Effects of Oxygen Partial Pressure and Thermal Annealing on the Electrical Properties and High-Temperature Stability of Pt Thin-Film Resistors
Abstract
:1. Introduction
2. Materials and Methods
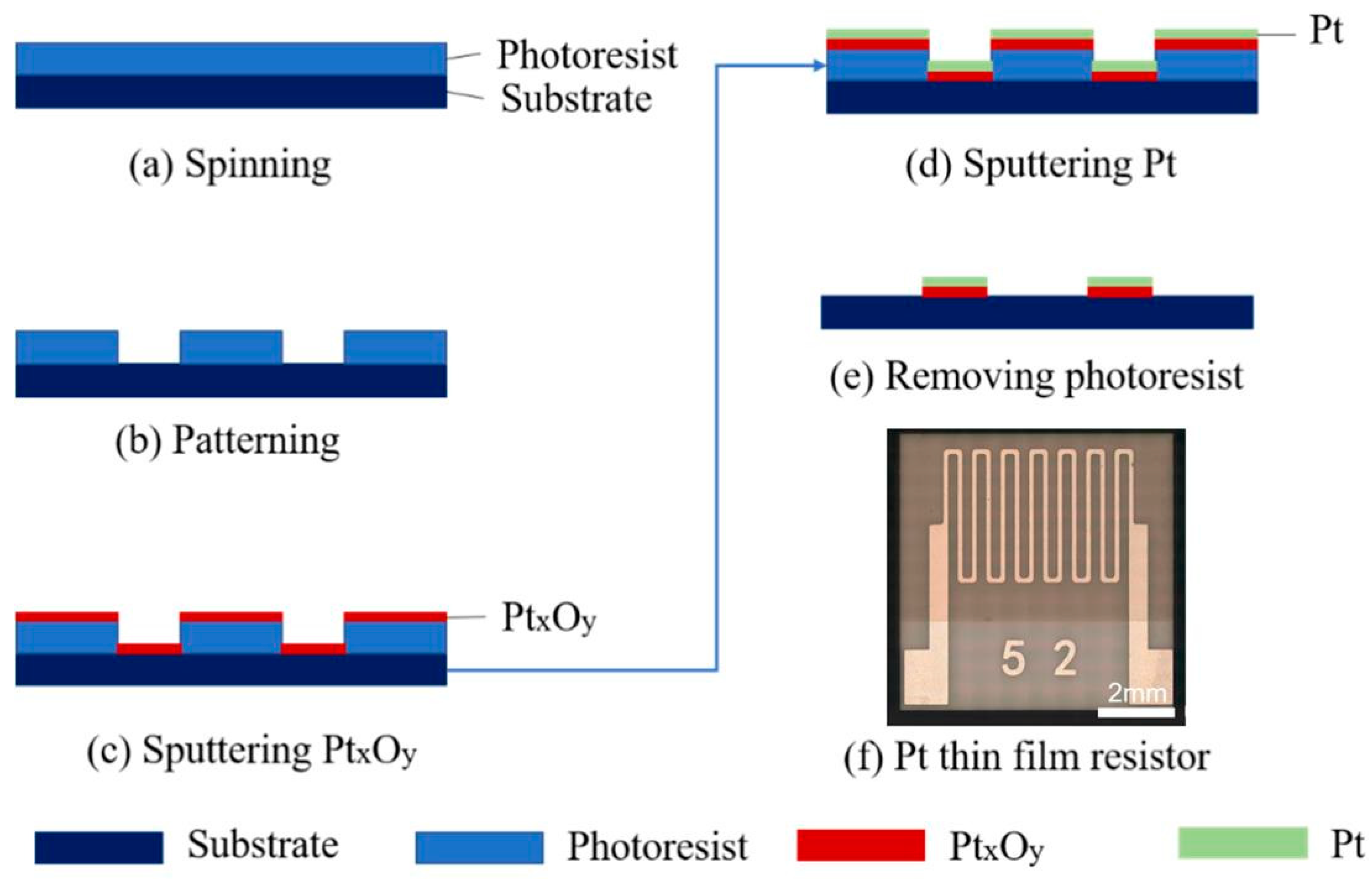
2.1. Pt Thin-Film Resistor Preparation
2.2. Characterization of Resistor
3. Results and Discussion
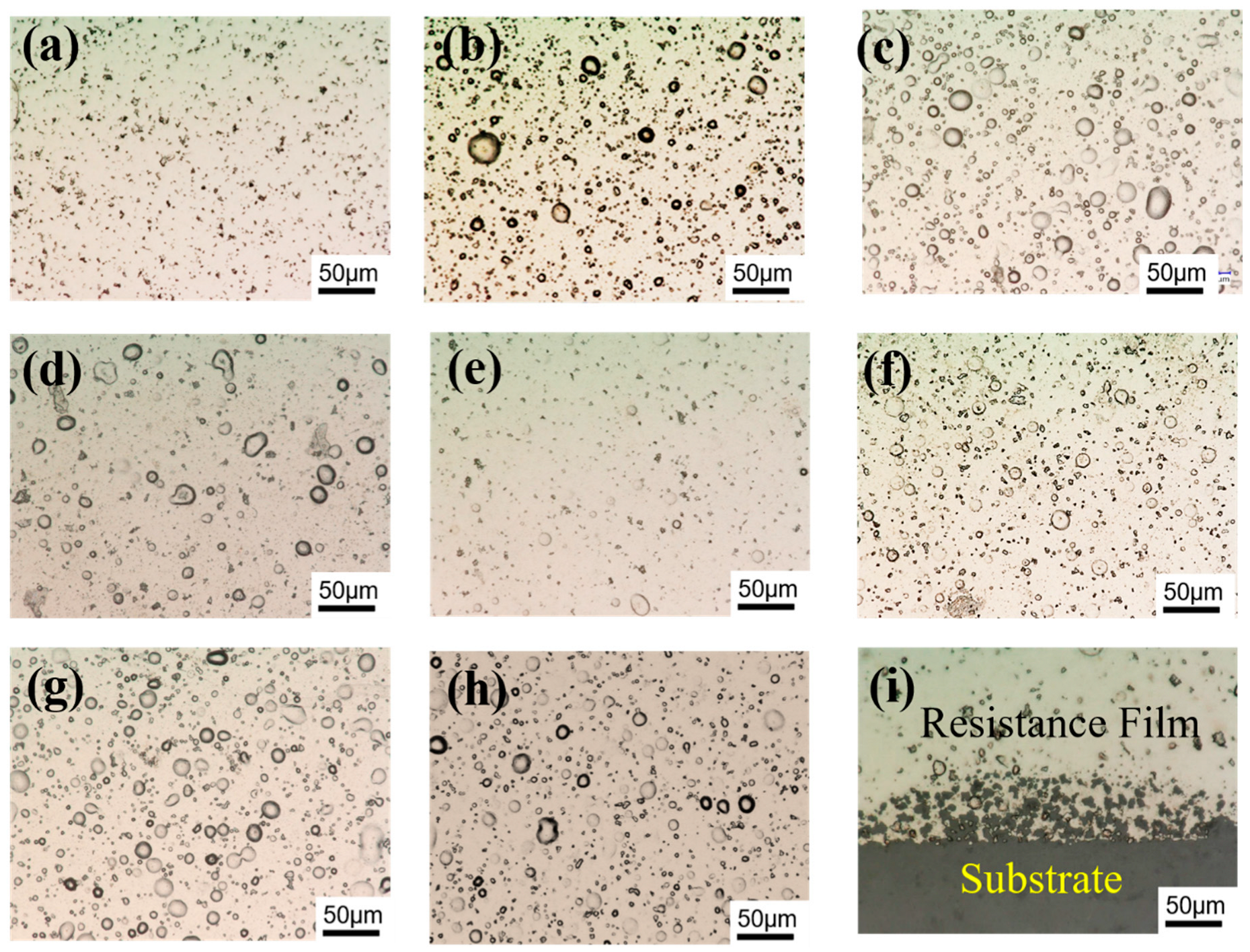
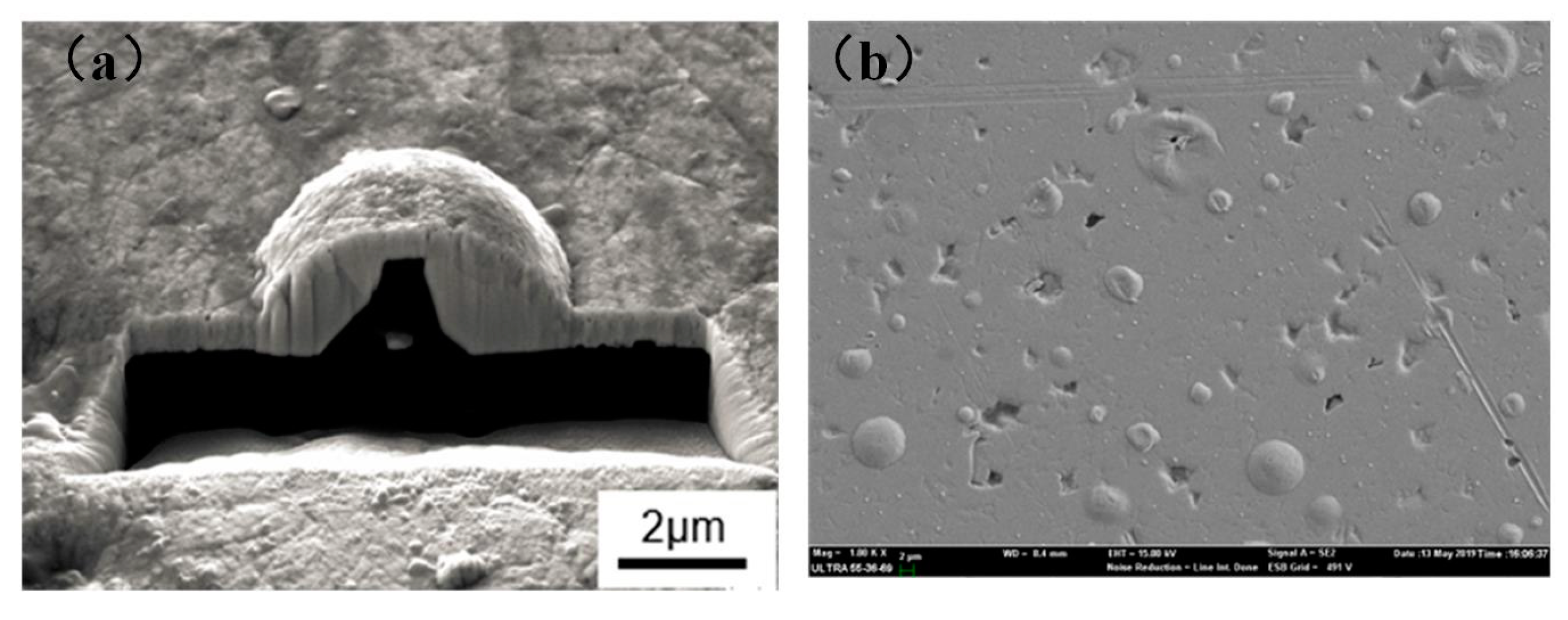
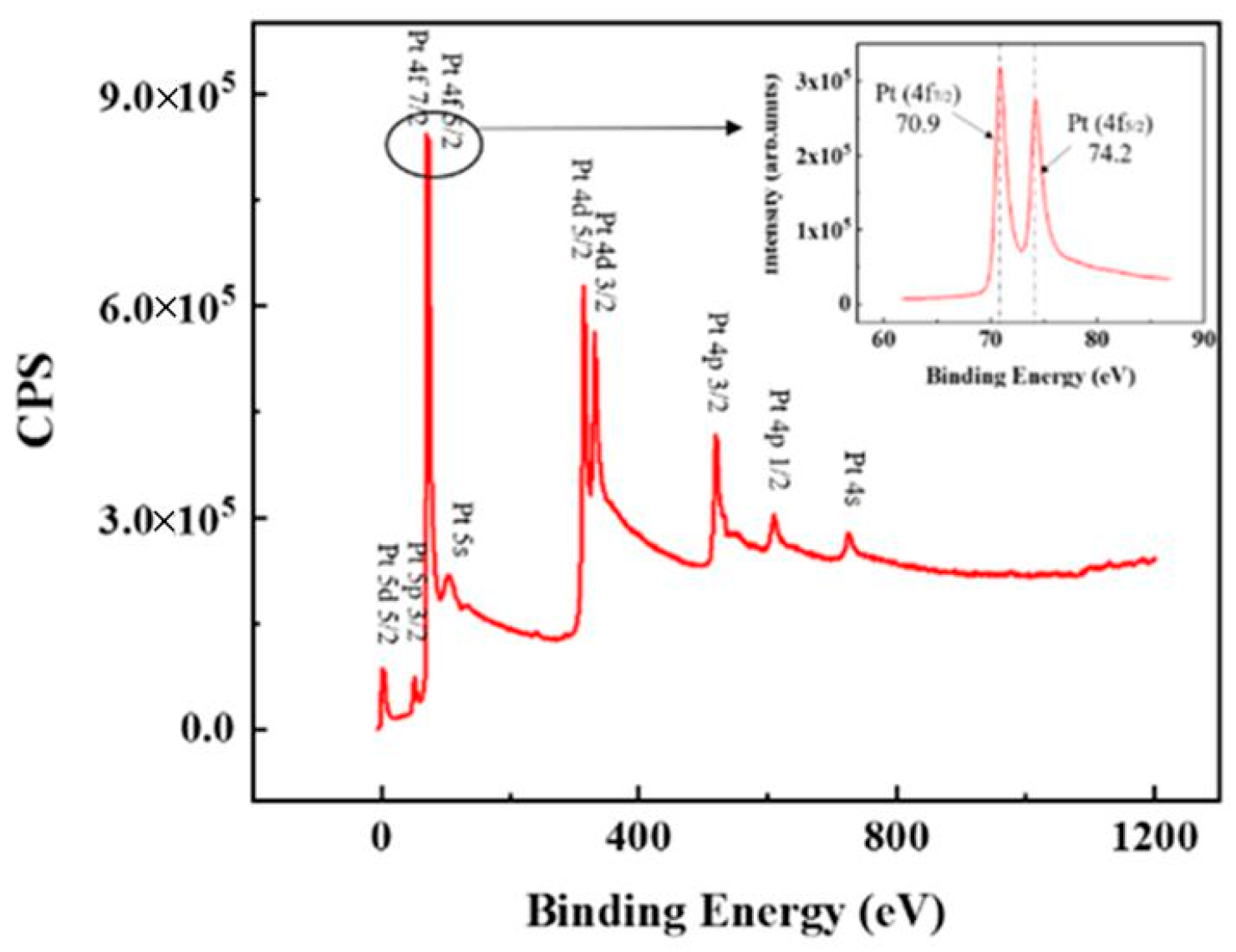
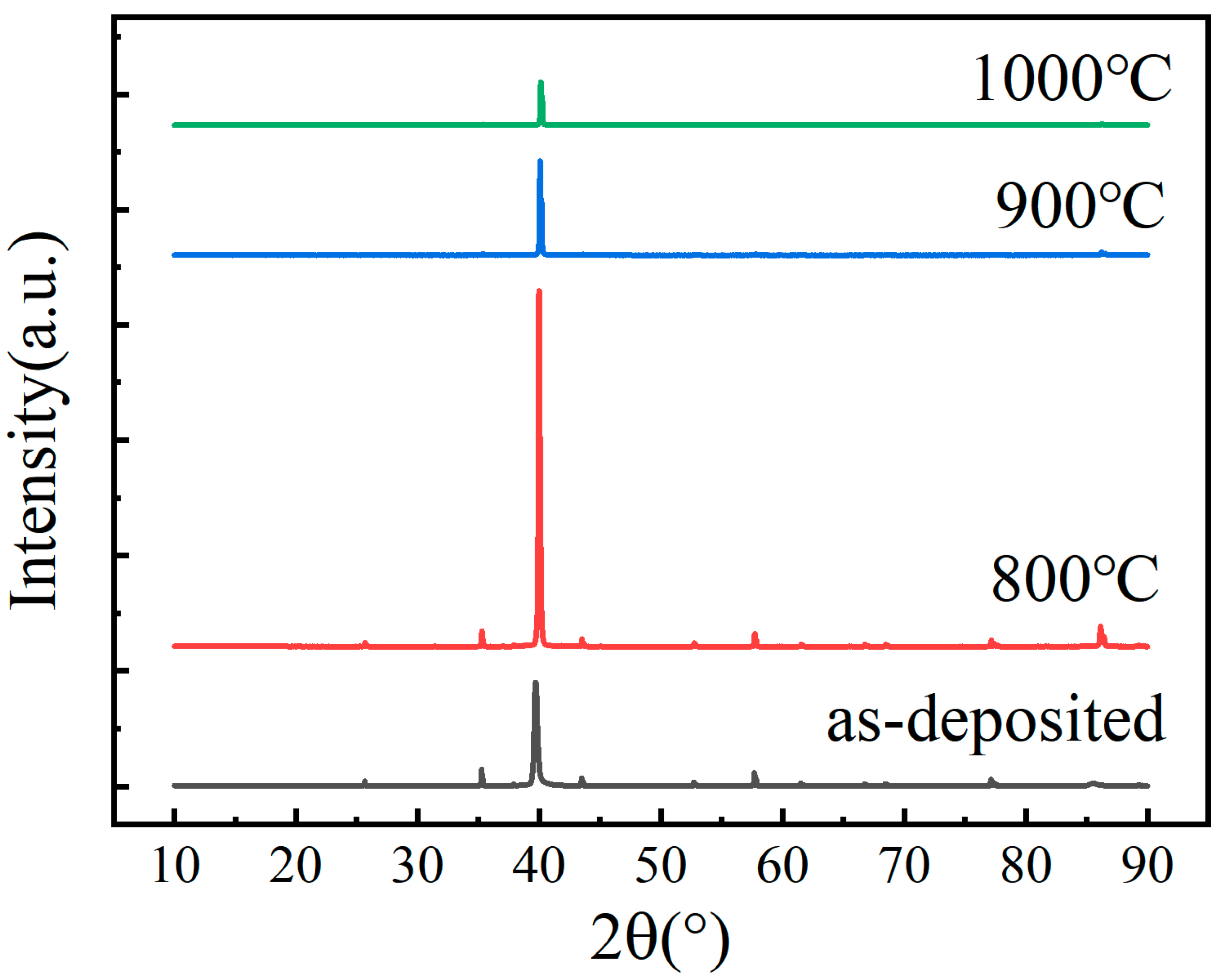
3.1. Microstructure and Chemical Composition
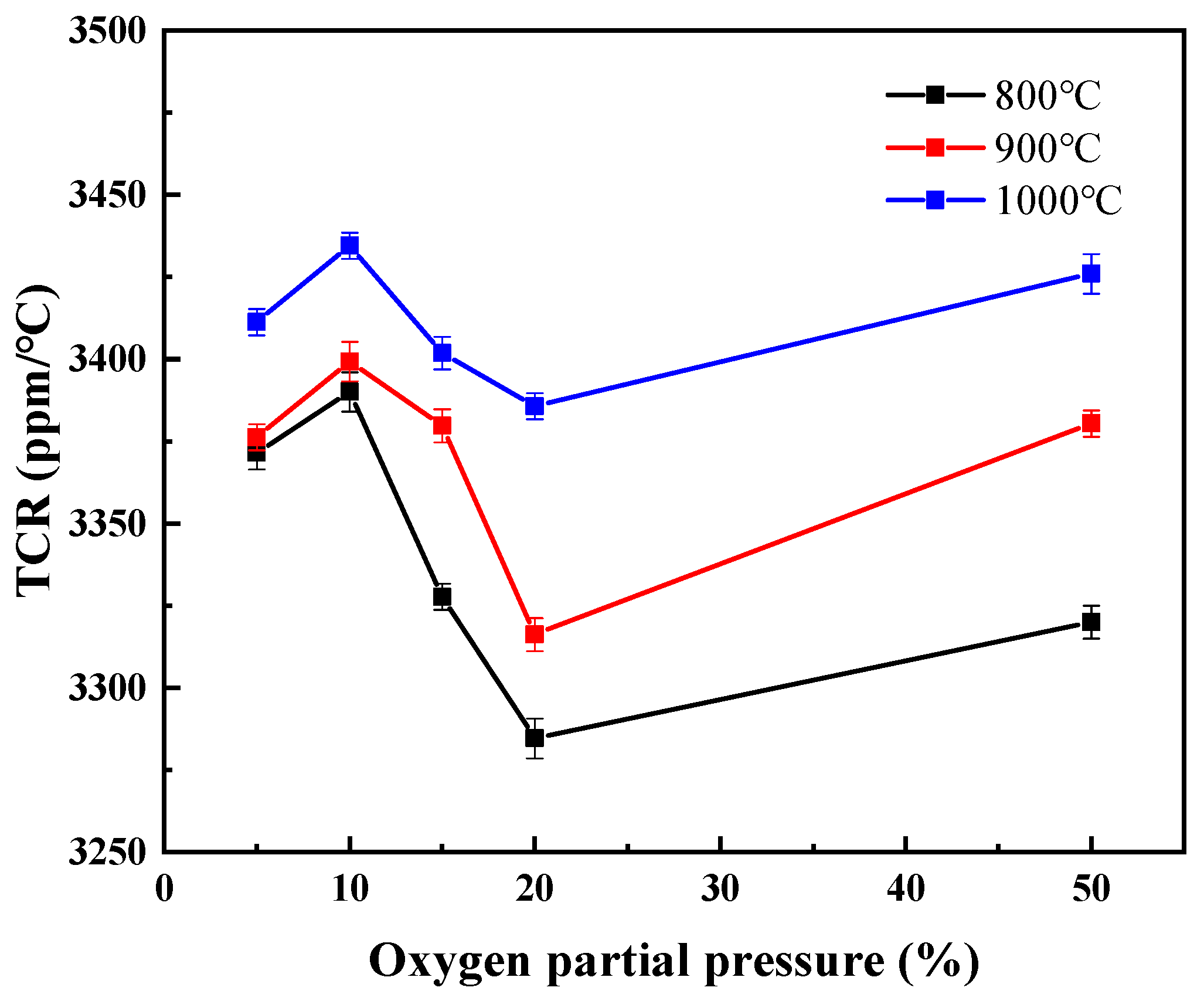
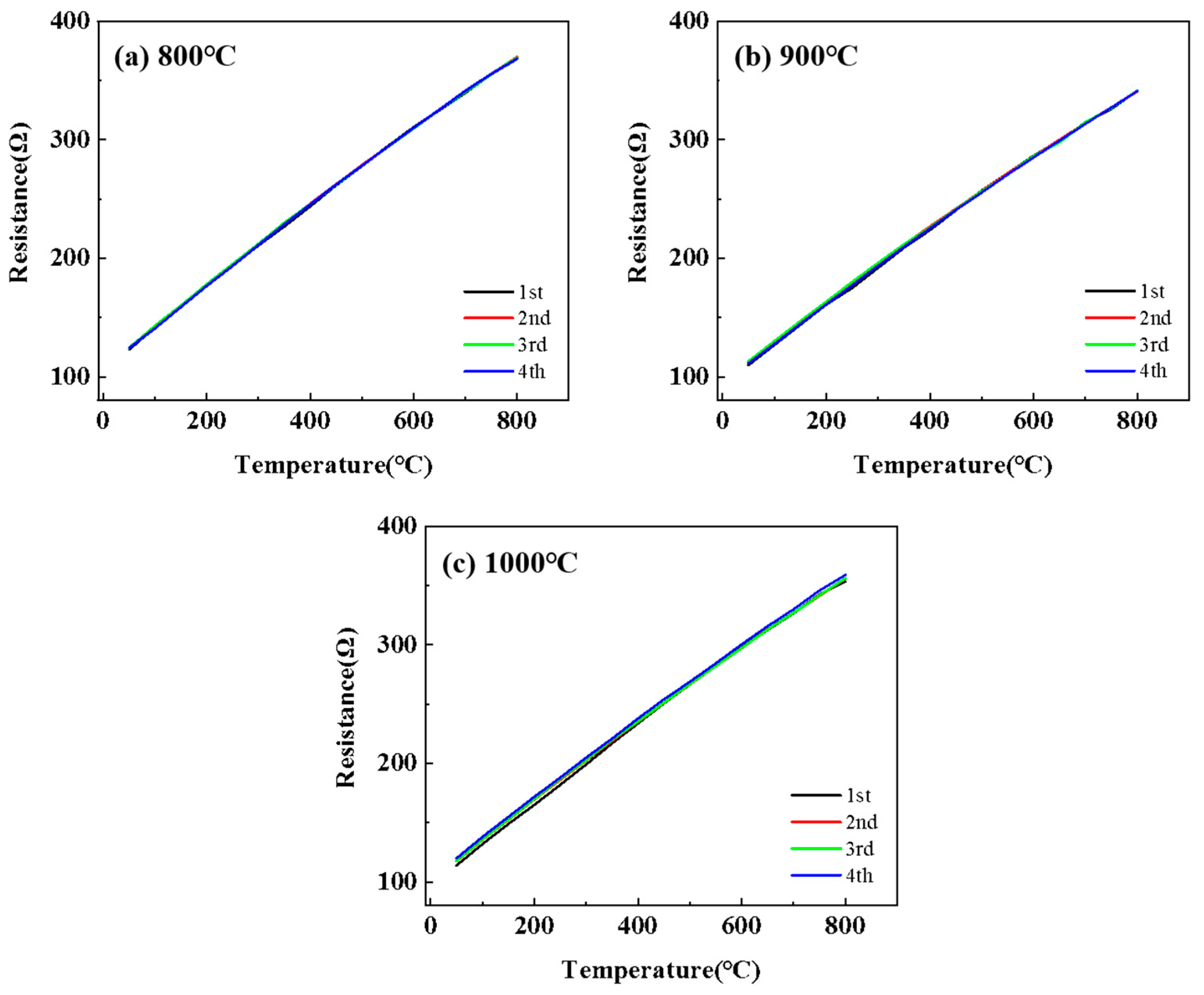
3.2. Electrical Properties and High-Temperature Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Hu, T. Research status and development trend of MEMS S&A devices: A review. Def. Technol. 2020, 17, 450–456. [Google Scholar]
- Lin, X.; Zhang, C.; Yang, S.; Guo, W.; Zhang, Y.; Yang, Z.; Ding, G. The impact of thermal annealing on the temperature dependent resistance behavior of Pt thin films sputtered on Si and Al2O3 substrates. Thin Solid Film. 2019, 685, 372–378. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, S.; Wang, H.; Jiang, H.; Zhang, W. Fabrication and microstructure evolution of Al2O3/ZrBN-SiCN/Al2O3 high-temperature oxidation-resistant composite coating. Ceram. Int. 2019, 45, 247–251. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Yamashita, A.; Ohji, H. The experimental study of high TCR Pt thin films for thermal sensors. In Proceedings of the Sensors, 2002 IEEE, Orlando, FL, USA, 12–14 June 2002; pp. 1002–1005. [Google Scholar]
- Purkl, F.; English, T.; Yama, G.; Provine, J.; Kenny, T.W. Sub-10 nanometer uncooled platinum bolometers via plasma enhanced atomic layer deposition. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems, Taipei, Taiwan, 20–24 January 2013. [Google Scholar]
- Rusanov, R.; Rank, H.; Fuchs, T.; Mueller-Fiedler, R.; Kraft, O. Reliability characterization of a soot particle sensor in terms of stress- and electromigration in thin-film platinum. Microsyst. Technol. 2016, 22, 481–493. (In English) [Google Scholar] [CrossRef]
- Fricke, S.; Friedberger, A.; Seidel, H.; Schmid, U. A robust pressure sensor for harsh environmental applications. Sens. Actuators A Phys. 2012, 184, 16–21. [Google Scholar] [CrossRef]
- Mattox, D.M. Influence of Oxygen on the Adherence of Gold Films to Oxide Substrates. J. Appl. Phys. 1966, 37, 3613–3615. [Google Scholar] [CrossRef]
- Schmidl, G.; Dellith, J.; Kessler, E.; Schinkel, U. The influence of deposition parameters on Ti/Pt film growth by confocal sputtering and the temperature dependent resistance behavior using SiOx and Al2O3 substrates. Appl. Surf. Sci. 2014, 313, 267–275. [Google Scholar] [CrossRef]
- Sreenivas, K.; Reaney, I.; Maeder, T.; Setter, N.; Jagadish, C.; Elliman, R.G. Investigation of Pt/Ti bilayer metallization on silicon for ferroelectric thin film integration. J. Appl. Phys. 1994, 75, 232–239. [Google Scholar] [CrossRef]
- Weng, S.; Qiao, L.; Wang, P. Thermal stability of Pt-Ti bilayer films annealing in vacuum and ambient atmosphere. Appl. Surf. Sci. A J. Devoted Prop. Interfaces Relat. Synth. Behav. Mater. 2018, 444, 721–728. [Google Scholar] [CrossRef]
- Martin, D.M.; Agham, P.; Thong, Q.N.; Ajit, D.; David, J.S.; Alexander, A.D.; John, G.E. Growth of epitaxial oxides on silicon using atomic layer deposition: Crystallization and annealing of TiO2 on SrTiO3-buffered Si(001). J. Vac. Sci. Technol. B. Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. JVST B 2012, 30, 04E111-1–04E111-6. (In English) [Google Scholar]
- Halder, S.; Schneller, T.; Waser, R. Enhanced stability of platinized silicon substrates using an unconventional adhesion layer deposited by CSD for high temperature dielectric thin film deposition. Appl. Phys. A. Mater. Sci. Process. 2007, 87, 705–708. (In English) [Google Scholar] [CrossRef]
- Ababneh, A.; Al-Omari, A.N.; Dagamseh, A.M.K.; Tantawi, M.; Pauly, C.; Mücklich, F.; Feili, D.; Seidel, H. Electrical and morphological characterization of platinum thin-films with various adhesion layers for high temperature applications. Microsyst. Technol. 2015, 23, 703–709. [Google Scholar] [CrossRef]
- Schmid, U.; Seidel, H. Effect of high temperature annealing on the electrical performance of titanium/platinum thin films. Thin Solid Films 2008, 516, 898–906. [Google Scholar] [CrossRef]
- Courbat, J.; Briand, D.; Rooij, N. Reliability improvement of suspended platinum-based micro-heating elements. Sens. Actuators A Phys. 2008, 142, 284–291. [Google Scholar] [CrossRef]
- Garraud, A.; Combette, P.; Giani, A. Thermal stability of Pt/Cr and Pt/Cr2O3 thin-film layers on a SiNx/Si substrate for thermal sensor applications. Thin Solid Films 2013, 540, 256–260. [Google Scholar] [CrossRef]
- Chung, G.S.; Kim, C.H. RTD characteristics for micro-thermal sensors. Microelectron. J. 2008, 39, 1560–1563. [Google Scholar] [CrossRef]
- Kuribayashi, K.; Kitamura, S. Preparation of Pt-PtOx thin films as electrode for memory capacitors. Thin Solid Films 2001, 400, 160–164. [Google Scholar] [CrossRef]
- Budhani, R.C.; Prakash, S.; Bunshah, R.F. Thin-film temperature sensors for gas turbine engines: Problems and prospects. J. Vac. Sci. Technol. A Vac. Surf. Film. 1986, 4, 2609–2617. [Google Scholar] [CrossRef]
- John, D.W.; Kimala, L.H. Preparation and Analysis of Platinum Thin Films for High Temperature Sensor Applications; National Aeronautic Space Administration (NASA): Washington, DC, USA, 2005. [Google Scholar]
- Grosser, M.; Schmid, U. The impact of annealing temperature and time on the electrical performance of Ti/Pt thin films. Appl. Surf. Sci. 2010, 256, 4564–4569. [Google Scholar] [CrossRef]
- Schssler, T.; Schn, F.; Lemier, C.; Urban, G. Effect of high temperature annealing on resistivity and temperature coefficient of resistance of sputtered platinum thin films of SiO2/Pt/SiOx interfaces. Thin Solid Films 2020, 698, 137877. [Google Scholar] [CrossRef]
- Pang, Y.; Zhang, C.; Lei, P.; Wang, Y.; Lv, Z. Effects of Thermal Annealing on the Electrical Properties and Stability of Pt Thin Film Resistors with Ti and PtxOy Interlayers. J. Phys. Conf. Ser. 2021, 2002, 012002. [Google Scholar] [CrossRef]
- Firebaugh, S.L.; Jensen, K.F.; Schmidt, M.A. Investigation of high-temperature degradation of platinum thin films with an in situ resistance measurement apparatus. J. Microelectr. Syst. 1998, 7, 128–135. [Google Scholar] [CrossRef]
- Jiran, E.; Carl, V. Thompson. Capillary instabilities in thin films. J. Electron. Mater. 1990, 19, 1153–1160. [Google Scholar] [CrossRef]
- Berry, R.J. Effect of Pt Oxidation on Pt Resistance Thermometry. Metrologia 1980, 16, 117–126. [Google Scholar] [CrossRef]
- Niu, Y.L.; Song, K.; Sun, J.P.; Xu, L.I.; Meng, Y.E. Research on the Decomposition Experiment of PtO2 in Air Environment. Acta Metrol. Sin. 2018, 39, 33–38. [Google Scholar]
- Mooiman, M.B.; Sole, K.C.; Dinham, N. The Precious Metals Industry: Global Challenges, Responses, and Prospects. In Metal Sustainability: Global Challenges, Consequences, and Prospects; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Joseph, R.D. Precious Metals and Alloys. In Metals Handbook Desk Edition; ASM International: Almere, The Netherlands, 1998. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, T.; Pandey, R.K.; Pathak, S.; Kumar, R. Roughening and Sputtering Kinetics of Pt Thin Films at different Angles of Ion Irradiation. Mater. Lett. 2021, 303, 130474. [Google Scholar] [CrossRef]
- Ruina, J.; Kunlun, W.; Yanqing, X.; Hui, S.; Jianhong, G.; Lan, Y.; Yong, W. Enhancing the temperature coefficient of resistance of Pt thin film resistance-temperature-detector by short-time annealing. Ceram. Int. 2023, 49, 12596–12603. [Google Scholar]
- Zhang, Y.; Ba, D.; Liu, X.; Hang, G. Latest Progress in Development of Thin Film Temperature Sensors. Vac. Sci. Technol. 2003, 23, 334–1124. [Google Scholar]

| Film | PtxOy Adhesion Layer | Pt Film | ||||
|---|---|---|---|---|---|---|
| Oxygen partial pressure | 5% | 10% | 15% | 20% | 50% | - |
| Deposition rate (nm/min) | 33 ± 4 | 34 ± 3 | 36 ± 3 | 55 ± 3 | 60 ± 4 | 48 ± 3 |
| Oxygen Partial Pressure | 5% | 10% | 15% | 20% | 50% | |
|---|---|---|---|---|---|---|
| Resistance at RT (as-deposited) (Ω) | 130.6 ± 3.5 | 146.9 ± 4.3 | 177.5 ± 4.3 | 196.3 ± 4.8 | 155.4 ± 4.6 | |
| Annealing temperature and resistance change rate | 800 °C | −19.8% | −32.4% | −41.0% | −45.9% | −47.9% |
| 900 °C | −19.0% | −32.9% | −34.3% | −44.9% | −49.0% | |
| 1000 °C | −17.6% | −32.6% | −34.1% | −43.7% | −49.1% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Y.; Zhao, N.; Ruan, Y.; Sun, L.; Zhang, C. Effects of Oxygen Partial Pressure and Thermal Annealing on the Electrical Properties and High-Temperature Stability of Pt Thin-Film Resistors. Chemosensors 2023, 11, 285. https://doi.org/10.3390/chemosensors11050285
Pang Y, Zhao N, Ruan Y, Sun L, Zhang C. Effects of Oxygen Partial Pressure and Thermal Annealing on the Electrical Properties and High-Temperature Stability of Pt Thin-Film Resistors. Chemosensors. 2023; 11(5):285. https://doi.org/10.3390/chemosensors11050285
Chicago/Turabian StylePang, Yawen, Nan Zhao, Yong Ruan, Limin Sun, and Congchun Zhang. 2023. "Effects of Oxygen Partial Pressure and Thermal Annealing on the Electrical Properties and High-Temperature Stability of Pt Thin-Film Resistors" Chemosensors 11, no. 5: 285. https://doi.org/10.3390/chemosensors11050285





