; Walter, M.; Biernat, M.; Cebrat, M.; Kuczer, M.; Modzel, M.; Bachor, R.; Kluczyk, A. Citius, Altius, Fortius—Advanced Mass Spectrometry in Service of Forensic Analysis. Chemosensors 2022, 10, 324. [Google Scholar] [CrossRef]
- Barea-Sepúlveda, M.; Duarte, H.; Aliaño-González, M.J.; Romano, A.; Medronho, B. Total Ion Chromatogram and Total Ion Mass Spectrum as Alternative Tools for Detection and Discrimination (A Review). Chemosensors 2022, 10, 465. [Google Scholar] [CrossRef]

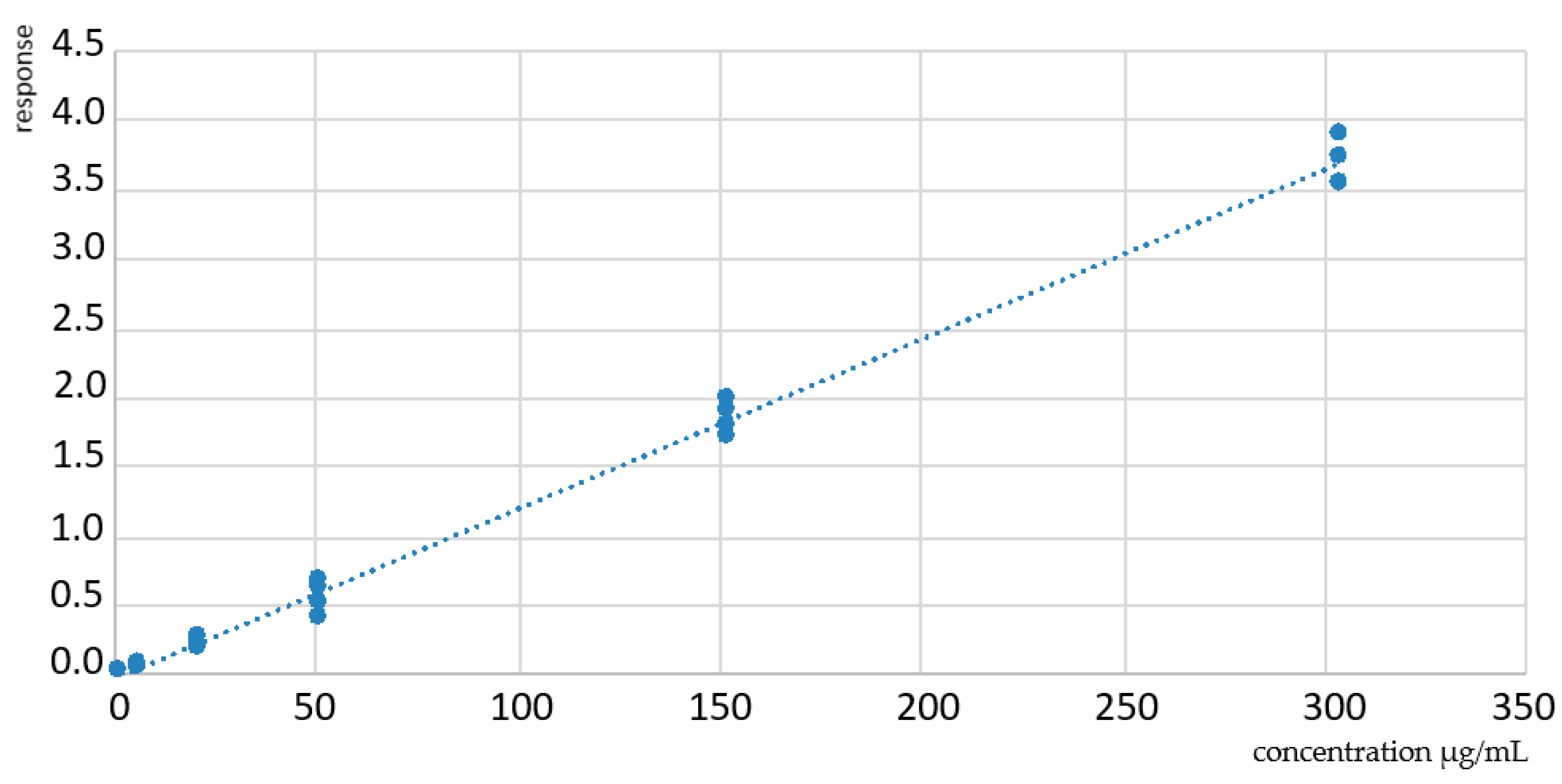
| Matrix | Equation (Mean ± SD, n = 5) | r2 (Mean ± SD, n = 5) |
|---|---|---|
| Blood | y = 0.012 (±0.002) x | 0.998 (±0.001) |
| Urine | y = 0.009 (±0.001) x | 0.998 (±0.001) |
| Brain | y = 0.013 (±0.001) x | 0.999 (±0.001) |
| Lung | y = 0.009 (±0.002) x | 0.999 (±<0.001) |
| Matrix and QC | Concentration (µg/mL or µg/mg) | Precision (Mean RSD%) | Accuracy (Mean BIAS%) | ||
|---|---|---|---|---|---|
| Intraday (n = 6) | Interday (n = 15) | Intraday (n = 6) | Interday (n = 15) | ||
| Blood | |||||
| LLOQ | 1.0 | 2.3 | 2.8 | 5.2 | 5.2 |
| Low | 3.0 | 6.2 | 7.9 | 18.2 | 18.9 |
| Medium | 152.0 | 6.5 | 12.9 | 6.3 | 7.1 |
| High | 304.0 | 6.5 | 10.2 | 2.5 | 3.0 |
| Urine | |||||
| LLOQ | 1.0 | 9.1 | 15.3 | 6.1 | 13.5 |
| Low | 3.0 | 5.5 | 8.6 | 14.2 | 18.4 |
| Medium | 152.0 | 2.6 | 9.4 | 14.0 | 11.2 |
| High | 304.0 | 3.1 | 11.0 | 7.8 | 9.3 |
| Brain | |||||
| LLOQ | 1.0 | 14.0 | 16.3 | 14.7 | 14.7 |
| Low | 3.0 | 12.3 | 14.4 | 12.2 | 12.6 |
| Medium | 152.0 | 3.6 | 5.0 | 9.2 | 19.2 |
| High | 304.0 | 4.1 | 5.0 | 3.5 | 3.6 |
| Lung | |||||
| LLOQ | 1.0 | 11.1 | 5.7 | 11.1 | 11.1 |
| Low | 3.0 | 8.8 | 10.2 | 15.8 | 16.2 |
| Medium | 152.0 | 8.7 | 10.4 | 8.7 | 8.7 |
| High | 304.0 | 7.1 | 8.7 | 7.1 | 8.8 |
| Matrix | Volume | Extraction Method | Separation and Detection | LOQ | |
|---|---|---|---|---|---|
| Saito et al. [25] | Blood | 0.03 mL (PH) 0.5 mL (HS) | PH HS | GC–MS | 1 μg/mL |
| Yang et al. [26] | Blood | 5 mL | HS | GC–MS | 4.8 μg/mL |
| Kojima et al. [27] | Blood | 0.5 mL | HS | GC–FID with COT | * |
| Kovatsi et al. [14] | Blood and urine | 1 mL | HS | GC–FID | 52 μg/mL blood 41 μg/mL urine |
| Bourdeaux et al. [28] | Plasma | 1 mL | HS | GC–MS | 1 μg/mL |
| Burrows et al. [6] | Blood Urine Tracheal aspirate Vitreous Liver Kidney | 1 g | HS | GC–FID | * |
| Rosales et al. [11] | Blood Brain Lungs | 2.14 g 8.12 g 4.10 g | HS | GC–FID | * |
| Levine et al. [12] | Blood Urine Bile Kidney Liver | 0.5 mL 0.5 mL 0.5 mL 0.5 g 0.5 g | HS | GC–MS | 1 μg/mL |
| Current method | Blood Urine Brain Lungs | 0.5 mL 0.5 mL 0.5 g 0.5 g | HS | GC–FID | 1 μg/mL 1 μg/g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelletti, G.; Barone, R.; Mohamed, S.; Rossi, F.; Garagnani, M.; Giorgetti, A.; Fais, P.; Pelotti, S. Validation of a HS–GC–FID Method for the Quantification of Sevoflurane in the Blood, Urine, Brain and Lungs for Forensic Purposes. Chemosensors 2023, 11, 133. https://doi.org/10.3390/chemosensors11020133
Pelletti G, Barone R, Mohamed S, Rossi F, Garagnani M, Giorgetti A, Fais P, Pelotti S. Validation of a HS–GC–FID Method for the Quantification of Sevoflurane in the Blood, Urine, Brain and Lungs for Forensic Purposes. Chemosensors. 2023; 11(2):133. https://doi.org/10.3390/chemosensors11020133
Chicago/Turabian StylePelletti, Guido, Rossella Barone, Susan Mohamed, Francesca Rossi, Marco Garagnani, Arianna Giorgetti, Paolo Fais, and Susi Pelotti. 2023. "Validation of a HS–GC–FID Method for the Quantification of Sevoflurane in the Blood, Urine, Brain and Lungs for Forensic Purposes" Chemosensors 11, no. 2: 133. https://doi.org/10.3390/chemosensors11020133






