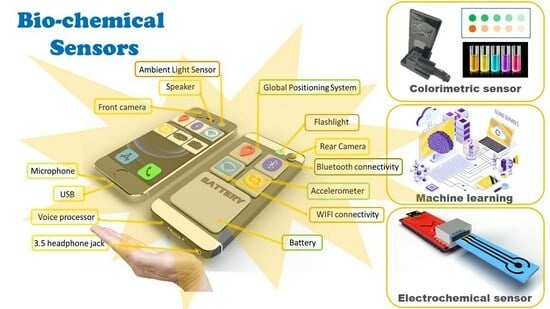
Smartphone-Based Portable Bio-Chemical Sensors: Exploring Recent Advancements
Abstract
:1. Introduction
2. Fundamental Concepts Underlying Optical Sensing Using a Smartphone
2.1. White LED Flash
2.2. Built-In Smartphone Digital Camera
2.3. Ambient Light Sensor of Smartphone
2.4. Utilizing Mobile Phone’s Optical System for Colorimetry and Spectrophotometry
3. Fundamental Concepts Underlying Electrochemical Sensing Using a Smartphone
3.1. Conductive Sensor
3.2. Potentiometric Sensor
3.3. Amperometric Sensor
3.4. Micro-USB Port, Bluetooth, and NFC
4. Applications of Bio-Chemical Optical Sensors Using a Smartphone
4.1. Smartphone-Camera-Based Biochemical Sensors
4.1.1. Healthcare/Biomedical Sensors
4.1.2. Cell Analysis
4.1.3. Chemical Sensors

4.1.4. Sensors with the Assistance of Deep Learning
4.2. Ambient Light Sensor of Smartphone-Based Biosensors
5. Applications of Bio-Chemical Electrochemical Sensors Using a Smartphone
6. Other Smartphone-Based Sensors for Biometrics
6.1. Acoustic Sensor
6.2. Accelerometer Sensors
7. Perspective and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Yadav, P.; Yadav, L.; Laddha, H.; Agarwal, M.; Gupta, R. Upsurgence of smartphone as an economical, portable, and consumer-friendly analytical device/interface platform for digital sensing of hazardous environmental ions. Trends Environ. Anal. Chem. 2022, 36, e00177. [Google Scholar] [CrossRef]
- Sun, B.R.; Zhou, A.G.; Li, X.; Yu, H.-Z. Development and Application of Mobile Apps for Molecular Sensing: A Review. ACS Sens. 2021, 6, 1731–1744. [Google Scholar] [CrossRef] [PubMed]
- Rasitanon, N.; Ittisoponpisan, S.; Kaewpradub, K.; Jeerapan, I. Wearable Electrodes for Lactate: Applications in Enzyme-Based Sensors and Energy Biodevices. Anal. Sens. 2023, 3, e202200066. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Montiel, V.R.-V.; Vargas, E.; Teymourian, H.; Wang, J. Wearable and Mobile Sensors for Personalized Nutrition. ACS Sens. 2021, 6, 1745–1760. [Google Scholar] [CrossRef] [PubMed]
- Son, M.H.; Park, S.W.; Sagong, H.Y.; Jung, Y.K. Recent Advances in Electrochemical and Optical Biosensors for Cancer Biomarker Detection. BioChip J. 2023, 17, 44–67. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, J.; Ouyang, X.; Liao, Y.; Feng, H.; Yu, J.; Chen, L.; Lu, Y.; Yi, Y.; Tang, L. Hollow NiCo@C Nanozyme-Embedded Paper-Based Colorimetric Aptasensor for Highly Sensitive Antibiotic Detection on a Smartphone Platform. Anal. Chem. 2022, 94, 16768–16777. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, K.; Wang, R.; Liu, T.; Wu, Z.; Ding, L.; Fang, Y. Dual-Mode Optical Sensor Array for Detecting and Identifying Perillaldehyde in Solution Phase and Plant Leaf with Smartphone. ACS Appl. Mater. Interfaces 2022, 14, 53323–53330. [Google Scholar] [CrossRef]
- Chu, S.; Wang, H.; Ling, X.; Yu, S.; Yang, L.; Jiang, C. A Portable Smartphone Platform Using a Ratiometric Fluorescent Paper Strip for Visual Quantitative Sensing. ACS Appl. Mater. Interfaces 2020, 12, 12962–12971. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Chu, S.; Liu, B.; Zhang, Q.; Zou, L.; Yu, S.; Jiang, C. Semiquantitative Visual Detection of Lead Ions with a Smartphone via a Colorimetric Paper-Based Analytical Device. Anal. Chem. 2019, 91, 9292–9299. [Google Scholar] [CrossRef]
- Pearsall, T. Photonics Essentials: An Introduction with Experiments; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Dutta, P.S.; Liotta, K.M. Full Spectrum White LEDs of Any Color Temperature with Color Rendering Index Higher than 90 Using a Single Broad-Band Phosphor. ECS J. Solid State Sci. Technol. 2018, 7, R3194. [Google Scholar] [CrossRef]
- Spring, K.R.; Davidson, M.W. Sources of Visible Light. 2023. Available online: https://micro.magnet.fsu.edu/primer/lightandcolor/lightsourcesintro.html (accessed on 22 June 2023).
- Spring, K.R.; Fellers, T.J.; Davidson, M.W. Non-Coherent Light Sources for Confocal Microscopy. Available online: https://www.olympus-lifescience.com/ko/microscope-resource/primer/techniques/confocal/noncoherentsources/ (accessed on 22 June 2023).
- Difference between Photovoltaic and Photoconductive Mode Photodiode. 2023. Available online: https://www.rfwireless-world.com/Terminology/Difference-between-Photovoltaic-mode-and-Photoconductive-mode.html (accessed on 14 August 2023).
- Ted_Pella, I. Background Information on CCD and CMOS Technology. 2023. Available online: https://www.tedpella.com/cameras_html/ccd_cmos.aspx (accessed on 22 June 2023).
- Turchetta, R.; Spring, K.R.; Davidson, M.W. Introduction to CMOS Image Sensors. Available online: https://micro.magnet.fsu.edu/primer/digitalimaging/cmosimagesensors.html (accessed on 22 June 2023).
- Grube, B. Ambient Light Sensor Calibration with Tunable Light Sources. Available online: https://www.energetiq.com/application-note-ambient-light-sensor-calibration-with-tunable-light-sources#:~:text=Ambient%20light%20sensors%20are%20used,impact%20on%20the%20user%20experience. (accessed on 22 June 2023).
- Enlitech. A Complete Introduction to Ambient Light Sensors (ALS)—Starting from the Working Principle, How to Test, Important Performance Parameters, Application Scope, Major Manufacturers and Industrial Structure of ALS. Available online: https://enlitech-sensor.com/blog-ambient-light-sensor-01/ (accessed on 13 June 2023).
- Zhang, R.; Song, B.; Yuan, J. Bioanalytical methods for hypochlorous acid detection: Recent advances and challenges. TrAC Trends Anal. Chem. 2018, 99, 1–33. [Google Scholar] [CrossRef]
- Chaudhary, M.; Kumar, A.; Devi, A.; Singh, B.P.; Malhotra, B.D.; Singhal, K.; Shukla, S.; Ponnada, S.; Sharma, R.K.; Vega-Olivencia, C.A.; et al. Prospects of nanostructure-based electrochemical sensors for drug detection: A review. Mater. Adv. 2023, 4, 432–457. [Google Scholar] [CrossRef]
- Di Francia, G.; Alfano, B.; Massera, E.; Miglietta, M.L.; Polichetti, T. Chemical Sensors: Conductometric Gas Sensors. In Encyclopedia of Sensors and Biosensors, 1st ed.; Narayan, R., Ed.; Elsevier: Oxford, UK, 2023; pp. 189–208. [Google Scholar]
- Yin, T.; Qin, W. Applications of nanomaterials in potentiometric sensors. TrAC Trends Anal. Chem. 2013, 51, 79–86. [Google Scholar] [CrossRef]
- Imanzadeh, H.; Sefid-Sefidehkhan, Y.; Afshary, H.; Afruz, A.; Amiri, M. Nanomaterial-based electrochemical sensors for detection of amino acids. J. Pharm. Biomed. Anal. 2023, 230, 115390. [Google Scholar] [CrossRef] [PubMed]
- Arduino Home. 2023. Available online: https://www.arduino.cc/ (accessed on 22 June 2023).
- USB Hardware. 2023. Available online: https://en.wikipedia.org/wiki/USB_hardware (accessed on 22 June 2023).
- Bluetooth. 2023. Available online: https://en.wikipedia.org/wiki/Bluetooth (accessed on 22 June 2023).
- Near Field Communication. 2023. Available online: https://en.wikipedia.org/wiki/Near-field_communication (accessed on 22 June 2023).
- Thang, L.T.H.; Han, W.; Shin, J.; Shin, J.H. Disposable, pressure-driven, and self-contained cartridge with pre-stored reagents for automated nucleic acid extraction. Sens. Actuators B 2023, 375, 132948. [Google Scholar] [CrossRef]
- Han, W.; Shin, J.; Ho Shin, J. Low-cost, open-source contact angle analyzer using a mobile phone, commercial tripods and 3D printed parts. HardwareX 2022, 12, e00327. [Google Scholar] [CrossRef]
- Park, S.B.; Shin, J.H. Pressed Lateral Flow Assay Strips for Flow Delay-Induced Signal Enhancement in Lateral Flow Assay Strips. BioChip J. 2022, 16, 480–489. [Google Scholar] [CrossRef]
- Erdem, A.; Yildiz, E.; Senturk, H.; Maral, M. Implementation of 3D printing technologies to electrochemical and optical biosensors developed for biomedical and pharmaceutical analysis. J. Pharm. Biomed. Anal. 2023, 230, 115385. [Google Scholar] [CrossRef]
- Bhandari, S.; Krishnanand; Singh, A.; Taufik, M. 3D printing methods and materials for sensor fabrication. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef]
- Wang, L.-J.; Chang, Y.-C.; Sun, R.; Li, L. A multichannel smartphone optical biosensor for high-throughput point-of-care diagnostics. Biosens. Bioelectron. 2017, 87, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Ngoc Nghia, N.; The Huy, B.; Thanh Phong, P.; Han, J.S.; Kwon, D.H.; Lee, Y.-I. Simple fluorescence optosensing probe for spermine based on ciprofloxacin-Tb3+ complexation. PLoS ONE 2021, 16, e0251306. [Google Scholar] [CrossRef] [PubMed]
- Nghia, N.N.; Huy, B.T.; Khanh, D.N.N.; Van Cuong, N.; Li, H.; Lee, Y.-I. Straightforward smartphone assay for quantifying tannic acid in beverages based on colour change of Eu3+/polyethyleneimine complex. Food Chem. 2023, 410, 135466. [Google Scholar] [CrossRef] [PubMed]
- Nghia, N.N.; The Huy, B.; Lee, Y.-I. Paper-based colorimetric probe for highly sensitive detection of folic acid based on open-ring form amplification of rhodamine B derivative. J. Ind. Eng. Chem. 2020, 81, 352–359. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, S.; Lu, L.; Li, C.; Wang, F. A dual-mode ratiometric fluorescence and smartphone-assisted colorimetric sensing platform based on bifunctional Fe,Co-CQD for glucose analysis at physiological pH. Anal. Chim. Acta 2023, 1239, 340701. [Google Scholar] [CrossRef]
- Duan, W.; Cheng, J.; Guo, J. Smartphone-based photochemical sensor for multiplex determination of glucose, uric acid, and total cholesterol in fingertip blood. Analyst 2022, 147, 3285–3290. [Google Scholar] [CrossRef]
- Tang, K.; Chen, Y.; Wang, X.; Zhou, Q.; Lei, H.; Yang, Z.; Zhang, Z. Smartphone-integrated tri-color fluorescence sensing platform based on acid-sensitive fluorescence imprinted polymers for dual-mode visual intelligent detection of ibuprofen, chloramphenicol and florfenicol. Anal. Chim. Acta 2023, 1260, 341174. [Google Scholar] [CrossRef]
- Liu, F.; Chen, R.; Song, W.; Li, L.; Lei, C.; Nie, Z. Modular Combination of Proteolysis-Responsive Transcription and Spherical Nucleic Acids for Smartphone-Based Colorimetric Detection of Protease Biomarkers. Anal. Chem. 2021, 93, 3517–3525. [Google Scholar] [CrossRef]
- Hong, K.-I.; Yang, A.H.; Kim, Y.; Ranathunga, M.; Kim, U.; Joo, C.; Jang, W.-D. Simple and effective detection of water in hygroscopic organic solvents using smartphone-based device with dithiophene-conjugated benzothiazole. Sens. Actuators B 2023, 387, 133756. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Hu, Y.; Moreddu, R.; Fan, Z.; Jiang, N.; Yetisen, A.K. Smartphone-based fluorescent sensing platforms for point-of-care ocular lactoferrin detection. Sens. Actuators B 2023, 378, 133128. [Google Scholar] [CrossRef]
- Biswas, S.K.; Chatterjee, S.; Bandyopadhyay, S.; Kar, S.; Som, N.K.; Saha, S.; Chakraborty, S. Smartphone-Enabled Paper-Based Hemoglobin Sensor for Extreme Point-of-Care Diagnostics. ACS Sens. 2021, 6, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, H.; Bayram, A.; Zhang, T.; Cai, Q.; Xie, C.; Huynh, H.; Peerzade, S.A.M.A.; Kahn, J.S.; Qin, Z. Gold Nanourchins Improve Virus Targeting and Plasmonic Coupling for Virus Diagnosis on a Smartphone Platform. ACS Sens. 2022, 7, 3741–3752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Z.; Wei, Q. Smartphone-based cytometric biosensors for point-of-care cellular diagnostics. Nanotechnol. Precis. Eng. 2020, 3, 32–42. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, L.; Cai, G.; Liu, N.; Liao, M.; Li, Y.; Zhang, X.; Lin, J. A microfluidic biosensor for online and sensitive detection of Salmonella typhimurium using fluorescence labeling and smartphone video processing. Biosens. Bioelectron. 2019, 140, 111333. [Google Scholar] [CrossRef]
- Ulep, T.-H.; Zenhausern, R.; Gonzales, A.; Knoff, D.S.; Lengerke Diaz, P.A.; Castro, J.E.; Yoon, J.-Y. Smartphone based on-chip fluorescence imaging and capillary flow velocity measurement for detecting ROR1+ cancer cells from buffy coat blood samples on dual-layer paper microfluidic chip. Biosens. Bioelectron. 2020, 153, 112042. [Google Scholar] [CrossRef]
- Needs, S.H.; Osborn, H.M.I.; Edwards, A.D. Counting bacteria in microfluidic devices: Smartphone compatible ‘dip-and-test’ viable cell quantitation using resazurin amplified detection in microliter capillary arrays. J. Microbiol. Methods 2021, 187, 106199. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chun, H.J.; Lee, K.W.; Yoon, H.C. Smartphone-integrated urinary CTX-II immunosensor based on wavelength filtering from chromogenic reaction. Biosens. Bioelectron. 2020, 150, 111932. [Google Scholar] [CrossRef]
- Freeman, E.E.; Semeere, A.; Osman, H.; Peterson, G.; Rajadhyaksha, M.; González, S.; Martin, J.N.; Anderson, R.R.; Tearney, G.J.; Kang, D. Smartphone confocal microscopy for imaging cellular structures in human skin in vivo. Biomed. Opt. Express 2018, 9, 1906–1915. [Google Scholar] [CrossRef]
- Hong, X.; Nagarajan, V.K.; Mugler, D.H.; Yu, B. Smartphone microendoscopy for high resolution fluorescence imaging. J. Innov. Opt. Health Sci. 2016, 09, 1650046. [Google Scholar] [CrossRef]
- Gong, C.; Kulkarni, N.; Zhu, W.; Nguyen, C.D.; Curiel-Lewandrowski, C.; Kang, D. Low-cost, high-speed near infrared reflectance confocal microscope. Biomed. Opt. Express 2019, 10, 3497–3505. [Google Scholar] [CrossRef]
- Wang, H.; Zou, Q.; Xiang, Y.; Yang, J.; Xu, Z.; Yang, W.; Wu, Y.; Wu, J.; Liu, D.; Hu, N.; et al. A smart tablet-phone-based system using dynamic light modulation for highly sensitive colorimetric biosensing. Talanta 2023, 252, 123862. [Google Scholar] [CrossRef] [PubMed]
- Shafizadeh, M.; Abbasi-Moayed, S.; Hamzei, Z.; Keshavarz, A.; Yousefi, S.; Hormozi-Nezhad, M.R.; Golmohammadi, H. Chlorophyll-based wicking sensing bioplatform coupled with a smartphone-based sample-to-answer analytical device for on-site detection of picric acid. Biosens. Bioelectron. X 2022, 11, 100150. [Google Scholar] [CrossRef]
- Duan, N.; Chen, X.; Lin, X.; Ying, D.; Wang, Z.; Yuan, W.; Wu, S. Paper-based fluorometric sensing of malachite green using synergistic recognition of aptamer-molecularly imprinted polymers and luminescent metal–organic frameworks. Sens. Actuators B 2023, 384, 133665. [Google Scholar] [CrossRef]
- Ma, J.; Guan, Y.; Xing, F.; Wang, Y.; Li, X.; Yu, Q.; Yu, X. Smartphone-based chemiluminescence detection of aflatoxin B1 via labelled and label-free dual sensing systems. Food Chem. 2023, 413, 135654. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Liu, Z.; Xu, N.; Jiang, L.; Yang, M.; Yi, C. A Smartphone-Based Sensing System for On-Site Quantitation of Multiple Heavy Metal Ions Using Fluorescent Carbon Nanodots-Based Microarrays. ACS Sens. 2020, 5, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Ke, Y.; Yue, C.; Xie, P.; Sheng, R.; Zeng, L. 5G smartphone-adaptable fluorescence sensing platform for simultaneous detection of toxic formaldehyde and phosgene in different emission channels. Dyes Pigm. 2022, 207, 110782. [Google Scholar] [CrossRef]
- Liu, J.; Zhan, Y.; Qiu, B.; Lin, Z.; Guo, L. Portable Smartphone Platform Based on Aggregation-Induced Enhanced Emission Carbon Dots for Ratiometric Quantitative Sensing of Fluoride Ions. ACS Sens. 2023, 8, 884–892. [Google Scholar] [CrossRef]
- Shan, Y.; Wang, B.; Huang, H.; Jian, D.; Wu, X.; Xue, L.; Wang, S.; Liu, F. On-site quantitative Hg2+ measurements based on selective and sensitive fluorescence biosensor and miniaturized smartphone fluorescence microscope. Biosens. Bioelectron. 2019, 132, 238–247. [Google Scholar] [CrossRef]
- Pennacchio, A.; Giampaolo, F.; Piccialli, F.; Cuomo, S.; Notomista, E.; Spinelli, M.; Amoresano, A.; Piscitelli, A.; Giardina, P. A machine learning-enhanced biosensor for mercury detection based on an hydrophobin chimera. Biosens. Bioelectron. 2022, 196, 113696. [Google Scholar] [CrossRef]
- Piccialli, F.; Giampaolo, F.; Di Cola, V.S.; Gatta, F.; Chiaro, D.; Prezioso, E.; Izzo, S.; Cuomo, S. A machine learning-based approach for mercury detection in marine waters. In Proceedings of the IEEE International Conference on Data Mining Workshops, ICDMW, Orlando, FL, USA, 28 November–1 December 2022. [Google Scholar]
- Lu, Z.; Chen, M.; Li, M.; Liu, T.; Sun, M.; Wu, C.; Su, G.; Yin, J.; Wu, M.; Zou, P.; et al. Smartphone-integrated multi-color ratiometric fluorescence portable optical device based on deep learning for visual monitoring of Cu2+ and thiram. Chem. Eng. J. 2022, 439, 135686. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, S.; Chen, M.; Ma, H.; Wang, T.; Liu, T.; Yin, J.; Sun, M.; Wu, C.; Su, G.; et al. Trichromatic ratiometric fluorescent sensor based on machine learning and smartphone for visual and portable monitoring of tetracycline antibiotics. Chem. Eng. J. 2023, 454, 140492. [Google Scholar] [CrossRef]
- Zhu, J.; Cho, M.; Li, Y.; He, T.; Ahn, J.; Park, J.; Ren, T.-L.; Lee, C.; Park, I. Machine learning-enabled textile-based graphene gas sensing with energy harvesting-assisted IoT application. Nano Energy 2021, 86, 106035. [Google Scholar] [CrossRef]
- Liu, T.; Chen, S.; Ruan, K.; Zhang, S.; He, K.; Li, J.; Chen, M.; Yin, J.; Sun, M.; Wang, X.; et al. A handheld multifunctional smartphone platform integrated with 3D printing portable device: On-site evaluation for glutathione and azodicarbonamide with machine learning. J. Hazard. Mater. 2022, 426, 128091. [Google Scholar] [CrossRef]
- Dutta, S. Point of care sensing and biosensing using ambient light sensor of smartphone: Critical review. TrAC Trends Anal. Chem. 2019, 110, 393–400. [Google Scholar] [CrossRef]
- Park, Y.M.; Han, Y.D.; Kim, K.R.; Zhang, C.; Yoon, H.C. An immunoblot-based optical biosensor for screening of osteoarthritis using a smartphone-embedded illuminometer. Anal. Methods 2015, 7, 6437–6442. [Google Scholar] [CrossRef]
- Pol, R.; Diez, L.; Gabriel, D.; Baeza, M. Versatile Three-Dimensional-Printed Platform for Nitrite Ion Analyses Using a Smartphone with Real-Time Location. Anal. Chem. 2019, 91, 13916–13923. [Google Scholar] [CrossRef]
- Gul, I.; Aer, L.; Zhang, M.; Jiang, H.; Khan, A.A.; Bilal, M.; Fang, R.; Feng, J.; Zeng, H.; Tang, L. Multifunctional 3D-printed platform integrated with a smartphone ambient light sensor for halocarbon contaminants monitoring. Environ. Technol. Innov. 2021, 24, 101883. [Google Scholar] [CrossRef]
- Hussain, I.; Das, M.; Ahamad, K.U.; Nath, P. Water salinity detection using a smartphone. Sens. Actuators B 2017, 239, 1042–1050. [Google Scholar] [CrossRef]
- Park, Y.M.; Han, Y.D.; Chun, H.J.; Yoon, H.C. Ambient light-based optical biosensing platform with smartphone-embedded illumination sensor. Biosens. Bioelectron. 2017, 93, 205–211. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, M.; Fu, Q.; Ouyang, H.; Wen, W.; Song, Y.; Zhu, C.; Lin, Y.; Du, D. A Nanozyme- and Ambient Light-Based Smartphone Platform for Simultaneous Detection of Dual Biomarkers from Exposure to Organophosphorus Pesticides. Anal. Chem. 2018, 90, 7391–7398. [Google Scholar] [CrossRef]
- Wang, T.-T.; Guo, K.; Hu, X.-M.; Liang, J.; Li, X.-D.; Zhang, Z.-F.; Xie, J. Label-Free Colorimetric Detection of Urine Glucose Based on Color Fading Using Smartphone Ambient-Light Sensor. Chemosensors 2020, 8, 10. [Google Scholar] [CrossRef]
- Madrid, R.E.; Ashur Ramallo, F.; Barraza, D.E.; Chaile, R.E. Smartphone-Based Biosensor Devices for Healthcare: Technologies, Trends, and Adoption by End-Users. Bioengineering 2022, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Celikbas, E.; Celik, E.G.; Timur, S. Paper-Based Analytical Methods for Smartphone Sensing with Functional Nanoparticles: Bridges from Smart Surfaces to Global Health. Anal. Chem. 2018, 90, 12325–12333. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Maduraiveeran, G. Nanomaterials-based portable electrochemical sensing and biosensing systems for clinical and biomedical applications. J. Anal. Sci. Technol. 2022, 13, 35. [Google Scholar] [CrossRef]
- Guo, J. Smartphone-Powered Electrochemical Dongle for Point-of-Care Monitoring of Blood β-Ketone. Anal. Chem. 2017, 89, 8609–8613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, Y.; Liu, Y.; Mugo, S.M.; Zhang, Q. Integrated Wearable Sensors for Sensing Physiological Pressure Signals and β-Hydroxybutyrate in Physiological Fluids. Anal. Chem. 2022, 94, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, X.; Qin, Y.; Xia, Y.; Xu, X.; Sun, X.; Yu, D.; Mugo, S.M.; Wang, D.; Zhang, Q. An Integrated Wearable Sweat Sensing Patch for Passive Continuous Analysis of Stress Biomarkers at Rest. Adv. Funct. Mater. 2023, 33, 2212083. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, J.; Luo, J.; Ming, T.; Yang, G.; Sun, S.; Xu, S.; Li, X.; He, E.; Kong, F.; et al. A Dual-Channel Intelligent Point-of-Care Testing System for Soluble Programmed Death-1 and Programmed Death-Ligand 1 Detection Based on Folding Paper-Based Immunosensors. ACS Sens. 2022, 7, 584–592. [Google Scholar] [CrossRef]
- He, W.; Liu, L.; Cao, Z.; Lin, Y.; Tian, Y.; Zhang, Q.; Zhou, C.; Ye, X.; Cui, T. Shrink polymer based electrochemical sensor for point-of-care detection of prostate-specific antigen. Biosens. Bioelectron. 2023, 228, 115193. [Google Scholar] [CrossRef]
- Liao, J.; Chang, F.; Han, X.; Ge, C.; Lin, S. Wireless water quality monitoring and spatial mapping with disposable whole-copper electrochemical sensors and a smartphone. Sens. Actuators B 2020, 306, 127557. [Google Scholar] [CrossRef]
- Yan, W.; Ma, C.; Cai, X.; Sun, Y.; Zhang, G.; Song, W. Self-powered and wireless physiological monitoring system with integrated power supply and sensors. Nano Energy 2023, 108, 108203. [Google Scholar] [CrossRef]
- Yu, Z.; Lin, Q.; Gong, H.; Li, M.; Tang, D. Integrated solar-powered MEMS-based photoelectrochemical immunoassay for point-of-care testing of cTnI protein. Biosens. Bioelectron. 2023, 223, 115028. [Google Scholar] [CrossRef] [PubMed]
- Vernon, J.; Canyelles-Pericas, P.; Torun, H.; Dai, X.; Ng, W.P.; Binns, R.; Busawon, K.; Fu, Y.Q. Acousto-Pi: An Opto-Acoustofluidic System Using Surface Acoustic Waves Controlled with Open-Source Electronics for Integrated In-Field Diagnostics. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Zida, S.I.; Lin, Y.-D.; Khung, Y.L. Current Trends on Surface Acoustic Wave Biosensors. Adv. Mater. Technol. 2021, 6, 2001018. [Google Scholar] [CrossRef]
- Handzel, O.; Franck, K. Smartphone based hearing evaluation. Oper. Tech. Otolaryngol. Head Neck Surg. 2021, 32, 87–91. [Google Scholar] [CrossRef]
- Jandas, P.J.; Luo, J.; Quan, A.; Li, C.; Fu, C.; Fu, Y.Q. Graphene oxide-Au nano particle coated quartz crystal microbalance biosensor for the real time analysis of carcinoembryonic antigen. RSC Adv. 2020, 10, 4118–4128. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Piskin, A.K.; Yavuz, H.; Denizli, A. Quartz crystal microbalance biosensor for label-free MDA MB 231 cancer cell detection via notch-4 receptor. Talanta 2019, 204, 840–845. [Google Scholar] [CrossRef]
- Nair, M.P.; Teo, A.J.T.; Li, K.H.H. Acoustic Biosensors and Microfluidic Devices in the Decennium: Principles and Applications. Micromachines 2022, 13, 24. [Google Scholar] [CrossRef]
- Li, F.; Chen, H.; Song, X.; Zhang, Q.; Li, Y.; Wang, Y. CondioSense: High-quality context-aware service for audio sensing system via active sonar. Pers. Ubiquitous Comput. 2017, 21, 17–29. [Google Scholar] [CrossRef]
- Stowell, D.; Giannoulis, D.; Benetos, E.; Lagrange, M.; Plumbley, M.D. Detection and Classification of Acoustic Scenes and Events. IEEE Trans. Multimed. 2015, 17, 1733–1746. [Google Scholar] [CrossRef]
- Nandakumar, R.; Gollakota, S.; Watson, N. Contactless Sleep Apnea Detection on Smartphones. In Proceedings of the 13th Annual International Conference on Mobile Systems, Applications, and Services, Florence, Italy, 18–22 May 2015; Association for Computing Machinery: Florence, Italy; pp. 45–57. [Google Scholar]
- Wang, W.; Liu, A.X.; Sun, K. Device-free gesture tracking using acoustic signals. In Proceedings of the 22nd Annual International Conference on Mobile Computing and Networking, New York, NY, USA, 3–7 October 2016; Association for Computing Machinery: New York, NY, USA; pp. 82–94. [Google Scholar]
- Nandakumar, R.; Gollakota, S.; Sunshine, J.E. Opioid overdose detection using smartphones. Sci. Transl. Med. 2019, 11, eaau8914. [Google Scholar] [CrossRef]
- Wang, X.; Huang, R.; Yang, C.; Mao, S. Smartphone Sonar-Based Contact-Free Respiration Rate Monitoring. ACM Trans. Comput. Healthc. 2021, 2, 15. [Google Scholar] [CrossRef]
- Mohammed, Z.; Elfadel, I.M.; Rasras, M. Monolithic Multi Degree of Freedom (MDoF) Capacitive MEMS Accelerometers. Micromachines 2018, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Grouios, G.; Ziagkas, E.; Loukovitis, A.; Chatzinikolaou, K.; Koidou, E. Accelerometers in Our Pocket: Does Smartphone Accelerometer Technology Provide Accurate Data? Sensors 2023, 23, 192. [Google Scholar] [CrossRef] [PubMed]
- Barrantes, S.; Sánchez Egea, A.J.; González Rojas, H.A.; Martí, M.J.; Compta, Y.; Valldeoriola, F.; Simo Mezquita, E.; Tolosa, E.; Valls-Solè, J. Differential diagnosis between Parkinson’s disease and essential tremor using the smartphone’s accelerometer. PLoS ONE 2017, 12, e0183843. [Google Scholar] [CrossRef] [PubMed]
- Omberg, L.; Chaibub Neto, E.; Perumal, T.M.; Pratap, A.; Tediarjo, A.; Adams, J.; Bloem, B.R.; Bot, B.M.; Elson, M.; Goldman, S.M.; et al. Remote smartphone monitoring of Parkinson’s disease and individual response to therapy. Nat. Biotechnol. 2022, 40, 480–487. [Google Scholar] [CrossRef]
- Kuosmanen, E.; Wolling, F.; Vega, J.; Kan, V.; Nishiyama, Y.; Harper, S.; Van Laerhoven, K.; Hosio, S.; Ferreira, D. Smartphone-Based Monitoring of Parkinson Disease: Quasi-Experimental Study to Quantify Hand Tremor Severity and Medication Effectiveness. JMIR Mhealth Uhealth 2020, 8, e21543. [Google Scholar] [CrossRef] [PubMed]
- Lipsmeier, F.; Taylor, K.I.; Postuma, R.B.; Volkova-Volkmar, E.; Kilchenmann, T.; Mollenhauer, B.; Bamdadian, A.; Popp, W.L.; Cheng, W.-Y.; Zhang, Y.-P.; et al. Reliability and validity of the Roche PD Mobile Application for remote monitoring of early Parkinson’s disease. Sci. Rep. 2022, 12, 12081. [Google Scholar] [CrossRef]








| Analyte | Approach | Limit of Detection | Ref |
|---|---|---|---|
| Lactate, chloride, glucose, and pH | Wireless-communication-integrated microfluidic. | 1.6 mM, 39 mM, 200 µM, pH: 5–7 | [33] |
| Protein and human interleukin-6 | Homemade multichannel smartphone spectrometer. | 2 µg/mL, 8.8 pg/mL | [34] |
| Spermine | Paper strip test—smartphone captures and analyze images. | 0.17 µM | [35] |
| Tannic acid | Portable homemade fluorescent reader based on smartphone. | 87 nM | [36] |
| Folic acid | Paper strip test—smartphone captures and analyze fluorescence of strip test. | 22 nM | [37] |
| Glucose | Portable kit—smartphone-assisted fluorescent/colorimetric device. | 0.093 µM/0.437 µM | [38] |
| Glucose, uric acid, and cholesterol | Transform photochemical signal with the assistance of dongle connected to the smartphone. | 1.67 mM, 119 μM, and 2.59 mM | [39] |
| Ibuprofen, chloramphenicol, and florfenicol | Portable device using the smartphone to capture and analyze the fluorescence of the sample. | 10 pM, 8.5 pM, and 5.5 nM | [40] |
| Protease biomarker | Smartphone-based colorimetric paper strip test. | 5.7 pM | [41] |
| Water in organic solvent | Smartphone adapter for fluorescence imaging. | 0.05% | [42] |
| Lactoferrin | Smartphone-based fluorescence portable device with the automation of region of interest. | 0.12 mg/mL | [43] |
| Hemoglobin | Paper strip test—light illumination control by smartphone. | <0.070 mg/mL | [44] |
| Respiratory syncytial virus | Smartphone-based colorimetric spectrometer using flash as an excitation source. | 1400 PFU/mL | [45] |
| Salmonella | Microfluidic integrated with the fabricated fluorescent microscope using smartphone. | 58 CFU/mL | [47] |
| Tyrosine-like orphan receptor one | Smartphone-based on-chip microscopic imaging. | 0.1 cell/µL | [48] |
| Bacteria | Smartphone-based fluorescence imaging. | 10 CFU/mL | [49] |
| Urinary C-telopeptide fragments | Colorimetric with the fabricated specific color filter using smartphone. | 0–10 ng/mL | [50] |
| Tissue imaging | Smartphone confocal microscope for two-dimensional confocal imaging. | Resolution of 1–5 µm | [51] |
| Tartrazine, amaranth, and phenol red | Smartphone-based colorimeter using smart tablet as multi-light excitation sources. | 0.44, 1.04, 47 (µg/mL) | [54] |
| Picric acid, malachite green, aflatoxin, formaldehyde, and phosgene | Smartphone captures and analyzes image-based analytical device. | 10 µM, 0.1 ng/mL, 0.35 ng/mL, 62 nM, and 23 nM | [55,56,57,59] |
| Hg2+, Pb2+, and Cu2+ ions | Smartphone-based portable device with the homogenized LED light sources by plastic diffuser. | 5.8 nM, 0.12 µM, and 76 nM | [58] |
| Fluoride | Smartphone-based fluorescence signal analysis. | 1.53 µM | [60] |
| Hg2+ ions | Smartphone fluorescence microscope. | 1 nM | [61] |
| Cu2+, thiaram, and tetracycline | Deep-learning-assisted smartphone-based colorimetric device. | 1.84 µM, 0.34 µM, 0.42 µM | [64,65] |
| Glutathione, and azodicarbonamide | Deep-learning-assisted smartphone-based fluorescence device. | 0.07 µM, 0.09 µM | [67] |
| Osteoarthritis | Ambient light sensor of smartphone-embedded illuminometer. | 0.3 ng/mL | [69] |
| Water salinity | Integrate an ambient light sensor and flash light of smartphone-based colorimetric device. | 0.04 ppt | [72] |
| Butyrylcholinesterase and glucose | Ambient light sensor of smartphone-based colorimetric device. | 0.028 nM, 5 µg/mL | [74,75] |
| Analyte | Approach | Performance | Ref |
|---|---|---|---|
| Blood ketone | Dongle-connected OTG port as an electrochemical system. | LOD: 1 µM | [80] |
| β-hydroxybutyrate | Homemade signal-processing-circuitry-integrated wearable amperometric sensor. | 322.01 nA/mM | [81] |
| Cortisol, Mg2+, and pH | Homemade signal-processing-circuitry-based amperometric wearable sensor controlled by a smartphone. | 2751 nA/decade, 47.3 mV/decade, 63.96 mV/pH | [82] |
| Programmed death-1 and programmed death-ligand 1 | Homemade dongle using paper-based three-electrode kit for voltammetry device controlled by a smartphone. | LOD: 10 pg/mL, 5 pg/mL | [83] |
| Prostate-specific antigen | Wrinkled electrode integrated with miniaturized electrochemical electric circuit controlled with a smartphone through Bluetooth. | LOD: 0.38 fg/mL | [84] |
| Water quality (Pb2+ and chemical oxygen demand-COD) | Whole-copper-electrode-connected chronoamperometric/voltammetric detector operated by Bluetooth. | LOD: 45 nM, 9 mg/L | [85] |
| Electrocardiogram and blood pressure | Self-powered wearable triboelectric sensor which transmitted output signal to smartphone via Bluetooth. | NA | [86] |
| Cardiac troponin I | Self-powered photo electrochemical sensor with assistance of artificial neural network model. | LOD: <2 pg/mL | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, T.H.; Thangavel, B.; Sharipov, M.; Chen, K.; Shin, J.H. Smartphone-Based Portable Bio-Chemical Sensors: Exploring Recent Advancements. Chemosensors 2023, 11, 468. https://doi.org/10.3390/chemosensors11090468
Bui TH, Thangavel B, Sharipov M, Chen K, Shin JH. Smartphone-Based Portable Bio-Chemical Sensors: Exploring Recent Advancements. Chemosensors. 2023; 11(9):468. https://doi.org/10.3390/chemosensors11090468
Chicago/Turabian StyleBui, The Huy, Balamurugan Thangavel, Mirkomil Sharipov, Kuangcai Chen, and Joong Ho Shin. 2023. "Smartphone-Based Portable Bio-Chemical Sensors: Exploring Recent Advancements" Chemosensors 11, no. 9: 468. https://doi.org/10.3390/chemosensors11090468