Label-Free Electrochemical Sensing Using Glassy Carbon Electrodes Modified with Multiwalled-Carbon Nanotubes Non-Covalently Functionalized with Human Immunoglobulin G
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
2.3. Preparation of MWCNT-IgG Hybrid and GCE/MWCNT-IgG Platform
2.4. Construction of the Genosensor (GCE/MWCNT-IgG/DNAp)
2.5. Voltammetric Detection of Do and UA
3. Results and Discussion
3.1. Electrooxidation of the DNA Probe at GCE/MWCNT-IgG
3.2. Construction of BRCA1 Gene Biosensor and Analytical Applications
3.3. Simultaneous Detection of Do and UA in the Presence of AA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramya, M.; Senthil, P.; Rangasamy, G.; Saravanan, A.; Krishnapandi, A. A recent advancement on the applications of nanomaterials in electrochemical sensors and biosensors. Chemosphere 2022, 308, 136416. [Google Scholar] [CrossRef] [PubMed]
- Safari, M.; Moghaddam, A.; Salehi Moghaddam, A.; Ruckdäschel, H.; Khonakdar, H.A. Carbon-based biosensors from graphene family to carbon dots: A viewpoint in cancer detection. Talanta 2023, 258, 124399. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, Y.; Fang, Y.; Zhang, D. Carbon-based electrochemical sensors for in vivo and in vitro neurotransmitter detection. Crit. Rev. Anal. Chem. 2023, 53, 955–974. [Google Scholar] [CrossRef] [PubMed]
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A review on carbon nanotubes: An overview of synthesis, properties, functionalization, characterization, and the application. Mater. Sci. Eng. B 2021, 268, 115095. [Google Scholar] [CrossRef]
- Palomar, Q.; Xu, X.; Selegård, R.; Aili, D.; Zhang, Z. Peptide decorated gold nanoparticle/carbon nanotube electrochemical sensor for ultrasensitive detection of matrix metalloproteinase-7. Sens. Actuators B Chem. 2020, 325, 128789. [Google Scholar] [CrossRef]
- Mujica, M.L.; Tamborelli, A.; Castellaro, A.; Barcudi, D.; Rubianes, M.D.; Rodríguez, M.C.; Bocco, J.L.; Dalmasso, P.R.; Rivas, G.A. Impedimetric and amperometric genosensors for the highly sensitive quantification of SARS-CoV-2 nucleic acid using an avidin-functionalized multi-walled carbon nanotubes biocapture platform. Biosens. Bioelectron. X 2022, 12, 100222. [Google Scholar] [CrossRef] [PubMed]
- Negahdary, M.; Agnes, L. Application of electrochemical biosensors for the detection of microRNAs (miRNAs) related to cancer. Coord. Chem. Rev. 2022, 464, 214565. [Google Scholar] [CrossRef]
- Banakar, M.; Hamidi, M.; Khurshid, Z.; Zafar, M.S.; Sapkota, J.; Azizian, R.; Rokaya, D. Electrochemical biosensors for pathogen detection: An updated review. Biosensors 2022, 12, 927. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, G.; Yang, X. Electrochemical immunosensor based on Fe3O4/MWCNTs-COOH/AuNPs nanocomposites for trace liver cancer marker alpha-fetoprotein detection. Talanta 2023, 259, 124492. [Google Scholar] [CrossRef]
- Peng, Y.; Ou, S.; Li, M.; Zeng, Z.; Feng, N. An electrochemical biosensor based on network-like DNA nanoprobes for detection of mesenchymal circulating tumor cells. Biosens. Bioelectron. 2023, 238, 115564. [Google Scholar] [CrossRef]
- Mujica, M.L.; Zhang, Y.; Gutiérrez, F.; Bédioui, F.; Rivas, G. Non-amplified impedimetric genosensor for quantification of miRNA-21 based on the use of reduced graphene oxide modified with chitosan. Microchem. J. 2021, 160, 105596. [Google Scholar] [CrossRef]
- Alosime, E.M. A review on surface functionalization of carbon nanotubes: Methods and applications. Nanoscale Res. Lett. 2023, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Kharlamova, M.V.; Paukov, M.; Burdanova, M.G. Nanotube functionalization: Investigation, methods and demonstrated applications. Materials 2022, 15, 5386. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fang, Y.; Ramasamy, R.P. Non-covalent functionalization of carbon nanotubes for electrochemical biosensor development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, F.; Rubianes, M.D.; Rivas, G.A. New bioanalytical platform based on the use of avidin for the successful exfoliation of multi-walled carbon nanotubes and the robust anchoring of biomolecules. Application for hydrogen peroxide biosensing. Anal. Chim. Acta 2019, 1065, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Eguílaz, M.; Gutierrez, A.; Rivas, A. Non-covalent functionalization of multi-walled carbon nanotubes with cytochrome c: Enhanced direct electron transfer and analytical applications. Sens. Actuators B Chem. 2016, 216, 629–637. [Google Scholar] [CrossRef]
- Primo, E.N.; Cañete-Rosales, P.; Bollo, S.; Rubianes, M.D.; Rivas, G.A. Dispersion of bamboo type multi-wall carbon nanotubes in calf-thymus double stranded DNA. Colloids Surf. B 2013, 108, 329–336. [Google Scholar] [CrossRef]
- Ortiz, E.; Gallay, P.; Galicia, L.; Eguílaz, M.; Rivas, G. Nanoarchitectures based on multi-walled carbon nanotubes non-covalently functionalized with Concanavalin A: A new building-block with supramolecular recognition properties for the development of electrochemical biosensors. Sens. Actuators B Chem. 2019, 292, 254–262. [Google Scholar] [CrossRef]
- Gallay, P.; Eguílaz, M.; Rivas, G. Designing electrochemical interfaces based on nanohybrids of avidin functionalized-carbon nanotubes and ruthenium nanoparticles as peroxidase-like nanozyme with supramolecular recognition properties for site-specific anchoring of biotinylated residues. Biosens. Bioelectron. 2020, 148, 111764. [Google Scholar] [CrossRef]
- López Mujica, M.; Rubianes, M.D.; Rivas, G. A multipurpose biocapture nanoplatform based on multiwalled-carbon nanotubes non-covalently functionalized with avidin: Analytical applications for the non-amplified and label-free impedimetric quantification of BRCA1. Sens. Actuators B Chem. 2022, 357, 131304. [Google Scholar] [CrossRef]
- López Mujica, M.; Tamborelli, A.; Espinosa, C.; Vaschetti, V.; Bollo, S.; Dalmasso, P.; Rivas, G. Two birds with one stone: Integrating exfoliation and immunoaffinity properties in multi-walled carbon nanotubes by non-covalent functionalization with human immunoglobulin G. Microchim. Acta 2023, 190, 73. [Google Scholar] [CrossRef] [PubMed]
- Edri, E.; Regev, O. “Shaken, not stable”: Dispersion mechanism and dynamics of protein-dispersed nanotubes studied via spectroscopy. Langmuir 2009, 25, 10459–10465. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, T.; Tischkowitz, M.; Antoniou, A.C. BRCA1 and BRCA2 pathogenic variants and prostate cancer risk: Systematic review and meta-analysis. Br. J. Cancer 2022, 126, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Glodzik, D.; Bosch, A.; Hartman, J.; Aine, M.; Vallon-Christersson, J.; Reuterswärd, C.; Karlsson, A.; Mitra, S.; Niméus, E.; Holm, K.; et al. Comprehensive molecular comparison of BRCA1 hypermethylated and BRCA1 mutated triple negative breast cancers. Nat. Commun. 2020, 11, 3747. [Google Scholar] [CrossRef] [PubMed]
- Madariaga, H.; Lheureux, S.; Oza, A.M. Tailoring ovarian cancer treatment: Implications of BRCA1/2 mutations. Cancer 2019, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Hawsawi, Y.M.; Al-Numair, N.S.; Sobahy, T.M.; Al-Ajmi, A.M.; Al-Harbi, R.M.; Baghdadi, M.A.; Oyouni, A.A.; Alamer, O.M. The role of BRCA1/2 in hereditary and familial breast and ovarian cancers. Mol. Genet. Genom. Med. 2019, 7, e879. [Google Scholar] [CrossRef] [PubMed]
- Venkitaraman, A.R. How do mutations affecting the breast cancer genes BRCA1 and BRCA2 cause cancer susceptibility? DNA Repair 2019, 81, 102668. [Google Scholar] [CrossRef]
- Philpott, S.; Raikou, M.; Manchanda, R.; Lockley, M.; Singh, N.; Scott, M.; Evans, D.G.; Adlard, J.; Ahmed, M.; Edmondson, R.; et al. The avoiding late diagnosis of ovarian cancer (ALDO) project; a pilot national surveillance programme for women with pathogenic germline variants in BRCA1 and BRCA2. J. Med. Genet. 2023, 60, 440–449. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Hui, N. A low fouling electrochemical biosensor based on the zwitterionic polypeptide doped conducting polymer PEDOT for breast cancer marker BRCA1 detection. Bioelectrochemistry 2020, 136, 107595. [Google Scholar] [CrossRef]
- Senel, M.; Dervisevic, M.; Kokkokoglu, F. Electrochemical DNA biosensors for label free breast cancer gene marker detection. Anal. Bioanal. Chem. 2019, 411, 2925–2935. [Google Scholar] [CrossRef]
- Işın, D.; Eksin, E.; Erdem, A. Graphene-oxide and ionic liquid modified electrodes for electrochemical sensing of breast cancer 1 gene. Biosensors 2022, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Ehzari, H.; Safari, M.; Shahlaei, M. A simple and label-free genosensor for BRCA1 related sequence based on electrospinned ribbon conductive nanofibers. Microchem. J. 2018, 143, 118–126. [Google Scholar] [CrossRef]
- Ehzari, H.; Safari, M.; Samini, M. Signal amplification of novel sandwich-type genosensor via catalytic redox-recycling on platform MWCNTs/Fe3O4@TMU-21 for BRCA1 gene detection. Talanta 2021, 234, 122698. [Google Scholar] [CrossRef] [PubMed]
- García-Mendiola, C.; Gutierrez-Sánchez, C.; Gibaja, I.; Torres, C.; Buso-Rogero, F.; Pariente, J.; Solera, Z.; Razavifar, J.J.; Palacios, F.; Zamora, E.; et al. Functionalization of a few-layer antimonene with oligonucleotides for DNA sensing. ACS Appl. Nano Mater. 2020, 3, 3625–3633. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, J.; Zhang, L.; Liu, Y.; Jia, Y.; Zhou, G. Signal “on-off-off” strategy for improving the sensitivity of BRCA1 electrochemical detection by combining gold substrate amplification, DNA conformational transformation and DSN enzymatic hydrolysis dual reduction. Anal. Chim. Acta 2022, 1235, 340461. [Google Scholar] [CrossRef]
- Xia, Y.M.; Li, M.Y.; Chen, C.L.; Xia, M.; Zhang, W.; Gao, W.W. Employing label-free electrochemical biosensor based on 3D-reduced graphene oxide and polyaniline nanofibers for ultrasensitive detection of breast cancer BRCA1 biomarker. Electroanalysis 2020, 32, 2045–2055. [Google Scholar] [CrossRef]
- Cui, M.; Wang, Y.; Wang, H.; Wua, Y.; Luo, X. A label-free electrochemical DNA biosensor for breast cancer marker BRCA1 based on self-assembled antifouling peptide monolayer. Sens. Actuators B Chem. 2017, 244, 742–749. [Google Scholar] [CrossRef]
- Divya, K.P.; Keerthana, S.; Viswanathan, C.; Ponpandian, N. MXene supported biomimetic bilayer lipid membrane biosensor for zeptomole detection of BRCA1 gene. Microchim. Acta 2023, 190, 116. [Google Scholar] [CrossRef]
- Benvidi, A.; Tezerjani, M.D.; Jahanbani, S.; Ardakani, M.M.; Moshtaghioun, S.M. Comparison of impedimetric detection of DNA hybridization on the various biosensors based on modified glassy carbon electrodes with PANHS and nanomaterials of RGO and MWCNTs. Talanta 2016, 147, 621–627. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.; Chen, C. Impedimetric biosensor modified with hydrophilic material of tannic acid/polyethylene glycol and dopamine-assisted deposition for detection of breast cancer-related BRCA1 gene. J. Electroanal. Chem. 2017, 791, 204–210. [Google Scholar] [CrossRef]
- Lee, J.Y.; Martín-Bastida, A.; Murueta-Goyena, A.; Gabilondo, I.; Cuenca, N.; Piccini, P.; Jean, B. Multimodal brain and retinal imaging of dopaminergic degeneration in Parkinson disease. Nat. Rev. Neurol. 2022, 18, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Broome, S.T.; Louangaphay, K.; Keay, K.A.; Leggio, G.A.; Musumeci, G.; Castorina, A. Dopamine: An immune transmitter. Neural. Regen. Res. 2020, 15, 2173–2185. [Google Scholar]
- Gonzalez-Lopez, E.; Vrana, K.E. Dopamine beta-hydroxylase and its genetic variants in human health and disease. J. Neurochem. 2020, 152, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, V.C.; Souvatzoglou, M.; Paraskevas, G.P.; Chalioti, M.; Stefanis, L.; Kapaki, E. Dopamine transporter SPECT imaging in Parkinson’s disease and atypical parkinsonism: A study of 137 patients. Neurol. Sci. 2023, 44, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Salazar, E.O.; Martínez-Nava, G.A. Uric acid extrarenal excretion: The gut microbiome as an evident yet understated factor in gout development. Rheumatol. Int. 2022, 42, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Andres-Hernando, A.; Kuwabara, M. Uric acid and hypertension. Hypertens. Res. 2020, 43, 832–834. [Google Scholar] [CrossRef]
- Saito, Y.; Tanaka, A.; Node, K.; Kobayashi, Y. Uric acid and cardiovascular disease: A clinical review. J. Cardiol. 2021, 78, 51–57. [Google Scholar] [CrossRef]
- Przewodowska, D.; Marzec, W.; Madetko, N. Novel therapies for parkinsonian syndromes-Recent progress and future perspectives. Front. Mol. Neurosci. 2021, 14, 720220. [Google Scholar] [CrossRef]
- Murugan, N.; Jerome, R.; Preethika, M.; Sundaramurthy, A.; Sundramoorthy, A.K. 2D-titanium carbide (MXene) based selective electrochemical sensor for simultaneous detection of ascorbic acid, dopamine and uric acid. J. Mater. Sci. Technol. 2021, 72, 122–131. [Google Scholar] [CrossRef]
- Li, M.; Xu, H.; Chen, G.; Sun, S.; Wang, Q.; Liu, B.; Wu, X.; Zhou, L.; Chai, Z.; Sun, X.; et al. Impaired D2 receptor-dependent dopaminergic transmission in prefrontal cortex of awake mouse model of Parkinson’s disease. Brain 2019, 142, 3099–3115. [Google Scholar] [CrossRef]
- Xie, X.; Wang, D.P.; Guo, C.; Liu, Y.; Rao, Q.; Lou, F.; Li, Q.; Dong, Y.; Li, Q.; Yang, H.B.; et al. Single-atom ruthenium biomimetic enzyme for simultaneous electrochemical detection of dopamine and uric acid. Anal. Chem. 2021, 93, 4916–4923. [Google Scholar] [CrossRef] [PubMed]
- Tchekep, A.G.K.; Suryanarayanan, V.; Pattanayak, D.K. Alternative approach for highly sensitive and free-interference electrochemical dopamine sensing. Carbon 2023, 204, 57–69. [Google Scholar] [CrossRef]
- Pan, J.; Liu, M.; Li, D.; Zheng, H.; Zhang, D. Overoxidized poly(3, 4-ethylenedioxythiophene)–gold nanoparticles–graphene-modified electrode for the simultaneous detection of dopamine and uric acid in the presence of ascorbic acid. J. Pharm. Anal. 2021, 11, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Chen, X.; Ren, T.; Zhang, P.; Yang, D. Carbon nanotube based biosensors. Sens. Actuators B Chem. 2015, 207, 690–715. [Google Scholar] [CrossRef]
- Dai, B.; Zhou, R.; Ping, J.; Ying, Y.; Xie, L. Recent advances in carbon nanotube-based biosensors for biomolecular detection. Trends Anal. Chem. 2022, 154, 116658. [Google Scholar] [CrossRef]
- Burns, G.; Ali, M.Y.; Howlader, M.M.R. Advanced functional materials for electrochemical dopamine sensors. Trends Anal. Chem. 2023, 169, 117367. [Google Scholar] [CrossRef]
- Olivera-Brett, A.M.; Dicolescu, V.; Piedade, J.V.A. Electrochemical oxidation mechanism of guanine and adenine using a glassy carbon microelectrode. Bioelectrochemistry 2022, 154, 61–62. [Google Scholar] [CrossRef]
- Wei, X.; Guo, H.; Lu, Z.; Sun, L.; Pan, Z.; Liu, B.; Peng, L.; Yang, W. A novel electrochemical sensor based on DUT-67/ZnCo2O4-MWCNTs modified glassy carbon electrode for the simultaneous sensitive detection of dopamine and uric acid. Colloids Surf. A 2023, 674, 131921. [Google Scholar] [CrossRef]
- Zhou, X.; Kuang, Y.; Li, J.; Hu, S.; Cheng, C.; Wang, J.; Qin, X.; Ou, L.; Su, Z. Melamine-based nanocomposites for selective dopamine and uric acid sensing. ACS Appl. Polym. Mater. 2023, 5, 5609–5619. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, J.; Liu, M.; Lin, L.; Wang, X.; Quan, Z. Iron nanoparticles encapsulated in boron-nitrogen co-doped carbon nanotubes biomimetic enzyme for electrochemical monitoring of dopamine and uric acid in human serum. Microchem. J. 2023, 185, 108184. [Google Scholar] [CrossRef]
- Guo, H.; Liu, B.; Pan, Z.; Sun, L.; Peng, L.; Chen, Y.; Wu, N.; Wang, M.; Yang, W. Electrochemical determination of dopamine and uric acid with covalent organic frameworks and ox-MWCNT co-modified glassy carbon electrode. Colloids Surf. A 2022, 648, 129316. [Google Scholar] [CrossRef]
- Ma, C.; Xu, P.; Chen, H.; Cui, J.; Guo, M.; Zhao, J. An electrochemical sensor based on reduced graphene oxide/β-cyclodextrin/multiwall carbon nanotubes/polyoxometalate tetracomponent hybrid: Simultaneous determination of ascorbic acid, dopamine and uric acid. Microchem. J. 2022, 180, 107533. [Google Scholar] [CrossRef]
- Rattanaumpa, T.; Maensiri, S.; Ngamchuea, K. Microporous carbon in the selective electro-oxidation of molecular biomarkers: Uric acid, ascorbic acid, and dopamine. RSC Adv. 2022, 12, 18709–18721. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Guo, H.; Sun, L.; Liu, B.; Chen, Y.; Zhang, T.; Wang, M.; Peng, L.; Yang, W. A novel electrochemical platform based on COF/La2O3/MWCNTs for simultaneous detection of dopamine and uric acid. Colloids Surf. A 2022, 6350, 128083. [Google Scholar] [CrossRef]
- You, Y.; Zou, J.; Li, W.-J.; Chen, J.; Jiang, X.-Y.; Yu, J.-G. Novel lanthanum vanadate-based nanocomposite for simultaneously electrochemical detection of dopamine and uric acid in fetal bovine serum. Int. J. Biol. Macromol. 2022, 195, 346–355. [Google Scholar] [CrossRef]
- Li, R.; Liang, H.; Zhu, M.; Lai, M.; Wang, S.; Zhang, H.; Ye, H.; Zhu, R.; Zhang, W. Electrochemical dual signal sensing platform for the simultaneous determination of dopamine, uric acid and glucose based on copper and cerium bimetallic carbon nanocomposites. Bioelectrochemistry 2021, 139, 107745. [Google Scholar] [CrossRef]
- Guan, Q.; Guo, H.; Xue, R.; Wang, M.; Zhao, X.; Fan, T.; Yang, W.; Xu, M.; Yang, W. Electrochemical sensor based on covalent organic frameworks-MWCNT-NH2/AuNPs for simultaneous detection of dopamine and uric acid. J. Electroanal. Chem. 2021, 880, 114932. [Google Scholar] [CrossRef]

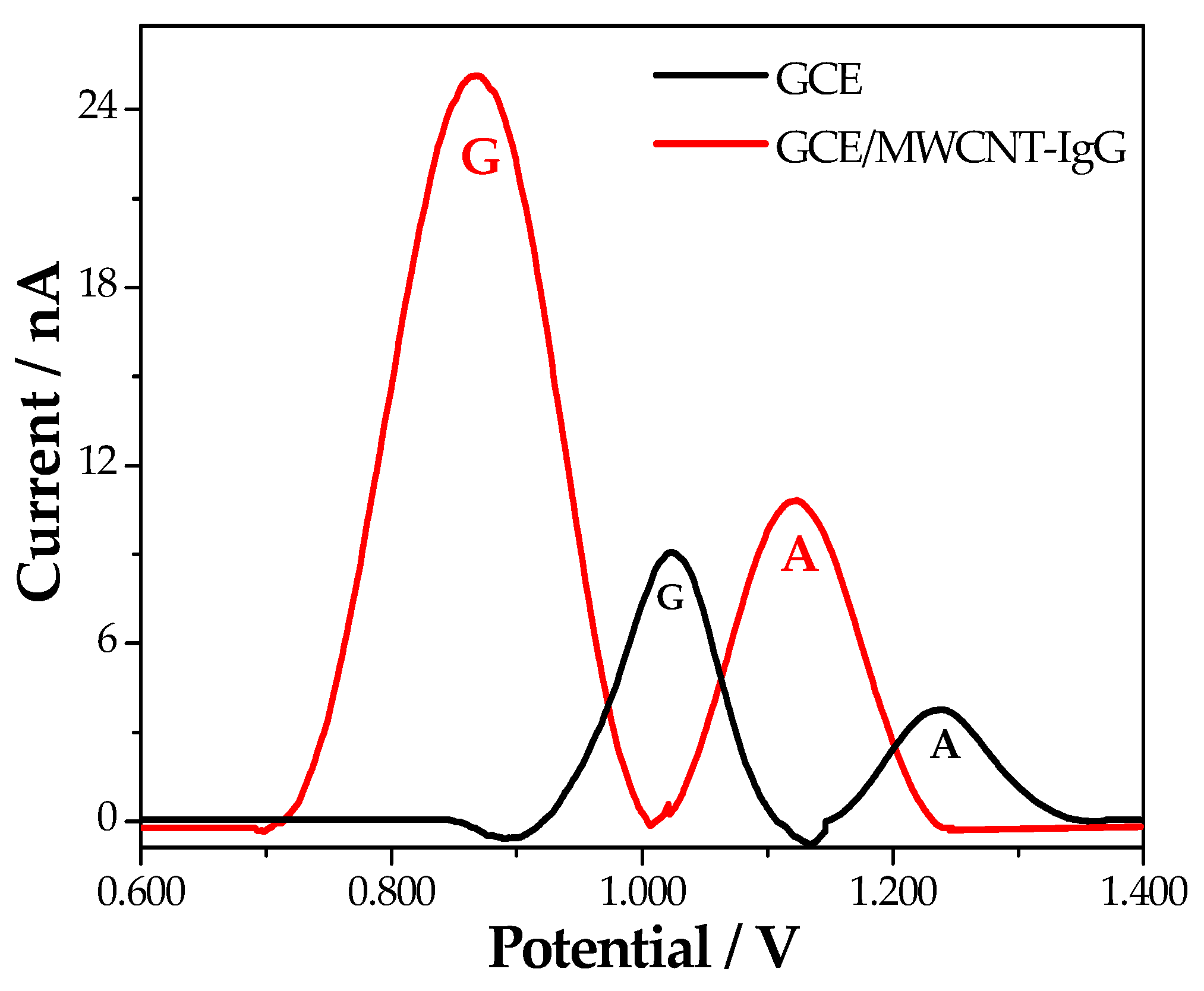
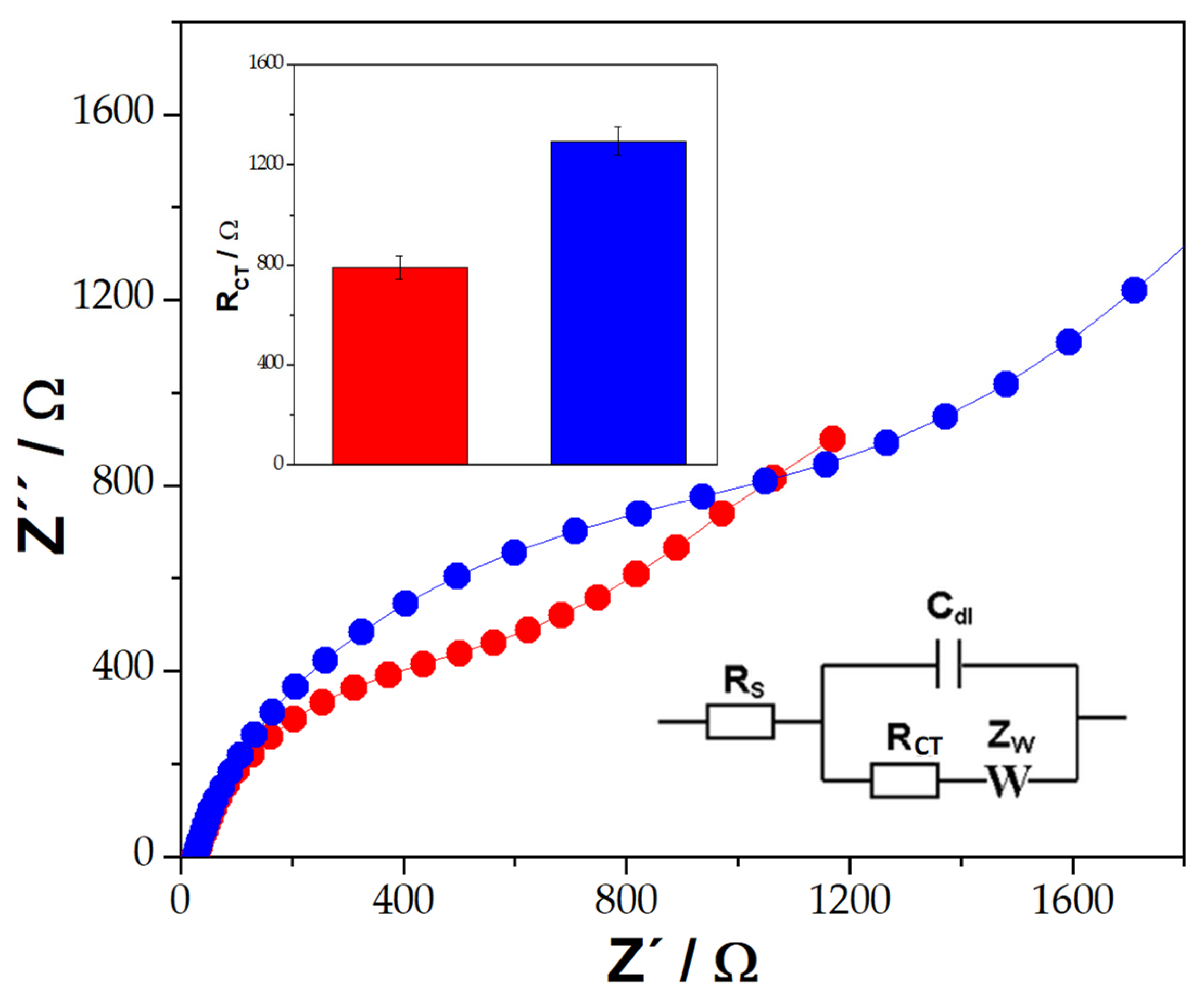
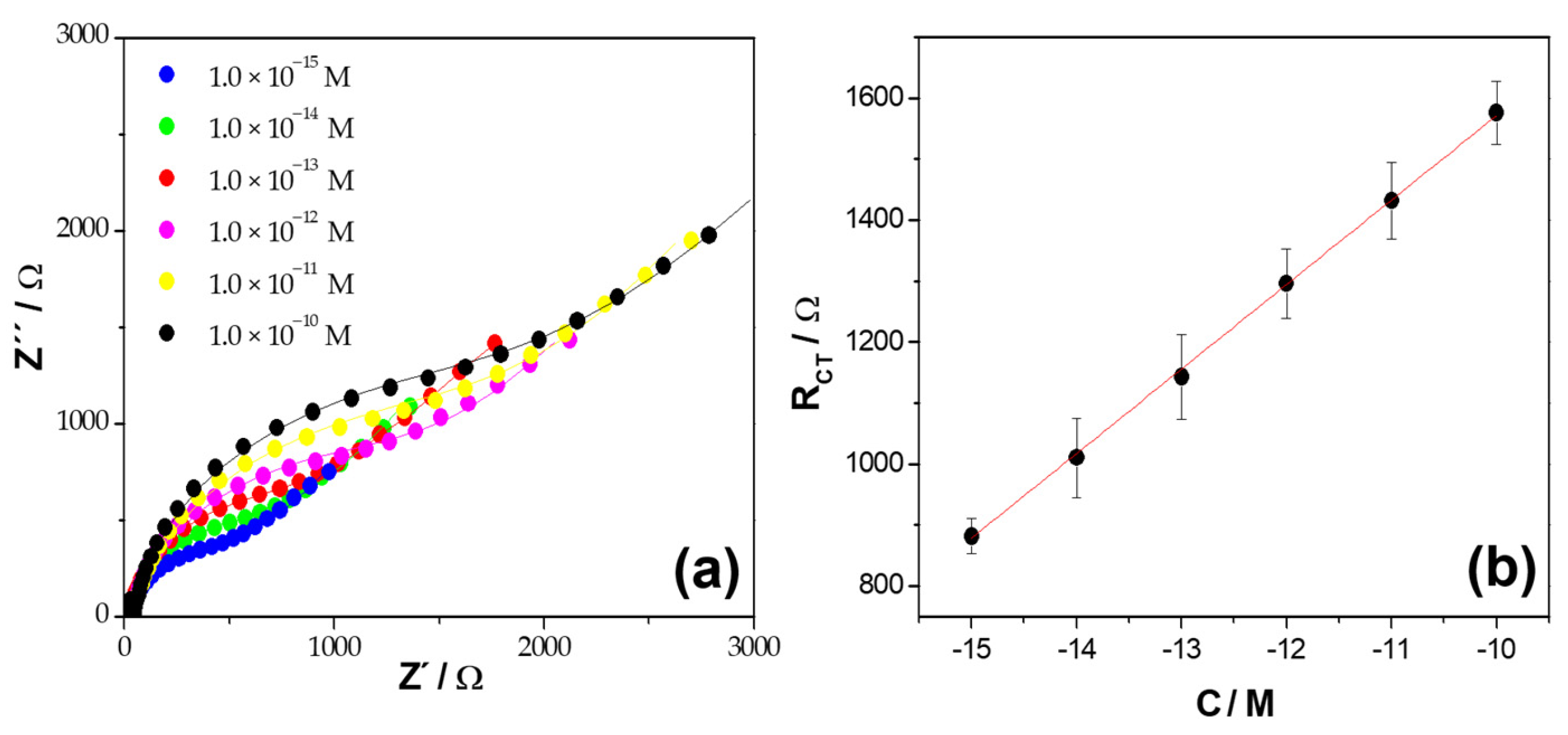

| Platform | RCT/Ω |
|---|---|
| GCE/MWCNT-IgG/BSA | (8.1 ± 0.2) × 102 |
| GCE/MWCNT-IgG/BSA/BRCA1 (1.0 × 10−12 M) | (1.3 ± 0.5) × 103 |
| GCE/MWCNT-IgG/BSA/NC (1.0 × 10−12 M) | (7.9 ± 0.5) × 102 |
| GCE/MWCNT-IgG/BSA/Mi (1.0 × 10−12 M) | (8.1 ± 0.3) × 102 |
| GCE/MWCNT-IgG/BSA/Diluted serum 1/100 | (8.0 ± 0.4) × 102 |
| GCE/MWCNT-IgG/BSA/Diluted serum 1/100 + BRCA1 (1.0 × 10−12 M) | (1.22 ± 0.09) × 103 |
| Platform | Detection Limit | Linear Range | Ref. |
|---|---|---|---|
| Electrochemical Technique: DPV | |||
| GCE modified with an electrodeposited nanocomposite of PEP-doped PEDOT | 3.4 fM | 0.01 pM to 1.0 nM | [29] |
| Au modified with Fc-cored PAMAM as support for DNAp | 0.38 nM | 1.3 nM to 20 nM | [30] |
| PGE modified with a GO and IL composite | 6.7 nM | --- | [31] |
| AuSPE modified with antimonene non-covalently functionalized with DNAp | 28.3 pg μL−1 (~128 pM) | 0.1 to 20.0 ng μL−1 (~452 pM to ~90 nM) | [34] |
| Electrodeposited nanoAu surface as support for a stem-loop DNAp modified with a thiol group (5′-end) and MB (3′-end) | 52 pM | 5.0 nM to 70 nM | [35] |
| GCE modified with 3D-rGO and PANI nanofibers | 0.301 fM | 1.0 fM to 100 nM | [36] |
| Electrochemical Technique: EIS | |||
| CPE modified with electrospun conductive nanofibers of PES and MWCNTs as platform to immobilize DNAp | 2.4 pM | 5.0 pM to 14 nM | [32] |
| GCE modified with MWCNTs and MOF with Fe3O4 nanoparticles core to immobilize DNAp | 0.57 fM | 1.0 fM to 100 pM | [33] |
| Au modified with an antifouling zwitterionic peptide SAM to covalently bind DNAp | 0.3 fM | 1.0 fM to 10.0 pM | [37] |
| Au modified with MXene/AuNP@BLM to immobilize SH-DNAp | 1.0 zM | 1.0 zM to 1.0 μM | [38] |
| GCE modified with rGO and PANHS to graft DNAp | 0.35 aM | 1.0 aM to 100 pM | [39] |
| GCE modified with ePDA/TA/tetraPEG/eAuNP to immobilize SH-DNAp | 0.05 fM | 0.1 fM to 10 pM | [40] |
| GCE modified with MWCNT-IgG/DNAp | 0.3 fM | 1.0 fM to 100 pM | This work |
| Platform | Detection Limit (μM) | Linear Range (μM) | Ref. | ||
|---|---|---|---|---|---|
| Do | UA | Do | UA | ||
| Electrochemical Technique: DPV | |||||
| GCE modified with MWCNTs and a Zr-based MOF (DUT-67) grown on ZnCo2O4 nanoflowers | 0.012 | 0.0087 | 1.0 to 180.0 | 1.0 to 180.0 | [58] |
| GCE modified with a cMWCNT/CD-PMEL nanocomposite obtained by polymerization of CD-fixed MEL residues | 0.023 | 0.064 | 0.1 to 10 | 0.1 to 200 | [59] |
| GCE modified with FeNPs encapsulated in BNC | 0.8 | 0.28 | 1 to 630 | 0.5 to 2065 | [60] |
| GCE modified with cMWCNTs and TFPB-TAPB-COF | 0.073 | 0.063 | 0.6 to 250 | 0.6 to 250 | [61] |
| GCE modified with a rGO-β-CD-cMWCNT-rPOM tetracomponent hybrid | 0.04 | 0.05 | 0.5 to 300 | 1 to 400 | [62] |
| GCE modified with microporous carbon | 0.2 | 1.7 | 10 to 150 | 10 to 150 | [63] |
| GCE modified with DBTA-TAPT-COF, La2O3, and cMWCNTs | 0.039 | 0.024 | 2 to 450 | 0.4 to 450 | [64] |
| GCE modified with a LaV-MWCNT nanocomposite | 0.046 | 0.025 | 2 to 100 | 2 to 100 | [65] |
| GCE modified with Gr, SWCNTs, eCeNPs, eCuNPs, and Tween 20 | 0.0072 | 0.0063 | 0.1 to 100 | 0.08 to 100 | [66] |
| GCE modified with eAuNPs and a TFPPy-PDA-COF—aMWCNT dispersion | 0.21 | 0.29 | 0.7 to 108 | 0.97 to 200 | [67] |
| Electrochemical Technique: LSV | |||||
| GCE modified with MWCNT-IgG | 0.33 | 0.33 | 1 to 500 | 1 to 500 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mujica, M.L.; Tamborelli, A.; Dalmasso, P.; Rivas, G. Label-Free Electrochemical Sensing Using Glassy Carbon Electrodes Modified with Multiwalled-Carbon Nanotubes Non-Covalently Functionalized with Human Immunoglobulin G. Chemosensors 2024, 12, 4. https://doi.org/10.3390/chemosensors12010004
Mujica ML, Tamborelli A, Dalmasso P, Rivas G. Label-Free Electrochemical Sensing Using Glassy Carbon Electrodes Modified with Multiwalled-Carbon Nanotubes Non-Covalently Functionalized with Human Immunoglobulin G. Chemosensors. 2024; 12(1):4. https://doi.org/10.3390/chemosensors12010004
Chicago/Turabian StyleMujica, Michael López, Alejandro Tamborelli, Pablo Dalmasso, and Gustavo Rivas. 2024. "Label-Free Electrochemical Sensing Using Glassy Carbon Electrodes Modified with Multiwalled-Carbon Nanotubes Non-Covalently Functionalized with Human Immunoglobulin G" Chemosensors 12, no. 1: 4. https://doi.org/10.3390/chemosensors12010004






