Visual Measurement of Fumonisin B1 with Bipolar Electrodes Array-Based Electrochemiluminescence Biosensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments
2.3. Synthesis of Zr-MOFs and MB@Zr-MOFs
2.4. Preparation of ITO Array Electrodes
2.5. Preparation of Au/ITO Array Electrodes
2.6. Preparation of DNA Mix
2.7. Fabrication of ECL Biosensor
2.8. ECL Imaging
2.9. Real Sample Detection
3. Results and Discussion
3.1. Principle of the BPE- ECL Biosensors Array for FB1 Detection
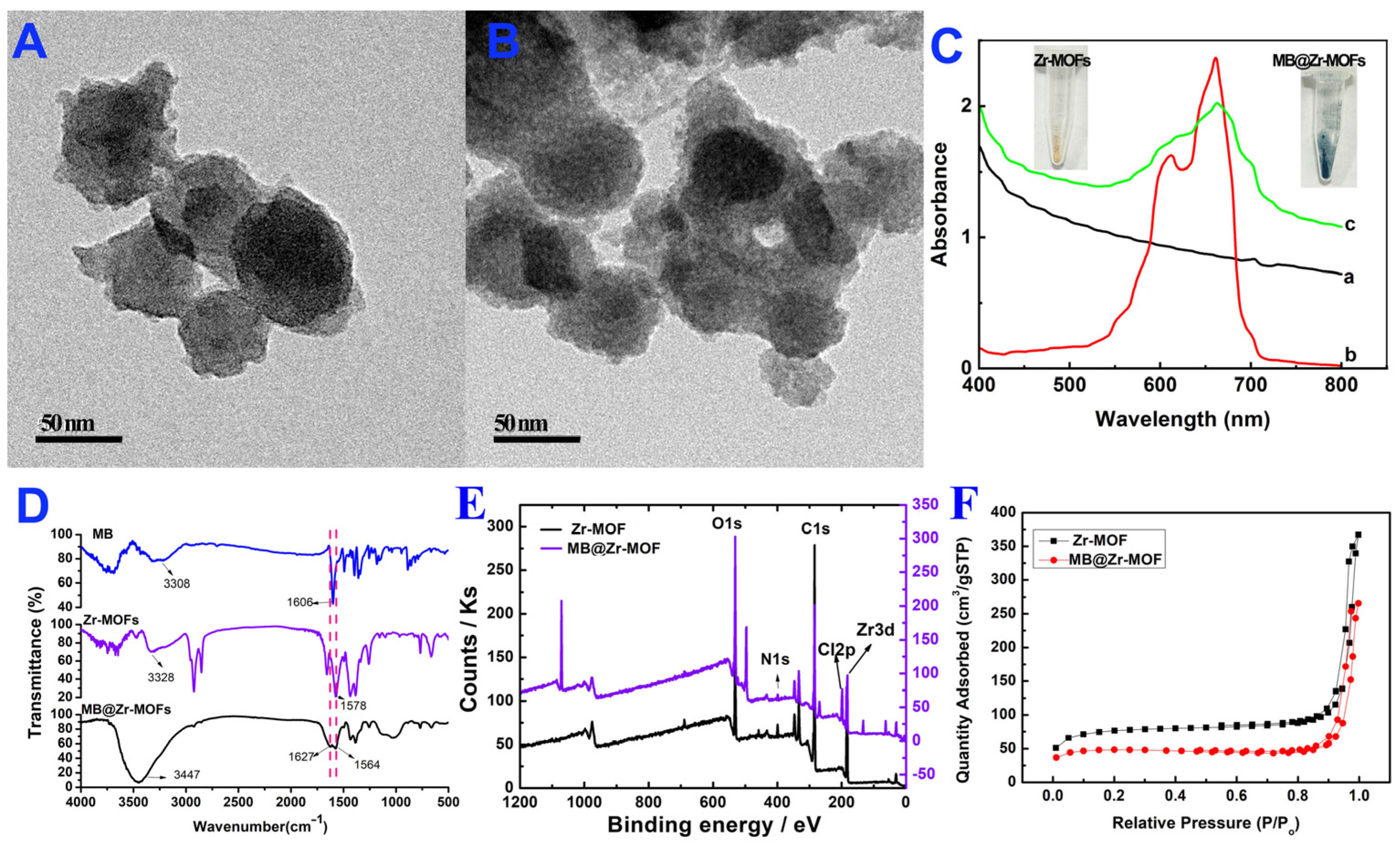
3.2. Characterization of MB@Zr-MOFs
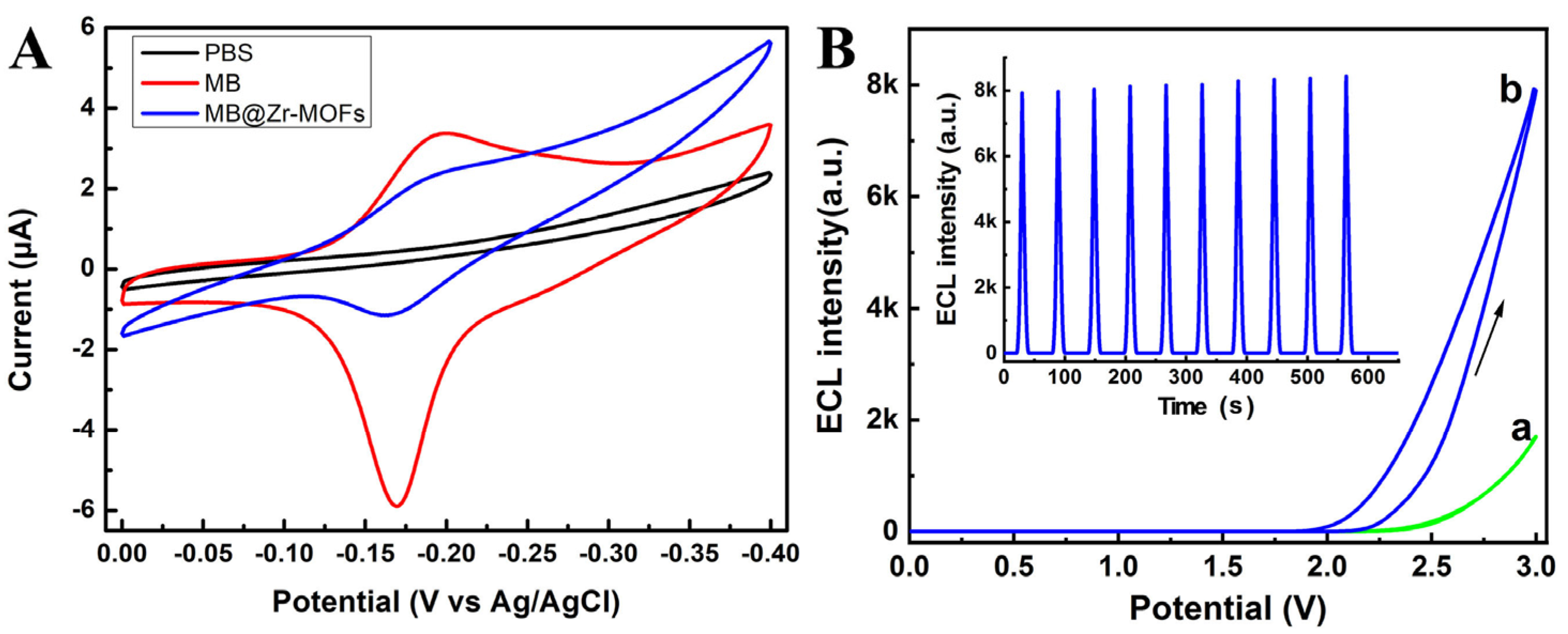
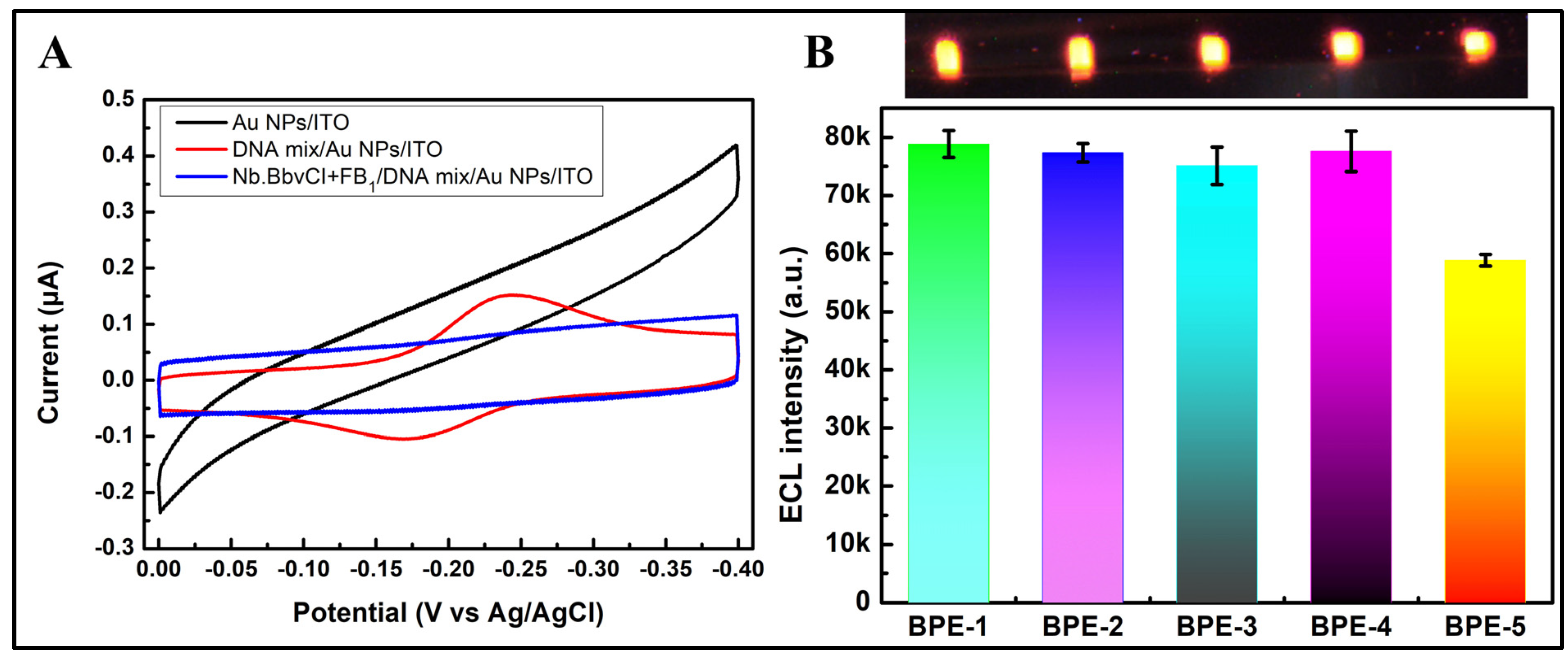
3.3. Feasibility of the BPE-ECL Device for FB1 Assay
3.4. Optimization of Experimental Conditions
3.5. Calibration Curve for FB1 Measurement
3.6. Application of the BPE Array Biosensor in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.C.; Luo, K.X.; Zhu, Q.X.; Peng, J.H.; Liu, C.; Wang, X.Y.; Li, S.J.; Zhang, H.Y. The natural occurrence, toxicity mechanisms and management strategies of Fumonisin B1: A review. Environ. Pollut. 2023, 320, 121065. [Google Scholar] [CrossRef]
- Lumsangkul, C.; Chiang, H.I.; Lo, N.W.; Fan, Y.K.; Ju, J.C. Developmental Toxicity of Mycotoxin Fumonisin B-1 in Animal Embryogenesis: An Overview. Toxins 2019, 11, 114. [Google Scholar] [CrossRef] [Green Version]
- Hao, K.; Suryoprabowo, S.; Hong, T.; Song, S.; Liu, L.; Zheng, Q.; Kuang, H. Immunochromatographic strip for ultrasensitive detection of fumonisin B-1. Food Agric. Immunol. 2018, 29, 699–710. [Google Scholar] [CrossRef] [Green Version]
- He, D.; Wu, Z.; Cui, B.; Xu, E. Aptamer and gold nanorod-based fumonisin B1 assay using both fluorometry and SERS. Microchim. Acta 2020, 187, 215. [Google Scholar] [CrossRef]
- Molina-Pintor, I.B.; Ruiz-Arias, M.A.; Guerrero-Flores, M.C.; Rojas-Garcia, A.E.; Barron-Vivanco, B.S.; Medina-Diaz, I.M.; Bernal-Hernandez, Y.Y.; Ortega-Cervantes, L.; Rodriguez-Cervantes, C.H.; Ramos, A.J.; et al. Preliminary survey of the occurrence of mycotoxins in cereals and estimated exposure in a northwestern region of Mexico. Int. J. Environ. Health Res. 2022, 32, 2271–2285. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, T.; Ghazi, T.; Chuturgoon, A.A. Molecular and epigenetic modes of Fumonisin B-1 mediated toxicity and carcinogenesis and detoxification strategies. Crit. Rev. Toxicol. 2021, 51, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Tang, Z.; Jiang, K.; Huang, Q.; Meng, J.; Nie, D.; Zhao, Z. Dual-target electrochemical aptasensor based on co-reduced molybdenum disulfide and Au NPs (rMoS2-Au) for multiplex detection of mycotoxins. Biosens. Bioelectron. 2020, 150, 111894. [Google Scholar] [CrossRef]
- De Rycke, E.; Foubert, A.; Dubruel, P.; Bol’hakov, O.I.; De Saeger, S.; Beloglazova, N. Recent advances in electrochemical monitoring of zearalenone in diverse matrices. Food Chem. 2021, 353, 129342. [Google Scholar] [CrossRef]
- Jiang, F.; Li, P.; Zong, C.; Yang, H. Surface-plasmon-coupled chemiluminescence amplification of silver nanoparticles modified immunosensor for high-throughput ultrasensitive detection of multiple mycotoxins. Anal. Chim. Acta 2020, 1114, 58–65. [Google Scholar] [CrossRef]
- Zong, C.; Jiang, F.; Wang, X.; Li, P.; Xu, L.; Yang, H. Imaging sensor array coupled with dual-signal amplification strategy for ultrasensitive chemiluminescence immunoassay of multiple mycotoxins. Biosens. Bioelectron. 2021, 177, 112998. [Google Scholar] [CrossRef]
- Jiang, P.; Luo, L.; Liu, X.; Zhao, W.; Bi, X.; Luo, L.; Li, L.; You, T. A potential-resolved ratiometric electrochemiluminescence aptasensor for Pb2+: Gold nanoclusters and amino-terminated perylene derivative as both emitters and resonance energy transfer donor-acceptor pair. Sens. Actuators B Chem. 2023, 386, 133758. [Google Scholar] [CrossRef]
- Luo, L.; Liu, X.; Bi, X.; Li, L.; You, T. Dual-quenching effects of methylene blue on the luminophore and co-reactant: Application for electrochemiluminescent-electrochemical ratiometric zearalenone detection. Biosens. Bioelectron. 2023, 222, 114991. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xiong, C.; Li, J.; Deng, Q.; Zhang, X.; Wang, S.; Chen, M.-M. High-performance electrochemiluminescence sensors based on ultra-stable perovskite quantum dots@ZIF-8 composites for aflatoxin B1 monitoring in corn samples. Food Chem. 2023, 410, 135325. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Wang, C.; Xu, E.; Chen, J.; Xu, X.; Wei, W.; Liu, S. A simple and sensitive electrochemiluminescence aptasensor for determination of ochratoxin A based on a nicking endonuclease-powered DNA walking machine. Food Chem. 2019, 282, 141–146. [Google Scholar] [CrossRef]
- Xi, M.; Wu, Z.; Luo, Z.; Ling, L.; Xu, W.; Xiao, R.; Wang, H.; Fang, Q.; Hu, L.; Gu, W.; et al. Water Activation for Boosting Electrochemiluminescence. Angew. Chem. Int. Ed. Engl. 2023, 62, e202302166. [Google Scholar] [CrossRef]
- Qin, X.; Gao, J.; Jin, H.-J.; Li, Z.-Q.; Xia, X.-H. Closed Bipolar Electrode Array for Optical Reporting Reaction-Coupled Electrochemical Sensing and Imaging. Chem. A Eur. J. 2022, 29, e202202687. [Google Scholar]
- Liu, Y.; Cheng, Q.-Y.; Gao, H.; Chen, H.-Y.; Xu, J.-J. Microfluidic Gradient Culture Arrays for Cell Pro-oxidation Analysis Using Bipolar Electrochemiluminescence. Anal. Chem. 2023, 95, 8376–8383. [Google Scholar] [CrossRef]
- Anderson, T.J.; Defnet, P.A.; Zhang, B. Electrochemiluminescence (ECL)-Based Electrochemical Imaging Using a Massive Array of Bipolar Ultramicroelectrodes. Anal. Chem. 2020, 92, 6748–6755. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, N.; Pan, J.-B.; Song, J.; Zhao, W.; Chen, H.-Y.; Xu, J.-J. Bipolar Electrode Array for Multiplexed Detection of Prostate Cancer Biomarkers. Anal. Chem. 2022, 94, 3005–3012. [Google Scholar] [CrossRef]
- Zhang, R.; Liang, Y.; Su, Y.; Lai, W.; Zhang, C. Cloth-based closed bipolar electrochemiluminescence DNA sensors (CCBEDSs): A new class of electrochemiluminescence gene sensors. J. Lumin. 2021, 238, 118209. [Google Scholar] [CrossRef]
- Li, X.; Du, Y.; Wang, H.; Ma, H.; Wu, D.; Ren, X.; Wei, Q.; Xu, J.-J. Self-Supply of H2O2 and O2 by Hydrolyzing CaO2 to Enhance the Electrochemiluminescence of Luminol Based on a Closed Bipolar Electrode. Anal. Chem. 2020, 92, 12693–12699. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Wang, L.; Zhu, J.; Zhao, J.; Zong, H.; Chen, C. Bipolar electrode ratiometric electrochemiluminescence biosensing analysis based on boron nitride quantum dots and biological release system. Biosens. Bioelectron. 2021, 191, 113393. [Google Scholar] [CrossRef] [PubMed]
- Khoshfetrat, S.M.; Bagheri, H.; Mehrgardi, M.A. Visual electrochemiluminescence biosensing of aflatoxin M1 based on luminol-functionalized, silver nanoparticle-decorated graphene oxide. Biosens. Bioelectron. 2018, 100, 382–388. [Google Scholar] [CrossRef]
- Wang, F.; Fu, C.; Huang, C.; Li, N.; Wang, Y.; Ge, S.; Yu, J. Paper-based closed Au-Bipolar electrode electrochemiluminescence sensing platform for the detection of miRNA-155. Biosens. Bioelectron. 2020, 150, 111917. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Tao, Q.; OuYang, S.; Wang, M.; Liu, Y.; Xiong, X.; Liu, S. Biocathodes reducing oxygen in BPE-ECL system for rapid screening of E. coli O157:H7. Biosens. Bioelectron. 2023, 221, 114940. [Google Scholar] [CrossRef]
- Che, Z.-Y.; Wang, X.-Y.; Ma, X.; Ding, S.-N. Bipolar electrochemiluminescence sensors: From signal amplification strategies to sensing formats. Coord. Chem. Rev. 2021, 446, 214116. [Google Scholar] [CrossRef]
- Jia, Y.-L.; Xu, C.-H.; Li, X.-Q.; Chen, H.-Y.; Xu, J.-J. Visual analysis of Alzheimer disease biomarker via low-potential driven bipolar electrode. Anal. Chim. Acta 2023, 1251, 340980. [Google Scholar] [CrossRef]
- Ge, S.; Zhao, J.; Wang, S.; Lan, F.; Yan, M.; Yu, J. Ultrasensitive electrochemiluminescence assay of tumor cells and evaluation of H2O2 on a paper-based closed-bipolar electrode by in-situ hybridization chain reaction amplification. Biosens. Bioelectron. 2018, 102, 411–417. [Google Scholar] [CrossRef]
- Zhang, S.; Rong, F.; Guo, C.; Duan, F.; He, L.; Wang, M.; Zhang, Z.; Kang, M.; Du, M. Metal–organic frameworks (MOFs) based electrochemical biosensors for early cancer diagnosis in vitro. Coord. Chem. Rev. 2021, 439, 213948. [Google Scholar] [CrossRef]
- He, B.S.; Dong, X.Z. Nb. BbvCI powered DNA walking machine-based Zr-MOFs-labeled electrochemical aptasensor using Pt@AuNRs/Fe-MOFs/PEI-rGO as electrode modification material for patulin detection. Chem. Eng. J. 2021, 405, 126642. [Google Scholar] [CrossRef]
- He, B.; Dong, X. Hierarchically porous Zr-MOFs labelled methylene blue as signal tags for electrochemical patulin aptasensor based on ZnO nano flower. Sens. Actuators B Chem. 2019, 294, 192–198. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Qiao, J.; Wu, M. Closed bipolar electrochemical biosensor based on ohmic loss mechanism for noncontact measurements. J. Electroanal. Chem. 2020, 860, 113873. [Google Scholar] [CrossRef]
- Yu, H.H.; Yang, H.J.; Liu, W.S.; Jin, L.S.; Jin, B.; Wu, M.S. Novel electrochemiluminescence biosensor of fumonisin B1 detection using MWCNTs-PDMS flexible bipolar electrode. Talanta 2023, 257, 124379. [Google Scholar] [CrossRef]
- Li, Y.C.; Zheng, C.R.; Liu, S.; Huang, L.; Fang, T.S.; Li, J.X.Z.; Xu, F.; Li, F. Smart Glove Integrated with Tunable MWNTs/PDMS Fibers Made of a One-Step Extrusion Method for Finger Dexterity, Gesture, and Temperature Recognition. ACS Appl. Mater. Interfaces 2020, 12, 23764–23773. [Google Scholar] [CrossRef]
- Ren, C.C.; Li, H.M.; Lu, X.T.; Qian, J.; Zhu, M.Y.; Chen, W.; Liu, Q.; Hao, N.; Li, H.N.; Wang, K. A disposable aptasensing device for label-free detection of fumonisin B1 by integrating PDMS film-based micro-cell and screen-printed carbon electrode. Sens. Actuators B Chem. 2017, 251, 192–199. [Google Scholar] [CrossRef]
- Al-Hazmi, G.A.A.; El-Zahhar, A.A.; El-Desouky, M.G.; El-Bindary, M.A.; El-Bindary, A.A. Adsorption of industrial dye onto a zirconium metal-organic framework: Synthesis, characterization, kinetics, thermodynamics, and DFT calculations. J. Coord. Chem. 2022, 75, 1203–1229. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Asadpour-Zeynali, K.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Non-enzymatic hydrogen peroxide sensor based on graphene quantum dots-chitosan/methylene blue hybrid nanostructures. Electrochimica Acta 2017, 246, 303–314. [Google Scholar] [CrossRef]




| Samples | Added (pg/mL) | Founded (ng/mL) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| Corn | 0 | Not detected | - | |
| 0.500 | 0.5207 ± 0.0104 | 2.0 | 104.1 | |
| 2.500 | 2.490 ± 0.085 | 3.4 | 99.6 | |
| 5.000 | 5.184 ± 0.454 | 8.7 | 103.7 | |
| Peanut | 0 | Not detected | - | |
| 0.500 | 0.4958 ± 0.0091 | 1.8 | 99.2 | |
| 2.500 | 2.646 ± 0.251 | 9.5 | 105.9 | |
| 5.000 | 5.530 ± 0.806 | 7.3 | 110.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Yu, H.; Liu, W.; Xiao, Z.; Yang, H.; Jin, B.; Wu, M. Visual Measurement of Fumonisin B1 with Bipolar Electrodes Array-Based Electrochemiluminescence Biosensor. Chemosensors 2023, 11, 451. https://doi.org/10.3390/chemosensors11080451
Jin L, Yu H, Liu W, Xiao Z, Yang H, Jin B, Wu M. Visual Measurement of Fumonisin B1 with Bipolar Electrodes Array-Based Electrochemiluminescence Biosensor. Chemosensors. 2023; 11(8):451. https://doi.org/10.3390/chemosensors11080451
Chicago/Turabian StyleJin, Longsheng, Huihui Yu, Weishuai Liu, Ziying Xiao, Haijian Yang, Bing Jin, and Meisheng Wu. 2023. "Visual Measurement of Fumonisin B1 with Bipolar Electrodes Array-Based Electrochemiluminescence Biosensor" Chemosensors 11, no. 8: 451. https://doi.org/10.3390/chemosensors11080451




